Central Neurocircuits Regulating Food Intake in Response to Gut Inputs—Preclinical Evidence
Abstract
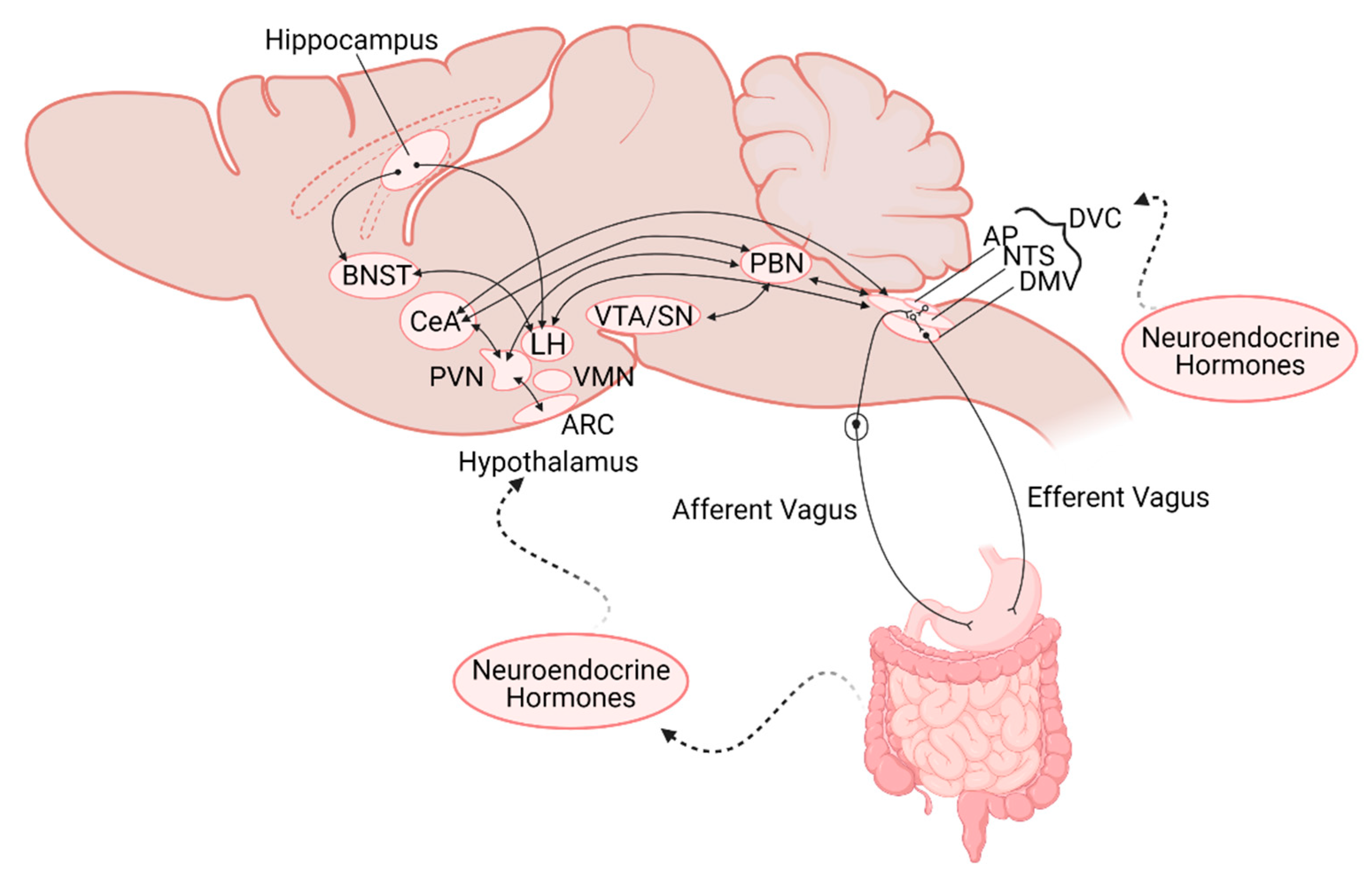
:1. Introduction
1.1. Afferent Inputs
1.2. Nucleus of the Tractus Solitarius (NTS)
1.3. Dorsal Motor Nucleus of the Vagus (DMV)
1.4. Area Postrema
1.5. Parabrachial Nucleus
1.6. Cerebellum
1.7. Hypothalamus
1.8. Hippocampus
1.9. Amygdala
2. Pathophysiology
2.1. Obesity, Diabetes, Inflammation
2.2. Developmental Modulation of Central Neurocircuits
2.3. Neurological Disorders
3. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Browning, K.N.; Travagli, R.A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol. 2014, 4, 1339–1368. [Google Scholar] [CrossRef] [Green Version]
- Brookes, S.J.; Spencer, N.J.; Costa, M.; Zagorodnyuk, V.P. Extrinsic primary afferent signalling in the gut. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Berthoud, H.R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef]
- Moran, T.H.; Ladenheim, E.E. Physiologic and Neural Controls of Eating. Gastroenterol. Clin. N. Am. 2016, 45, 581–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.B.; de Lartigue, G.; Page, A.J. Dissecting the Role of Subtypes of Gastrointestinal Vagal Afferents. Front. Physiol. 2020, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Mesgarzadeh, S.; Ramesh, K.S.; Huey, E.L.; Liu, Y.; Gray, L.A.; Aitken, T.J.; Chen, Y.; Beutler, L.R.; Ahn, J.S.; et al. Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell 2019, 179, 1129–1143.e1123. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.K.; Chang, R.B.; Strochlic, D.E.; Umans, B.D.; Lowell, B.B.; Liberles, S.D. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell 2016, 166, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Vincent, K.M.; Sharp, J.W.; Raybould, H.E. Intestinal glucose-induced calcium-calmodulin kinase signaling in the gut-brain axis in awake rats. Neurogastroenterol. Motil. 2011, 23, e282–e293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raybould, H.E. Gut chemosensing: Interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. 2010, 153, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Dockray, G.J. Enteroendocrine cell signalling via the vagus nerve. Curr. Opin. Pharm. 2013, 13, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Dockray, G.J. Luminal sensing in the gut: An overview. J. Physiol. Pharmacol. 2003, 54 (Suppl. 4), 9–17. [Google Scholar] [PubMed]
- Lassman, D.J.; McKie, S.; Gregory, L.J.; Lal, S.; D’Amato, M.; Steele, I.; Varro, A.; Dockray, G.J.; Williams, S.C.; Thompson, D.G. Defining the role of cholecystokinin in the lipid-induced human brain activation matrix. Gastroenterology 2010, 138, 1514–1524. [Google Scholar] [CrossRef]
- Fernandes, A.B.; Alves da Silva, J.; Almeida, J.; Cui, G.; Gerfen, C.R.; Costa, R.M.; Oliveira-Maia, A.J. Postingestive Modulation of Food Seeking Depends on Vagus-Mediated Dopamine Neuron Activity. Neuron 2020, 106, 778–788.e776. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361. [Google Scholar] [CrossRef] [Green Version]
- Kalia, M.; Mesulam, M.M. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J. Comp. Neurol. 1980, 193, 435–465. [Google Scholar] [CrossRef]
- Norgren, R.; Smith, G.P. Central distribution of subdiaphragmatic vagal branches in the rat. J. Comp. Neurol. 1988, 273, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Altschuler, S.M.; Bao, X.M.; Bieger, D.; Hopkins, D.A.; Miselis, R.R. Viscerotopic representation of the upper alimentary tract in the rat: Sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J. Comp. Neurol. 1989, 283, 248–268. [Google Scholar] [CrossRef]
- Broussard, D.L.; Altschuler, S.M. Brainstem viscerotopic organization of afferents and efferents involved in the control of swallowing. Am. J. Med. 2000, 108 (Suppl. 4a), 79s–86s. [Google Scholar] [CrossRef]
- Jean, A. The nucleus tractus solitarius: Neuroanatomic, neurochemical and functional aspects. Arch. Int. Physiol. Biochim. Biophys. 1991, 99, A3–A52. [Google Scholar] [CrossRef]
- Andresen, M.C.; Yang, M.Y. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am. J. Physiol. 1990, 259, H1307–H1311. [Google Scholar] [CrossRef] [PubMed]
- Andresen, M.C.; Kunze, D.L. Nucleus tractus solitarius—Gateway to neural circulatory control. Annu. Rev. Physiol. 1994, 56, 93–116. [Google Scholar] [CrossRef]
- Travagli, R.A.; Gillis, R.A.; Rossiter, C.D.; Vicini, S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am. J. Physiol. 1991, 260, G531–G536. [Google Scholar] [CrossRef]
- Clyburn, C.; Travagli, R.A.; Browning, K.N. Acute high-fat diet upregulates glutamatergic signaling in the dorsal motor nucleus of the vagus. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G623–G634. [Google Scholar] [CrossRef]
- Wright, J.; Campos, C.; Herzog, T.; Covasa, M.; Czaja, K.; Ritter, R.C. Reduction of food intake by cholecystokinin requires activation of hindbrain NMDA-type glutamate receptors. Am. J. Physiol. Regul. Integr Comp. Physiol. 2011, 301, R448–R455. [Google Scholar] [CrossRef]
- Guard, D.B.; Swartz, T.D.; Ritter, R.C.; Burns, G.A.; Covasa, M. Blockade of hindbrain NMDA receptors containing NR2 subunits increases sucrose intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R921–R928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Peters, J.H.; Zhu, M.; Page, S.J.; Ritter, R.C.; Appleyard, S.M. Frequency-dependent facilitation of synaptic throughput via postsynaptic NMDA receptors in the nucleus of the solitary tract. J. Physiol. 2015, 593, 111–125. [Google Scholar] [CrossRef]
- Glaum, S.R.; Miller, R.J. Activation of metabotropic glutamate receptors produces reciprocal regulation of ionotropic glutamate and GABA responses in the nucleus of the tractus solitarius of the rat. J. Neurosci. 1993, 13, 1636–1641. [Google Scholar] [CrossRef] [Green Version]
- Foley, C.M.; Moffitt, J.A.; Hay, M.; Hasser, E.M. Glutamate in the nucleus of the solitary tract activates both ionotropic and metabotropic glutamate receptors. Am. J. Physiol. 1998, 275, R1858–R1866. [Google Scholar] [CrossRef] [PubMed]
- De Lartigue, G. Putative roles of neuropeptides in vagal afferent signaling. Physiol. Behav. 2014, 136, 155–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babic, T.; Troy, A.E.; Fortna, S.R.; Browning, K.N. Glucose-dependent trafficking of 5-HT3 receptors in rat gastrointestinal vagal afferent neurons. Neurogastroenterol. Motil. 2012, 24, e476–e488. [Google Scholar] [CrossRef] [Green Version]
- Troy, A.E.; Simmonds, S.S.; Stocker, S.D.; Browning, K.N. High fat diet attenuates glucose-dependent facilitation of 5-HT3 -mediated responses in rat gastric vagal afferents. J. Physiol. 2016, 594, 99–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, S.; Browning, K.N. D-glucose modulates synaptic transmission from the central terminals of vagal afferent fibers. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G757–G763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, S.; Browning, K.N. Glucose increases synaptic transmission from vagal afferent central nerve terminals via modulation of 5-HT3 receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1050–G1057. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.L.; Zhu, M.; Zhao, H.; Dillon, C.; Appleyard, S.M. High glucose increases action potential firing of catecholamine neurons in the nucleus of the solitary tract by increasing spontaneous glutamate inputs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R229–R239. [Google Scholar] [CrossRef] [Green Version]
- Hosford, P.S.; Mifflin, S.W.; Ramage, A.G. 5-hydroxytryptamine-mediated neurotransmission modulates spontaneous and vagal-evoked glutamate release in the nucleus of the solitary tract effect of uptake blockade. J. Pharmacol. Exp. Ther. 2014, 349, 288–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Bado, A.; Levasseur, S.; Attoub, S.; Kermorgant, S.; Laigneau, J.P.; Bortoluzzi, M.N.; Moizo, L.; Lehy, T.; Guerre-Millo, M.; Le Marchand-Brustel, Y.; et al. The stomach is a source of leptin. Nature 1998, 394, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Neyens, D.; Zhao, H.; Huston, N.J.; Wayman, G.A.; Ritter, R.C.; Appleyard, S.M. Leptin Sensitizes NTS Neurons to Vagal Input by Increasing Postsynaptic NMDA Receptor Currents. J. Neurosci. 2020, 40, 7054–7064. [Google Scholar] [CrossRef]
- Cheng, W.; Ndoka, E.; Hutch, C.; Roelofs, K.; MacKinnon, A.; Khoury, B.; Magrisso, J.; Kim, K.S.; Rhodes, C.J.; Olson, D.P.; et al. Leptin receptor-expressing nucleus tractus solitarius neurons suppress food intake independently of GLP1 in mice. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyda, T.D.; Smith, P.M.; Ferguson, A.V. Gastrointestinal hormone actions in the central regulation of energy metabolism: Potential sensory roles for the circumventricular organs. Int. J. Obes. 2009, 33 (Suppl. 1), S16–S21. [Google Scholar] [CrossRef] [Green Version]
- Cottrell, G.T.; Ferguson, A.V. Sensory circumventricular organs: Central roles in integrated autonomic regulation. Regul. Pept. 2004, 117, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Fry, M.; Ferguson, A.V. The sensory circumventricular organs: Brain targets for circulating signals controlling ingestive behavior. Physiol. Behav. 2007, 91, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Baptista, V.; Browning, K.N.; Travagli, R.A. Effects of cholecystokinin-8s in the nucleus tractus solitarius of vagally deafferented rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1092–R1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapp, S.; Richards, J.E. The gut hormone glucagon-like peptide-1 produced in brain: Is this physiologically relevant? Curr. Opin. Pharmacol. 2013, 13, 964–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browning, K.N.; Travagli, R.A. Central control of gastrointestinal motility. Curr. Opin. Endocrinol. Diabetes Obes. 2019. [Google Scholar] [CrossRef]
- Roman, C.W.; Derkach, V.A.; Palmiter, R.D. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat. Commun. 2016, 7, 11905. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Y.-Y.; Lan, J.-N.; Liu, H.-M.; Li, W.; Wu, Y.; Leng, Y.; Tang, L.-H.; Hou, J.-B.; Sun, Q.; et al. Ischemic Postconditioning Alleviates Intestinal Ischemia-Reperfusion Injury by Enhancing Autophagy and Suppressing Oxidative Stress through the Akt/GSK-/Nrf2 Pathway in Mice. Oxidative Med. Cell. Longev. 2020, 2020, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Fox, E.A.; Powley, T.L. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res. 1985, 341, 269–282. [Google Scholar] [CrossRef]
- Shapiro, R.E.; Miselis, R.R. The central organization of the vagus nerve innervating the stomach of the rat. J. Comp. Neurol. 1985, 238, 473–488. [Google Scholar] [CrossRef]
- Browning, K.N.; Renehan, W.E.; Travagli, R.A. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J. Physiol. 1999, 517 Pt 2, 521–532. [Google Scholar] [CrossRef]
- Fogel, R.; Zhang, X.; Renehan, W.E. Relationships between the morphology and function of gastric and intestinal distention-sensitive neurons in the dorsal motor nucleus of the vagus. J. Comp. Neurol. 1996, 364, 78–91. [Google Scholar] [CrossRef]
- Valenzuela, I.M.; Browning, K.N.; Travagli, R.A. Morphological differences between planes of section do not influence the electrophysiological properties of identified rat dorsal motor nucleus of the vagus neurons. Brain Res. 2004, 1003, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travagli, R.A.; Hermann, G.E.; Browning, K.N.; Rogers, R.C. Brainstem circuits regulating gastric function. Annu. Rev. Physiol. 2006, 68, 279–305. [Google Scholar] [CrossRef] [Green Version]
- Cruz, M.T.; Murphy, E.C.; Sahibzada, N.; Verbalis, J.G.; Gillis, R.A. A reevaluation of the effects of stimulation of the dorsal motor nucleus of the vagus on gastric motility in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R291–R307. [Google Scholar] [CrossRef] [Green Version]
- Krowicki, Z.K.; Sharkey, K.A.; Serron, S.C.; Nathan, N.A.; Hornby, P.J. Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function. J. Comp. Neurol. 1997, 377, 49–69. [Google Scholar] [CrossRef]
- Rogers, R.C.; Hermann, G.E.; Travagli, R.A. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J. Physiol. 1999, 514 Pt 2, 369–383. [Google Scholar] [CrossRef]
- Rogers, R.C.; Travagli, R.A.; Hermann, G.E. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R479–R489. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.J.; Browning, K.N.; Rogers, R.C.; Travagli, R.A. Catecholaminergic neurons in rat dorsal motor nucleus of vagus project selectively to gastric corpus. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G361–G367. [Google Scholar] [CrossRef]
- Babic, T.; Browning, K.N.; Travagli, R.A. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G21–G32. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, R.; Fortna, S.R.; Browning, K.N. Exposure to a high fat diet during the perinatal period alters vagal motoneurone excitability, even in the absence of obesity. J. Physiol. 2015, 593, 285–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K.W.; Zsombok, A.; Smith, B.N. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology 2007, 148, 1868–1881. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kaneko, K.; Lin, H.Y.; Mo, Q.; Xu, Y.; Suganami, T.; Ravn, P.; Fukuda, M. Gut Hormone GIP Induces Inflammation and Insulin Resistance in the Hypothalamus. Endocrinology 2020, 161. [Google Scholar] [CrossRef] [PubMed]
- Clyburn, C.; Browning, K.N. Role of astroglia in diet-induced central neuroplasticity. J. Neurophysiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J.; Hoyda, T.D.; Ferguson, A.V. The area postrema: A brain monitor and integrator of systemic autonomic state. Neuroscientist 2008, 14, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Babic, T.; Browning, K.N. The role of vagal neurocircuits in the regulation of nausea and vomiting. Eur. J. Pharmacol. 2014, 722, 38–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, D.R.; Campbell, N.J.; Shaw, T.M.; Woodruff, G.N. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists. J. Neurosci. 1987, 7, 2967–2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercer, L.D.; Beart, P.M. Immunolocalization of CCK1R in rat brain using a new anti-peptide antibody. Neurosci. Lett. 2004, 359, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Covasa, M.; Ritter, R.C. Reduced CCK-induced Fos expression in the hindbrain, nodose ganglia, and enteric neurons of rats lacking CCK-1 receptors. Brain Res. 2005, 1051, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Kishi, T.; Lee, C.E.; Choi, B.J.; Fang, H.; Hollenberg, A.N.; Drucker, D.J.; Elmquist, J.K. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J. Neurosci. 2003, 23, 2939–2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaz, B.; Taylor, I.; Taché, Y. Peripheral peptide YY induces c-fos-like immunoreactivity in the rat brain. Neurosci. Lett. 1993, 163, 77–80. [Google Scholar] [CrossRef]
- Gilg, S.; Lutz, T.A. The orexigenic effect of peripheral ghrelin differs between rats of different age and with different baseline food intake, and it may in part be mediated by the area postrema. Physiol. Behav. 2006, 87, 353–359. [Google Scholar] [CrossRef]
- Van der Kooy, D.; Koda, L.Y. Organization of the projections of a circumventricular organ: The area postrema in the rat. J. Comp. Neurol. 1983, 219, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Menani, J.V.; Thunhorst, R.L.; Johnson, A.K. Lateral parabrachial nucleus and serotonergic mechanisms in the control of salt appetite in rats. Am. J. Physiol. 1996, 270, R162–R168. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Takayama, K. Circulatory and respiratory responses to glutamate stimulation of the lateral parabrachial nucleus of the cat. J. Auton. Nerv. Syst 1991, 32, 121–133. [Google Scholar] [CrossRef]
- Vigier, D.; Portalier, P. Efferent projections of the area postrema demonstrated by autoradiography. Arch. Ital. Biol. 1979, 117, 308–324. [Google Scholar] [PubMed]
- Andermann, M.L.; Lowell, B.B. toward a wiring diagram understanding of appetite control. Neuron 2017. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Heo, G.; Kim, M.; Kim, H.; Jin, J.A.; Kim, H.K.; Jung, S.; An, M.; Ahn, B.H.; Park, J.H.; et al. A neural circuit mechanism for mechanosensory feedback control of ingestion. Nature 2020, 580, 376–380. [Google Scholar] [CrossRef]
- Kreisler, A.D.; Davis, E.A.; Rinaman, L. Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non-entrained rats after intake of satiating vs. non-satiating meals. Physiol. Behav. 2014, 136, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Haubensak, W.; Anthony, T.E.; Anderson, D.J. Central amygdala PKC-δ(+) neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci. 2014, 17, 1240–1248. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.A.; Bowen, A.J.; Schwartz, M.W.; Palmiter, R.D. Parabrachial CGRP Neurons Control Meal Termination. Cell Metab. 2016, 23, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.W.; Gao, X.B.; et al. A Neural Circuit for Gut-Induced Reward. Cell 2018, 175, 665–678.e623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.N.; Yung, W.H.; Kwok-Chong Chow, B.; Chan, Y.S.; Wang, J.J. The cerebellar-hypothalamic circuits: Potential pathways underlying cerebellar involvement in somatic-visceral integration. Brain Res. Rev. 2006, 52, 93–106. [Google Scholar] [CrossRef]
- Dietrichs, E.; Haines, D.E.; Røste, G.K.; Røste, L.S. Hypothalamocerebellar and cerebellohypothalamic projections—Circuits for regulating nonsomatic cerebellar activity? Histol. Histopathol. 1994, 9, 603–614. [Google Scholar] [PubMed]
- Haines, D.E.; Dietrichs, E.; Mihailoff, G.A.; McDonald, E.F. The cerebellar-hypothalamic axis: Basic circuits and clinical observations. Int. Rev. Neurobiol. 1997, 41, 83–107. [Google Scholar] [CrossRef] [PubMed]
- Cavdar, S.; San, T.; Aker, R.; Sehirli, U.; Onat, F. Cerebellar connections to the dorsomedial and posterior nuclei of the hypothalamus in the rat. J. Anat. 2001, 198, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Cavdar, S.; Onat, F.; Aker, R.; Sehirli, U.; San, T.; Yananli, H.R. The afferent connections of the posterior hypothalamic nucleus in the rat using horseradish peroxidase. J. Anat. 2001, 198, 463–472. [Google Scholar] [CrossRef]
- Li, B.; Zhuang, Q.X.; Gao, H.R.; Wang, J.J.; Zhu, J.N. Medial cerebellar nucleus projects to feeding-related neurons in the ventromedial hypothalamic nucleus in rats. Brain Struct. Funct. 2017, 222, 957–971. [Google Scholar] [CrossRef]
- Zhu, J.N.; Li, H.Z.; Ding, Y.; Wang, J.J. Cerebellar modulation of feeding-related neurons in rat dorsomedial hypothalamic nucleus. J. Neurosci. Res. 2006, 84, 1597–1609. [Google Scholar] [CrossRef]
- Zhu, J.N.; Guo, C.L.; Li, H.Z.; Wang, J.J. Dorsomedial hypothalamic nucleus neurons integrate important peripheral feeding-related signals in rats. J. Neurosci. Res. 2007, 85, 3193–3204. [Google Scholar] [CrossRef]
- Li, B.; Guo, C.L.; Tang, J.; Zhu, J.N.; Wang, J.J. Cerebellar fastigial nuclear inputs and peripheral feeding signals converge on neurons in the dorsomedial hypothalamic nucleus. Neurosignals 2009, 17, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sugiyama, Y.; Yates, B.J. Integrative responses of neurons in parabrachial nuclei to a nauseogenic gastrointestinal stimulus and vestibular stimulation in vertical planes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R965–R975. [Google Scholar] [CrossRef] [Green Version]
- Mihalache, L.; Gherasim, A.; Niţă, O.; Ungureanu, M.C.; Pădureanu, S.S.; Gavril, R.S.; Arhire, L.I. Effects of ghrelin in energy balance and body weight homeostasis. Hormones 2016, 15, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Teubner, B.J.; Bartness, T.J. PYY(3-36) into the arcuate nucleus inhibits food deprivation-induced increases in food hoarding and intake. Peptides 2013, 47, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.S.; Nunn, N.; Chambers, A.P.; Østergaard, S.; Wulff, B.S.; Luckman, S.M. Modified Peptide YY Molecule Attenuates the Activity of NPY/AgRP Neurons and Reduces Food Intake in Male Mice. Endocrinology 2019, 160, 2737–2747. [Google Scholar] [CrossRef] [Green Version]
- Chronwall, B.M. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides 1985, 6 (Suppl. 2), 1–11. [Google Scholar] [CrossRef]
- DeFalco, J.; Tomishima, M.; Liu, H.; Zhao, C.; Cai, X.; Marth, J.D.; Enquist, L.; Friedman, J.M. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science 2001, 291, 2608–2613. [Google Scholar] [CrossRef]
- Aklan, I.; Sayar Atasoy, N.; Yavuz, Y.; Ates, T.; Coban, I.; Koksalar, F.; Filiz, G.; Topcu, I.C.; Oncul, M.; Dilsiz, P.; et al. NTS Catecholamine Neurons Mediate Hypoglycemic Hunger via Medial Hypothalamic Feeding Pathways. Cell Metab. 2020, 31, 313–326.e315. [Google Scholar] [CrossRef]
- Tsang, A.H.; Nuzzaci, D.; Darwish, T.; Samudrala, H.; Blouet, C. Nutrient sensing in the nucleus of the solitary tract mediates non-aversive suppression of feeding via inhibition of AgRP neurons. Mol. Metab. 2020, 42, 101070. [Google Scholar] [CrossRef]
- Harris, R.B.S. Loss of leptin receptor-expressing cells in the hindbrain decreases forebrain leptin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E806–E816. [Google Scholar] [CrossRef]
- Swanson, L.W.; Sawchenko, P.E. Hypothalamic integration: Organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 1983, 6, 269–324. [Google Scholar] [CrossRef]
- D’Agostino, G.; Lyons, D.J.; Cristiano, C.; Burke, L.K.; Madara, J.C.; Campbell, J.N.; Garcia, A.P.; Land, B.B.; Lowell, B.B.; Dileone, R.J.; et al. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. Elife 2016, 5. [Google Scholar] [CrossRef]
- Luiten, P.G.; ter Horst, G.J.; Karst, H.; Steffens, A.B. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985, 329, 374–378. [Google Scholar] [CrossRef]
- Saper, C.B.; Loewy, A.D.; Swanson, L.W.; Cowan, W.M. Direct hypothalamo-autonomic connections. Brain Res. 1976, 117, 305–312. [Google Scholar] [CrossRef]
- Heymann-Mönnikes, I.; Taché, Y.; Trauner, M.; Weiner, H.; Garrick, T. CRF microinjected into the dorsal vagal complex inhibits TRH analog- and kainic acid-stimulated gastric contractility in rats. Brain Res. 1991, 554, 139–144. [Google Scholar] [CrossRef]
- Lewis, M.W.; Hermann, G.E.; Rogers, R.C.; Travagli, R.A. In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J. Physiol. 2002, 543, 135–146. [Google Scholar] [CrossRef]
- Holmes, G.M.; Browning, K.N.; Babic, T.; Fortna, S.R.; Coleman, F.H.; Travagli, R.A. Vagal afferent fibres determine the oxytocin-induced modulation of gastric tone. J. Physiol. 2013, 591, 3081–3100. [Google Scholar] [CrossRef]
- Peters, J.H.; McDougall, S.J.; Kellett, D.O.; Jordan, D.; Llewellyn-Smith, I.J.; Andresen, M.C. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J. Neurosci. 2008, 28, 11731–11740. [Google Scholar] [CrossRef]
- Richard, P.; Moos, F.; Freund-Mercier, M.J. Central effects of oxytocin. Physiol. Rev. 1991, 71, 331–370. [Google Scholar] [CrossRef]
- Zheng, H.; Patterson, L.M.; Berthoud, H.R. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J. Comp. Neurol. 2005, 485, 127–142. [Google Scholar] [CrossRef]
- Grill, H.J.; Hayes, M.R. Hindbrain Neurons as an Essential Hub in the Neuroanatomically Distributed Control of Energy Balance. Cell Metab. 2012, 16, 296–309. [Google Scholar] [CrossRef] [Green Version]
- Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef]
- Page, A.J.; Christie, S.; Symonds, E.; Li, H. Circadian regulation of appetite and time restricted feeding. Physiol. Behav. 2020, 220, 112873. [Google Scholar] [CrossRef] [PubMed]
- Min, D.K.; Tuor, U.I.; Chelikani, P.K. Gastric distention induced functional magnetic resonance signal changes in the rodent brain. Neuroscience 2011, 179, 151–158. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Grill, H.J. Hippocampus Contributions to Food Intake Control: Mnemonic, Neuroanatomical, and Endocrine Mechanisms. Biol. Psychiatry 2017, 81, 748–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cenquizca, L.A.; Swanson, L.W. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J. Comp. Neurol. 2006, 497, 101–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, T.M.; Hahn, J.D.; Konanur, V.R.; Noble, E.E.; Suarez, A.N.; Thai, J.; Nakamoto, E.M.; Kanoski, S.E. Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Radley, J.J.; Sawchenko, P.E. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J. Neurosci. 2011, 31, 9683–9695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannapel, R.C.; Henderson, Y.H.; Nalloor, R.; Vazdarjanova, A.; Parent, M.B. Ventral hippocampal neurons inhibit postprandial energy intake. Hippocampus 2017, 27, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.N.; Liu, C.M.; Cortella, A.M.; Noble, E.E.; Kanoski, S.E. Ghrelin and Orexin Interact to Increase Meal Size Through a Descending Hippocampus to Hindbrain Signaling Pathway. Biol. Psychiatry 2020, 87, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.A.; Wald, H.S.; Suarez, A.N.; Zubcevic, J.; Liu, C.M.; Cortella, A.M.; Kamitakahara, A.K.; Polson, J.W.; Arnold, M.; Grill, H.J.; et al. Ghrelin Signaling Affects Feeding Behavior, Metabolism, and Memory through the Vagus Nerve. Curr. Biol. 2020, 30, 4510–4518.e4516. [Google Scholar] [CrossRef] [PubMed]
- Kanoski, S.E.; Hayes, M.R.; Greenwald, H.S.; Fortin, S.M.; Gianessi, C.A.; Gilbert, J.R.; Grill, H.J. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology 2011, 36, 1859–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, T.M.; Noble, E.E.; Liu, C.M.; Cortella, A.M.; Konanur, V.R.; Suarez, A.N.; Reiner, D.J.; Hahn, J.D.; Hayes, M.R.; Kanoski, S.E. A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Mol. Psychiatry 2018, 23, 1555–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, Y.O.; Smith, G.P.; Parent, M.B. Hippocampal neurons inhibit meal onset. Hippocampus 2013, 23, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.B. Cognitive control of meal onset and meal size: Role of dorsal hippocampal-dependent episodic memory. Physiol. Behav. 2016, 162, 112–119. [Google Scholar] [CrossRef]
- Van den Burg, E.H.; Stoop, R. Neuropeptide signalling in the central nucleus of the amygdala. Cell Tissue Res. 2019, 375, 93–101. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, J.; Schmit, M.B.; Cho, T.S.; Fang, C.; Cai, H. A bed nucleus of stria terminalis microcircuit regulating inflammation-associated modulation of feeding. Nat. Commun. 2019, 10, 2769. [Google Scholar] [CrossRef]
- De Lartigue, G.; Xu, C. Mechanisms of vagal plasticity influencing feeding behavior. Brain Res. 2018, 1693, 146–150. [Google Scholar] [CrossRef]
- Kentish, S.; Li, H.; Philp, L.K.; O’Donnell, T.A.; Isaacs, N.J.; Young, R.L.; Wittert, G.A.; Blackshaw, L.A.; Page, A.J. Diet-induced adaptation of vagal afferent function. J. Physiol. 2012, 590, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Feinle-Bisset, C. Upper gastrointestinal sensitivity to meal-related signals in adult humans—Relevance to appetite regulation and gut symptoms in health, obesity and functional dyspepsia. Physiol. Behav. 2016, 162, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Daly, D.M.; Park, S.J.; Valinsky, W.C.; Beyak, M.J. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J. Physiol. 2011, 589, 2857–2870. [Google Scholar] [CrossRef]
- Browning, K.N.; Fortna, S.R.; Hajnal, A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J. Physiol. 2013, 591, 2357–2372. [Google Scholar] [CrossRef] [PubMed]
- Waise, T.M.Z.; Toshinai, K.; Naznin, F.; NamKoong, C.; Md Moin, A.S.; Sakoda, H.; Nakazato, M. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem. Biophys. Res. Commun. 2015, 464, 1157–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, B.E.; Triscari, J.; Hogan, S.; Sullivan, A.C. Resistance to diet-induced obesity: Food intake, pancreatic sympathetic tone, and insulin. Am. J. Physiol. 1987, 252, R471–R478. [Google Scholar] [CrossRef]
- Lee, S.J.; Jokiaho, A.J.; Sanchez-Watts, G.; Watts, A.G. Catecholaminergic projections into an interconnected forebrain network control the sensitivity of male rats to diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R811–R823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaman, L. Postnatal development of hypothalamic inputs to the dorsal vagal complex in rats. Physiol. Behav. 2003, 79, 65–70. [Google Scholar] [CrossRef]
- Levin, B.E. Developmental gene x environment interactions affecting systems regulating energy homeostasis and obesity. Front. Neuroendocrinol. 2010, 31, 270–283. [Google Scholar] [CrossRef] [Green Version]
- Levin, B.E. Metabolic imprinting: Critical impact of the perinatal environment on the regulation of energy homeostasis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1107–1121. [Google Scholar] [CrossRef] [Green Version]
- Bocarsly, M.E.; Barson, J.R.; Hauca, J.M.; Hoebel, B.G.; Leibowitz, S.F.; Avena, N.M. Effects of perinatal exposure to palatable diets on body weight and sensitivity to drugs of abuse in rats. Physiol. Behav. 2012, 107, 568–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clyburn, C.; Howe, C.A.; Arnold, A.C.; Lang, C.H.; Travagli, R.A.; Browning, K.N. Perinatal high-fat diet alters development of GABA A receptor subunits in dorsal motor nucleus of vagus. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G40–G50. [Google Scholar] [CrossRef]
- Poon, K. Behavioral Feeding Circuit: Dietary Fat-Induced Effects of Inflammatory Mediators in the Hypothalamus. Front. Endocrinol. 2020, 11, 591559. [Google Scholar] [CrossRef]
- Poon, K.; Mandava, S.; Chen, K.; Barson, J.R.; Buschlen, S.; Leibowitz, S.F. Prenatal exposure to dietary fat induces changes in the transcriptional factors, TEF and YAP, which may stimulate differentiation of peptide neurons in rat hypothalamus. PLoS ONE 2013, 8, e77668. [Google Scholar] [CrossRef] [Green Version]
- Stump, M.; Guo, D.F.; Lu, K.T.; Mukohda, M.; Cassell, M.D.; Norris, A.W.; Rahmouni, K.; Sigmund, C.D. Nervous System Expression of PPARγ and Mutant PPARγ Has Profound Effects on Metabolic Regulation and Brain Development. Endocrinology 2016, 157, 4266–4275. [Google Scholar] [CrossRef]
- Sullivan, E.L.; Rivera, H.M.; True, C.A.; Franco, J.G.; Baquero, K.; Dean, T.A.; Valleau, J.C.; Takahashi, D.L.; Frazee, T.; Hanna, G.; et al. Maternal and postnatal high-fat diet consumption programs energy balance and hypothalamic melanocortin signaling in nonhuman primate offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R169–R179. [Google Scholar] [CrossRef]
- Juan De Solis, A.; Baquero, A.F.; Bennett, C.M.; Grove, K.L.; Zeltser, L.M. Postnatal undernutrition delays a key step in the maturation of hypothalamic feeding circuits. Mol. Metab. 2016, 5, 198–209. [Google Scholar] [CrossRef]
- Franklin, T.B.; Saab, B.J.; Mansuy, I.M. Neural mechanisms of stress resilience and vulnerability. Neuron 2012, 75, 747–761. [Google Scholar] [CrossRef] [Green Version]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Greenwood-Van Meerveld, B.; Johnson, A.C.; Travagli, R.A. Role of estrogen and stress on the brain-gut axis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G203–G209. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukudo, S. Stress and visceral pain: Focusing on irritable bowel syndrome. Pain 2013, 154 (Suppl. 1), S63–S70. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Travagli, R.A. Hypothalamic-vagal oxytocinergic neurocircuitry modulates gastric emptying and motility following stress. J. Physiol. 2020, 598, 4941–4955. [Google Scholar] [CrossRef] [PubMed]
- Horvath, K.; Perman, J.A. Autistic disorder and gastrointestinal disease. Curr. Opin. Pediatr. 2002, 14, 583–587. [Google Scholar] [CrossRef]
- Chistol, L.T.; Bandini, L.G.; Must, A.; Phillips, S.; Cermak, S.A.; Curtin, C. Sensory Sensitivity and Food Selectivity in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 583–591. [Google Scholar] [CrossRef]
- Ristori, M.V.; Quagliariello, A.; Reddel, S.; Ianiro, G.; Vicari, S.; Gasbarrini, A.; Putignani, L. Autism, Gastrointestinal Symptoms and Modulation of Gut Microbiota by Nutritional Interventions. Nutrients 2019, 11, 2812. [Google Scholar] [CrossRef] [Green Version]
- Lefter, R.; Ciobica, A.; Timofte, D.; Stanciu, C.; Trifan, A. A Descriptive Review on the Prevalence of Gastrointestinal Disturbances and Their Multiple Associations in Autism Spectrum Disorder. Medicina 2019, 56, 11. [Google Scholar] [CrossRef] [Green Version]
- Horvath, K.; Papadimitriou, J.C.; Rabsztyn, A.; Drachenberg, C.; Tildon, J.T. Gastrointestinal abnormalities in children with autistic disorder. J. Pediatr. 1999, 135, 559–563. [Google Scholar] [CrossRef]
- Rodier, P.M.; Ingram, J.L.; Tisdale, B.; Nelson, S.; Romano, J. Embryological origin for autism: Developmental anomalies of the cranial nerve motor nuclei. J. Comp. Neurol. 1996, 370, 247–261. [Google Scholar] [CrossRef]
- Kushki, A.; Brian, J.; Dupuis, A.; Anagnostou, E. Functional autonomic nervous system profile in children with autism spectrum disorder. Mol. Autism 2014, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Modahl, C.; Green, L.; Fein, D.; Morris, M.; Waterhouse, L.; Feinstein, C.; Levin, H. Plasma oxytocin levels in autistic children. Biol. Psychiatry 1998, 43, 270–277. [Google Scholar] [CrossRef]
- Fetissov, S.O.; Averina, O.V.; Danilenko, V.N. Neuropeptides in the microbiota-brain axis and feeding behavior in autism spectrum disorder. Nutrition 2019, 61, 43–48. [Google Scholar] [CrossRef]
- Cersosimo, M.G.; Benarroch, E.E. Neural control of the gastrointestinal tract: Implications for Parkinson disease. Mov. Disord. 2008, 23, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, L.T.; Rueb, U.; Alho, A.T.; Heinsen, H. Brainstem pathology and non-motor symptoms in PD. J. Neurol. Sci. 2010, 289, 81–88. [Google Scholar] [CrossRef]
- Jellinger, K.A. Synuclein deposition and non-motor symptoms in Parkinson disease. J. Neurol. Sci. 2011, 310, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Pasquali, L.; Ruggieri, S.; Paparelli, A.; Fornai, F. Parkinson’s disease and the gut: A well known clinical association in need of an effective cure and explanation. Neurogastroenterol. Motil. 2008, 20, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Travagli, R.A.; Browning, K.N.; Camilleri, M. Parkinson disease and the gut: New insights into pathogenesis and clinical relevance. Nat. Rev. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Pathoanatomy of Parkinson’s disease. J. Neurol. 2000, 247 (Suppl. 2), II3–II10. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, L.; Toti, L.; Bove, C.; Hampton, J.; Travagli, R.A. A Nigro-Vagal Pathway Controls Gastric Motility and Is Affected in a Rat Model of Parkinsonism. Gastroenterology 2017, 153, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Toti, L.; Travagli, R.A. Gastric dysregulation induced by microinjection of 6-OHDA in the substantia nigra pars compacta of rats is determined by alterations in the brain-gut axis. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G1013–G1023. [Google Scholar] [CrossRef]
- Bove, C.; Travagli, R.A. Neurophysiology of the brain stem in Parkinson’s disease. J. Neurophysiol. 2019, 121, 1856–1864. [Google Scholar] [CrossRef]
- Tan, H.-E.; Sisti, A.C.; Jin, H.; Vignovich, M.; Villavicencio, M.; Tsang, K.S.; Goffer, Y.; Zuker, C.S. The gut-brain axis mediates sugar preference. Nature 2020, 580, 511–516. [Google Scholar] [CrossRef]
- Aguilera-Lizarraga, J.; Florens, M.V.; Viola, M.F.; Jain, P.; Decraecker, L.; Appeltans, I.; Cuende-Estevez, M.; Fabre, N.; Van Beek, K.; Perna, E.; et al. Local immune response to food antigens drives-meal-induced abdominal pain. Nature 2021, 591, 151–156. [Google Scholar] [CrossRef]
- Worthington, J.J.; Reimann, F.; Gribble, F.M. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 2018, 11, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Powley, T.L.; Jaffey, D.M.; McAdams, J.; Baronowsky, E.A.; Black, D.; Chesney, L.; Evans, C.; Phillips, R.J. Vagal innervation of the stomach reassessed: Brain-gut connectome uses smart terminals. Ann. N. Y. Acad. Sci. 2019, 1451, 14–30. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Browning, K.N.; Carson, K.E. Central Neurocircuits Regulating Food Intake in Response to Gut Inputs—Preclinical Evidence. Nutrients 2021, 13, 908. https://doi.org/10.3390/nu13030908
Browning KN, Carson KE. Central Neurocircuits Regulating Food Intake in Response to Gut Inputs—Preclinical Evidence. Nutrients. 2021; 13(3):908. https://doi.org/10.3390/nu13030908
Chicago/Turabian StyleBrowning, Kirsteen N., and Kaitlin E. Carson. 2021. "Central Neurocircuits Regulating Food Intake in Response to Gut Inputs—Preclinical Evidence" Nutrients 13, no. 3: 908. https://doi.org/10.3390/nu13030908
APA StyleBrowning, K. N., & Carson, K. E. (2021). Central Neurocircuits Regulating Food Intake in Response to Gut Inputs—Preclinical Evidence. Nutrients, 13(3), 908. https://doi.org/10.3390/nu13030908





