A Multi-Mineral Intervention to Modulate Colonic Mucosal Protein Profile: Results from a 90-Day Trial in Human Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Interventions
2.2. Trial Design
2.3. Histology and Quantitative Immunohistochemistry
2.4. Quantitative Morphometry
2.5. Proteomic Assessment
2.6. Data Analysis and Statistical Evaluation
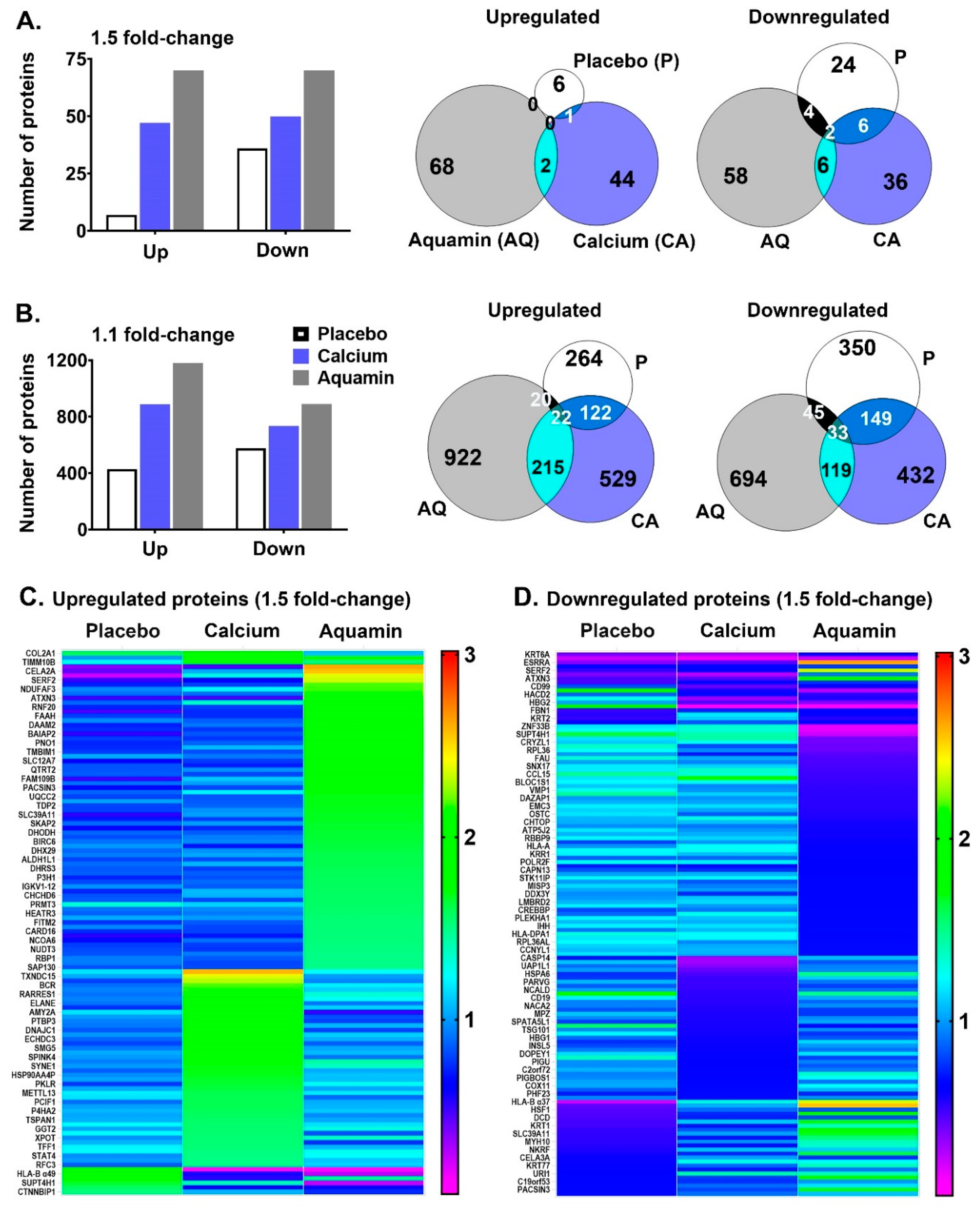
3. Results
3.1. Histological and Immunohistological Findings: Comparison of Aquamin® with Calcium Alone and Placebo
3.2. Proteomic Findings: Unbiased Comparison of Aquamin® with Calcium Alone and Placebo
3.3. Proteomic Findings: Directed Search
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heaney, R.P. Long-latency deficiency disease: Insights from calcium and vitamin D. Am. J. Clin. Nutr. 2003, 78, 912–919. [Google Scholar] [CrossRef]
- Peterlik, M.; Cross, H.S. Vitamin D and calcium insufficiency-related chronic diseases: Molecular and cellular pathophysiology. Eur. J. Clin. Nutr. 2009, 63, 1377–1386. [Google Scholar] [CrossRef]
- Peterlik, M.; Cross, H.S. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur. J. Clin. Investig. 2005, 35, 290–304. [Google Scholar] [CrossRef]
- Peterlik, M.; Boonen, S.; Cross, H.S.; Lamberg-Allardt, C. Vitamin D and Calcium Insufficiency-Related Chronic Diseases: An Emerging World-Wide Public Health Problem. Int. J. Environ. Res. Public Health 2009, 6, 2585–2607. [Google Scholar] [CrossRef]
- Aslam, M.; Varani, J. The Western-Style Diet, Calcium Deficiency and Chronic Disease. J. Nutr. Food Sci. 2016, 6, 2. [Google Scholar]
- Keum, N.; Aune, D.; Greenwood, D.C.; Ju, W.; Giovannucci, E.L. Calcium intake and colorectal cancer risk: Dose-response meta-analysis of prospective observational studies. Int. J. Cancer 2014, 135, 1940–1948. [Google Scholar] [CrossRef]
- Kesse, E.; Boutron-Ruault, M.; Norat, T.; Riboli, E.; Clavel-Chapelon, F. E3N Group Dietary calcium, phosphorus, vitamin D, dairy products and the risk of colorectal adenoma and cancer among French women of the E3N-EPIC prospective study. Int. J. Cancer 2005, 117, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Flood, A.; Peters, U.; Chatterjee, N.; Lacey, J.V.; Schairer, C.; Schatzkin, A. Calcium from diet and supplements is associated with reduced risk of colorectal cancer in a prospective cohort of women. Cancer Epidemiol. Biomark. Prev. 2005, 14, 126–132. [Google Scholar]
- Shaukat, A.; Scouras, N.; Schunemann, H.J. Role of Supplemental Calcium in the Recurrence of Colorectal Adenomas: A Metaanalysis of Randomized Controlled Trials. Am. J. Gastroenterol. 2005, 100, 390–394. [Google Scholar] [CrossRef]
- Larsson, S.C.; Bergkvist, L.; Rutegård, J.; Giovannucci, E.; Wolk, A. Calcium and dairy food intakes are inversely associated with colorectal cancer risk in the Cohort of Swedish Men. Am. J. Clin. Nutr. 2006, 83, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Murphy, S.P.; Wilkens, L.R.; Nomura, A.M.Y.; Henderson, B.E.; Kolonel, L.N. Calcium and Vitamin D Intake and Risk of Colorectal Cancer: The Multiethnic Cohort Study. Am. J. Epidemiol. 2007, 165, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Lennie, T.A.; Andreae, C.; Rayens, M.K.; Song, E.K.; Dunbar, S.B.; Pressler, S.J.; Heo, S.; Kim, J.; Moser, D.K. Micronutrient Deficiency Independently Predicts Time to Event in Patients With Heart Failure. J. Am. Hear. Assoc. 2018, 7, e007251. [Google Scholar] [CrossRef]
- Swaminath, S.; Um, C.Y.; Prizment, A.E.; Lazovich, D.; Bostick, R.M. Combined Mineral Intakes and Risk of Colorectal Cancer in Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 2019, 28, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Crowley, E.K.; Long-Smith, C.M.; Murphy, A.; Patterson, E.; Murphy, K.; O’Gorman, D.M.; Stanton, C.; Nolan, Y.M. Dietary Supplementation with a Magnesium-Rich Marine Mineral Blend Enhances the Diversity of Gastrointestinal Microbiota. Mar. Drugs 2018, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Biesalski Hans, K.; Jana, T. Micronutrients in the life cycle: Requirements and sufficient supply. NFS J. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Balk, E.M.; Adam, G.P.; Langberg, V.N.; Earley, A.; Clark, P.; Ebeling, P.R.; Mithal, A.; Rizzoli, R.; Zerbini, C.A.F.; Pierroz, D.D.; et al. Global dietary calcium intake among adults: A systematic review. Osteoporos. Int. 2017, 28, 3315–3324. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, N. and Allergies. Scientific Opinion on Dietary Reference Values for calcium. EFSA J. 2015, 13, 4101.
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015—2020 Dietary Guidelines for Americans. December 2015, 8th ed.; U.S. Department of Health and Human Services: Washington, DC, USA; U.S. Department of Agriculture: Washington, DC, USA, 2015. Available online: https://health.gov/our-work/food-and-nutrition/2015-2020-dietary-guidelines/. (accessed on 21 February 2021).
- Dai, Q.; Zhu, X.; Manson, J.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence vitamin D status and metabolism: Results from a randomized trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258. [Google Scholar] [CrossRef]
- Aslam, M.N.; Bhagavathula, N.; Paruchuri, T.; Hu, X.; Chakrabarty, S.; Varani, J. Growth-inhibitory effects of a mineralized extract from the red marine algae, Lithothamnion calcareum, on Ca2+-sensitive and Ca2+-resistant human colon carcinoma cells. Cancer Lett. 2009, 283, 186–192. [Google Scholar] [CrossRef]
- Singh, N.; Aslam, M.N.; Varani, J.; Chakrabarty, S. Induction of calcium sensing receptor in human colon cancer cells by calcium, vitamin D and aquamin: Promotion of a more differentiated, less malignant and indolent phenotype. Mol. Carcinog. 2013, 54, 543–553. [Google Scholar] [CrossRef][Green Version]
- McClintock, S.D.; Colacino, J.A.; Attili, D.; Dame, M.K.; Richter, A.; Reddy, A.R.; Basrur, V.; Rizvi, A.H.; Turgeon, D.K.; Varani, J.; et al. Calcium-Induced Differentiation of Human Colon Adenomas in Colonoid Culture: Calcium Alone versus Calcium with Additional Trace Elements. Cancer Prev. Res. 2018, 11, 413–428. [Google Scholar] [CrossRef]
- Attili, D.; McClintock, S.D.; Rizvi, A.H.; Pandya, S.; Rehman, H.; Nadeem, D.M.; Richter, A.; Thomas, D.; Dame, M.K.; Turgeon, D.K.; et al. Calcium-induced differentiation in normal human colonoid cultures: Cell-cell/cell-matrix adhesion, barrier formation and tissue integrity. PLoS ONE 2019, 14, e0215122. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.N.; McClintock, S.D.; Attili, D.; Pandya, S.; Rehman, H.; Nadeem, D.M.; Jawad-Makki, M.A.H.; Rizvi, A.H.; Berner, M.M.; Dame, M.K.; et al. Ulcerative Colitis-Derived Colonoid Culture: A Multi-Mineral-Approach to Improve Barrier Protein Expression. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- McClintock, S.D.; Attili, D.; Dame, M.K.; Richter, A.; Silvestri, S.S.; Berner, M.M.; Bohm, M.S.; Karpoff, K.; McCarthy, C.L.; Spence, J.R.; et al. Differentiation of human colon tissue in culture: Effects of calcium on trans-epithelial electrical resistance and tissue cohesive properties. PLoS ONE 2020, 15, e0222058. [Google Scholar] [CrossRef]
- Aslam, M.N.; Paruchuri, T.; Bhagavathula, N.; Varani, J. A Mineral-Rich Red Algae Extract Inhibits Polyp Formation and Inflammation in the Gastrointestinal Tract of Mice on a High-Fat Diet. Integr. Cancer Ther. 2010, 9, 93–99. [Google Scholar] [CrossRef]
- Aslam, M.N.; Bergin, I.; Naik, M.; Paruchuri, T.; Hampton, A.; Rehman, M.; Dame, M.K.; Rush, H.; Varani, J. A Multimineral Natural Product from Red Marine Algae Reduces Colon Polyp Formation in C57BL/6 Mice. Nutr. Cancer 2012, 64, 1020–1028. [Google Scholar] [CrossRef]
- Newmark, H.L.; Yang, K.; Kurihara, N.; Fan, K.; Augenlicht, L.H.; Lipkin, M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: A preclinical model for human sporadic colon cancer. Carcinogenesis 2008, 30, 88–92. [Google Scholar] [CrossRef]
- Mariadason, J.M.; Bordonaro, M.; Aslam, F.; Shi, L.; Kuraguchi, M.; Velcich, A.; Augenlicht, L.H. Down-regulation of beta-catenin TCF signaling is linked to colonic epithelial cell differentiation. Cancer Res. 2001, 61, 3465–3471. [Google Scholar] [PubMed]
- Grau, M.V.; Baron, J.A.; Sandler, R.S.; Haile, R.W.; Beach, M.L.; Church, T.R.; Heber, D. Vitamin D, Calcium Supplementation, and Colorectal Adenomas: Results of a Randomized Trial. J. Natl. Cancer Inst. 2003, 95, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.; Beach, M.; Mandel, J.; Van Stolk, R.; Haile, R.; Sandler, R.; Rothstein, R.; Summers, R.; Snover, D.; Beck, G.; et al. Calcium Supplements for the Prevention of Colorectal Adenomas. N. Engl. J. Med. 1999, 340, 101–107. [Google Scholar] [CrossRef]
- Pommergaard, H.-C.; Burcharth, J.; Rosenberg, J.; Raskov, H. Aspirin, Calcitriol, and Calcium Do Not Prevent Adenoma Recurrence in a Randomized Controlled Trial. Gastroenterology 2016, 150, 114–122. [Google Scholar] [CrossRef]
- Baron, J.A.; Barry, E.L.; Mott, L.A.; Rees, J.R.; Sandler, R.S.; Snover, D.C.; Bostick, R.M.; Ivanova, A.; Cole, B.F.; Ahnen, D.J.; et al. A Trial of Calcium and Vitamin D for the Prevention of Colorectal Adenomas. N. Engl. J. Med. 2015, 373, 1519–1530. [Google Scholar] [CrossRef]
- Nimptsch, K.; Lee, D.H.; Zhang, X.; Song, M.; Farvid, M.S.; Rezende, L.F.M.; Cao, Y.; Chan, A.T.; Fuchs, C.; Meyerhardt, J.; et al. Dairy intake during adolescence and risk of colorectal adenoma later in life. Br. J. Cancer 2021, 1–9. [Google Scholar] [CrossRef]
- Aslam, M.N.; Bassis, C.M.; Bergin, I.L.; Knuver, K.; Zick, S.M.; Sen, A.; Turgeon, D.K.; Varani, J. A Calcium-Rich Multimineral Intervention to Modulate Colonic Microbial Communities and Metabolomic Profiles in Humans: Results from a 90-Day Trial. Cancer Prev. Res. 2020, 13, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Bostick, R.M. Effects of supplemental vitamin D and calcium on normal colon tissue and circulating biomarkers of risk for colorectal neoplasms. J. Steroid Biochem. Mol. Biol. 2015, 148, 86–95. [Google Scholar] [CrossRef]
- Fedirko, V.; Bostick, R.M.; Flanders, W.D.; Long, Q.; Sidelnikov, E.; Shaukat, A.; Daniel, C.R.; Rutherford, R.E.; Woodard, J.J. Effects of vitamin d and calcium on proliferation and differentiation in normal colon mucosa: A randomized clinical trial. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Ahearn, T.U.; Bostick, R.M. Effects of calcium and vitamin D supplementation on crypt morphology in normal colon mucosa: A randomized clinical trial. Mol. Carcinog. 2013, 54, 242–247. [Google Scholar] [CrossRef]
- Adey, W.H.; McKibbin, D.L. Studies on the Maerl Species Phymatolithon calcareum (Pallas) nov. comb. and Lithothamnium coralloides Crouan in the Ria de Vigo. Bot. Mar. 1970, 13, 100–106. [Google Scholar] [CrossRef]
- Frestedt, J.L.; Walsh, M.; Kuskowski, M.A.; Zenk, J.L. A natural mineral supplement provides relief from knee osteoarthritis symptoms: A randomized controlled pilot trial. Nutr. J. 2008, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Frestedt, J.L.; Kuskowski, M.A.; Zenk, J.L. A natural seaweed derived mineral supplement (Aquamin F) for knee osteoarthritis: A randomized, placebo controlled pilot study. Nutr. J. 2009, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Slevin, M.M.; Allsopp, P.J.; Magee, P.J.; Bonham, M.P.; Naughton, V.R.; Strain, J.J.; Duffy, M.E.; Wallace, J.M.; Mc Sorley, E.M. Supplementation with Calcium and Short-Chain Fructo-Oligosaccharides Affects Markers of Bone Turnover but Not Bone Mineral Density in Postmenopausal Women. J. Nutr. 2013, 144, 297–304. [Google Scholar] [CrossRef] [PubMed]
- McAlister, G.C.; Nusinow, D.P.; Jedrychowski, M.P.; Wühr, M.; Huttlin, E.L.; Erickson, B.K.; Rad, R.; Haas, W.; Gygi, S.P. MultiNotch MS3 Enables Accurate, Sensitive, and Multiplexed Detection of Differential Expression across Cancer Cell Line Proteomes. Anal. Chem. 2014, 86, 7150–7158. [Google Scholar] [CrossRef]
- Yurchenco, P.D.; Patton, B.L. Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharm. Des. 2009, 15, 1277–1294. [Google Scholar] [CrossRef] [PubMed]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef]
- Chu, I.M.; Hengst, L.; Slingerland, J.M. The Cdk inhibitor p27 in human cancer: Prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer 2008, 8, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Z.; Han, Y.; Yao, R.; Yue, L.; Xu, Y.; Zhang, J. Rnf2 knockdown reduces cell viability and promotes cell cycle arrest in gastric cancer cells. Oncol. Lett. 2017, 13, 3817–3822. [Google Scholar] [CrossRef]
- Zhou, G.; Yang, W.; Yu, L.; Yu, T.; Liu, Z. CD99 refers to the activity of inflammatory bowel disease. Scand. J. Gastroenterol. 2016, 52, 359–364. [Google Scholar] [CrossRef]
- Huijbers, E.J.M.; Van Der Werf, I.M.; Faber, L.D.; Sialino, L.D.; Van Der Laan, P.; Holland, H.A.; Cimpean, A.M.; Thijssen, V.L.J.L.; Van Beijnum, J.R.; Griffioen, A.W. Targeting Tumor Vascular CD99 Inhibits Tumor Growth. Front. Immunol. 2019, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, S.; Itatani, Y.; Yamamoto, T.; Minamiguchi, S.; Hirai, H.; Iwamoto, M.; Hasegawa, S.; Taketo, M.M.; Sakai, Y.; Kawada, K. Loss of SMAD4 Promotes Colorectal Cancer Progression by Accumulation of Myeloid-Derived Suppressor Cells through the CCL15-CCR1 Chemokine Axis. Clin. Cancer Res. 2016, 22, 492–501. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, L.; Zhou, W.; Huang, Z.; Hu, J.; Dai, S.; Wang, X.; Huang, X.; He, C. Over-expression of the ATP5J gene correlates with cell migration and 5-fluorouracil sensitivity in colorectal cancer. PLoS ONE 2013, 8, e76846. [Google Scholar] [CrossRef]
- Jamal, B.; Sengupta, P.K.; Gao, Z.-N.; Nita-Lazar, M.; Amin, B.; Jalisi, S.; Bouchie, M.P.; Kukuruzinska, M.A. Aberrant amplification of the crosstalk between canonical Wnt signaling and N-glycosylation gene DPAGT1 promotes oral cancer. Oral Oncol. 2012, 48, 523–529. [Google Scholar] [CrossRef]
- Wang, B.; Pelletier, J.; Massaad, M.J.; Herscovics, A.; Shore, G.C. The Yeast Split-Ubiquitin Membrane Protein Two-Hybrid Screen Identifies BAP31 as a Regulator of the Turnover of Endoplasmic Reticulum-Associated Protein Tyrosine Phosphatase-Like, B. Mol. Cell. Biol. 2004, 24, 2767–2778. [Google Scholar] [CrossRef][Green Version]
- Pantaleo, M.A.; Astolfi, A.; Nannini, M.; Paterini, P.; Piazzi, G.; Ercolani, G.; Brandi, G.; Martinelli, G.; Pession, A.; Pinna, A.D.; et al. Gene expression profiling of liver metastases from colorectal cancer as potential basis for treatment choice. Br. J. Cancer 2008, 99, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Pedrazza, L.; Schneider, T.; Bartrons, R.; Ventura, F.; Rosa, J.L. The ubiquitin ligase HERC1 regulates cell migration via RAF-dependent regulation of MKK3/p38 signaling. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.X.; Sun, W.; Wang, S.H.; Liu, P.J.; Wang, Y.C. Differential expression and clinical significance of COX6C in human diseases. Am. J. Transl. Res. 2021, 13, 1–10. [Google Scholar]
- Tian, M.; Wang, X.; Sun, J.; Lin, W.; Chen, L.; Liu, S.; Wu, X.; Shi, L.; Xu, P.; Cai, X.; et al. IRF3 prevents colorectal tumorigenesis via inhibiting the nuclear translocation of β-catenin. Nat. Commun. 2020, 11, 5762. [Google Scholar] [CrossRef]
- Aslam, M.N.; Bergin, I.; Naik, M.; Hampton, A.; Allen, R.; Kunkel, S.L.; Rush, H.; Varani, J. A Multi-Mineral Natural Product Inhibits Liver Tumor Formation in C57BL/6 Mice. Biol. Trace Elem. Res. 2012, 147, 267–274. [Google Scholar] [CrossRef]
- Aslam, M.N.; Bergin, I.; Jepsen, K.J.; Kreider, J.M.; Graf, K.H.; Naik, M.; Goldstein, S.A.; Varani, J. Preservation of Bone Structure and Function by Lithothamnion sp. Derived Minerals. Biol. Trace Elem. Res. 2013, 156, 210–220. [Google Scholar] [CrossRef]
- Aslam, M.N.; Kreider, J.M.; Paruchuri, T.; Bhagavathula, N.; DaSilva, M.; Zernicke, R.F.; Goldstein, S.A.; Varani, J. A Mineral-Rich Extract from the Red Marine Algae Lithothamnion calcareum Preserves Bone Structure and Function in Female Mice on a Western-Style Diet. Calcif. Tissue Int. 2010, 86, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.N.; Jepsen, K.J.; Khoury, B.; Graf, K.H.; Varani, J. Bone structure and function in male C57BL/6 mice: Effects of a high-fat Western-style diet with or without trace minerals. Bone Rep. 2016, 5, 141–149. [Google Scholar] [CrossRef]
- Hampton, A.L.; Aslam, M.N.; Naik, M.K.; Bergin, I.L.; Allen, R.M.A.; Craig, R.; Kunkel, S.L.; Veerapaneni, I.; Paruchuri, T.A.; Patterson, K.; et al. Ulcerative Dermatitis in C57BL/6NCrl Mice on a Low-Fat or High-Fat Diet With or Without a Mineralized Red-Algae Supplement. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 487–496. [Google Scholar] [PubMed]
- Huh, J.W.; Lee, J.H.; Kim, H.R. Expression of p16, p53, and Ki-67 in colorectal adenocarcinoma: A study of 356 surgically resected cases. Hepatogastroenterology 2010, 57, 734–740. [Google Scholar] [PubMed]
- Luo, Z.W.; Zhu, M.G.; Zhang, Z.Q.; Ye, F.J.; Huang, W.H.; Luo, X.Z. Increased expression of Ki-67 is a poor prognostic marker for colorectal cancer patients: A me-ta-analysis. BMC Cancer 2019, 19, 123. [Google Scholar] [CrossRef]
- Varani, J.; McClintock, S.D.; Aslam, M.N. Organoid culture to study epithelial cell differentiation and barrier formation in the colon: Bridging the gap between monolayer cell culture and human subject research. In Vitr. Cell. Dev. Biol. Anim. 2021, 57, 174–190. [Google Scholar] [CrossRef]
- Aggarwal, A.; Prinz-Wohlgenannt, M.; Tennakoon, S.; Höbaus, J.; Boudot, C.; Mentaverri, R.; Brown, E.M.; Baumgartner-Parzer, S.; Kállay, E. The calcium-sensing receptor: A promising target for prevention of colorectal cancer. Biochim. Biophys. Acta Bioenerg. 2015, 1853, 2158–2167. [Google Scholar] [CrossRef]
- Iamartino, L.; Elajnaf, T.; Gall, K.; David, J.; Manhardt, T.; Heffeter, P.; Grusch, M.; Derdak, S.; Baumgartner-Parzer, S.; Schepelmann, M.; et al. Effects of pharmacological calcimimetics on colorectal cancer cells over-expressing the human calcium-sensing receptor. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118836. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.C.; Thiem, U.; Roth, S.; Aggarwal, A.; Fetahu, I.S.; Tennakoon, S.; Gomes, A.R.; Brandi, M.L.; Bruggeman, F.; Mentaverri, R.; et al. Calcium sensing receptor signalling in physiology and cancer. Biochim. Biophys. Acta Bioenerg. 2013, 1833, 1732–1744. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, Y.; Castiblanco, A.; Yang, W.; Brown, E.M.; Yang, J.J. Multiple Ca2+-Binding Sites in the Extracellular Domain of the Ca2+-Sensing Receptor Corresponding to Cooperative Ca2+Response†. Biochemistry 2008, 48, 388–398. [Google Scholar] [CrossRef]
- Carrillo-Lopez, N.; Fernandez-Martin, J.L.; Alvarez-Hernandez, D.; Gonzalez-Suarez, I.; Castro-Santos, P.; Roman-Garcia, P.; Lopez-Novoa, J.M.; Cannata-Andía, J.B. Lanthanum activates calcium-sensing receptor and enhances sensitivity to calcium. Nephrol. Dial. Transpl. 2010, 25, 2930–2937. [Google Scholar] [CrossRef]
- Attili, D.; Jenkins, B.; Aslam, M.N.; Dame, M.K.; Varani, J. Growth Control in Colon Epithelial Cells: Gadolinium Enhances Calcium-Mediated Growth Regulation. Biol. Trace Elem. Res. 2012, 150, 467–476. [Google Scholar] [CrossRef]
- Davis, C.D.; Feng, Y. Dietary Copper, Manganese and Iron Affect the Formation of Aberrant Crypts in Colon of Rats Administered 3,2′-Dimethyl-4-Aminobiphenyl. J. Nutr. 1999, 129, 1060–1067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alwahaibi, N.; Mohamed, J.; Alhamadani, A. Supplementation of selenium reduces chemical hepatocarcinogenesis in male Sprague-Dawley rats. J. Trace Elem. Med. Biol. 2010, 24, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.Y.; Soderholm, J.D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel. Dis. 2011, 17, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Dunlop, S.P.; Hebden, J.; Campbell, E.; Naesdal, J.; Olbe, L.; Perkins, A.C.; Spiller, R.C. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am. J. Gastroenterol. 2006, 101, 1288–1294. [Google Scholar] [CrossRef]
- De Arcangelis, A.; Hamade, H.; Alpy, F.; Normand, S.; Bruyère, E.; Lefebvre, O.; Méchine-Neuville, A.; Siebert, S.; Pfister, V.; Lepage, P.; et al. Hemidesmosome integrity protects the colon against colitis and colorectal cancer. Gut 2017, 66, 1748–1760. [Google Scholar] [CrossRef] [PubMed]


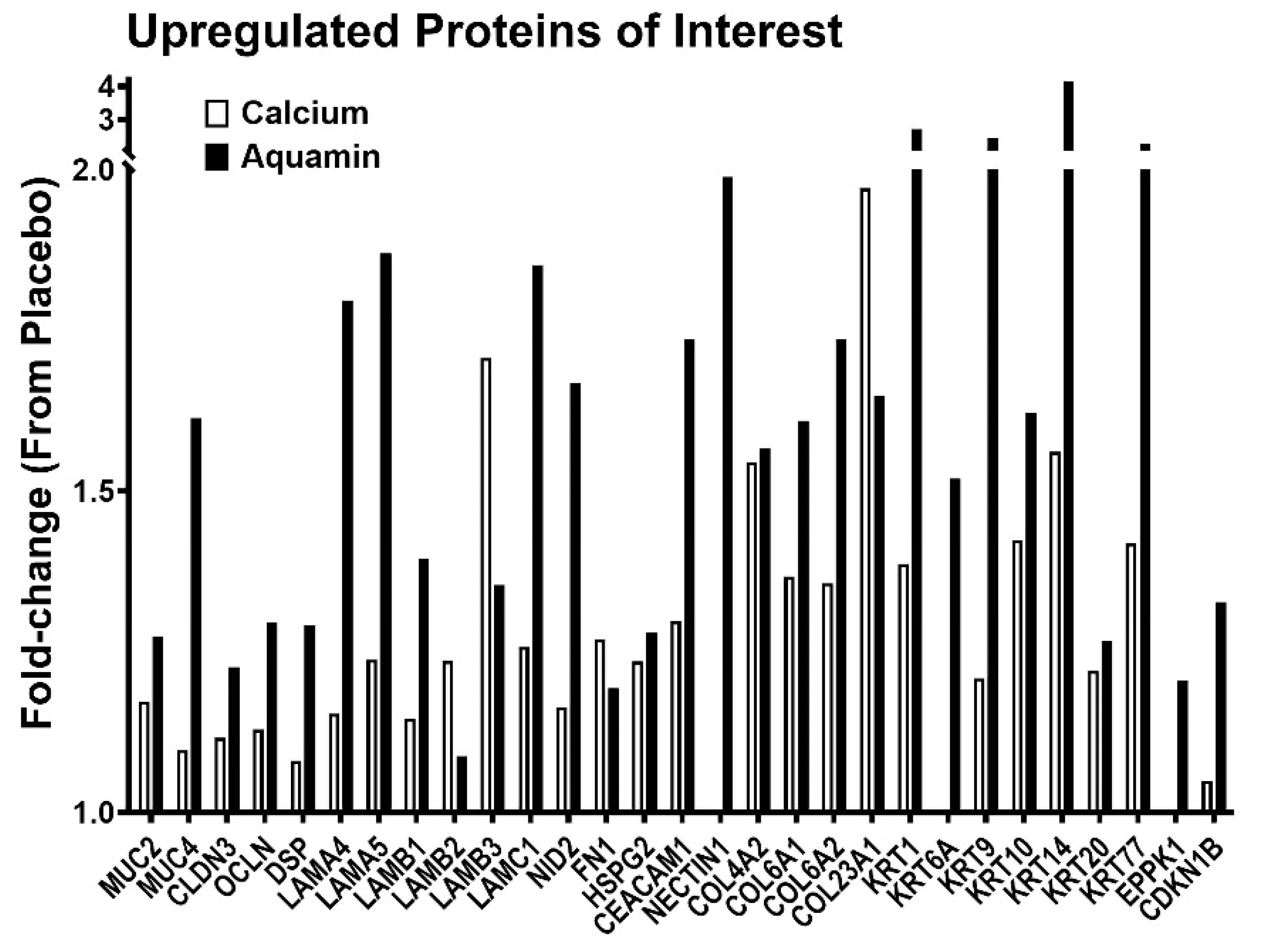

| Pathway Name | Entities p Value | Entities FDR | Mapped Entities |
|---|---|---|---|
| Laminin interactions | 1.1 × 10−16 | 1.9 × 10−14 | COL4A2; HSPG2; LAMA4; LAMA5; LAMB1; LAMB2; LAMB3; LAMC1; NID2 |
| ECM proteoglycans | 3.0 × 10−15 | 2.3 × 10−13 | COL4A2; COL6A1; COL6A2; FN1; HSPG2; LAMA4; LAMA5; LAMB1; LAMB2; LAMC1 |
| Extracellular matrix organization | 4.1 × 10−15 | 2.3 × 10−13 | CEACAM1; COL4A2; COL6A1; COL6A2; COL23A1; FN1; HSPG2; LAMA4; LAMA5; LAMB1; LAMB2; LAMB3; LAMC1; NID2 |
| Non-integrin membrane-ECM interactions | 2.4 × 10−14 | 9.9 × 10−13 | COL4A2; FN1; HSPG2; LAMA4; LAMA5; LAMB1; LAMB2; LAMB3; LAMC1 |
| Degradation of the extracellular matrix | 1.2 × 10−12 | 3.6 × 10−11 | COL4A2; COL6A1; COL6A2; COL23A1; FN1; HSPG2; LAMA5; LAMB1; LAMB3; LAMC1 |
| MET activates PTK2 signaling | 1.3 × 10−12 | 3.6 × 10−11 | FN1; LAMA4; LAMA5; LAMB1; LAMB2; LAMB3; LAMC1 |
| MET promotes cell motility | 1.1 × 10−11 | 2.7 × 10−10 | FN1; LAMA4; LAMA5; LAMB1; LAMB2; LAMB3; LAMC1 |
| Formation of the cornified envelope | 9.1 × 10−10 | 1.9 × 10−8 | DSP; KRT1; KRT10; KRT14; KRT20; KRT6A; KRT9; KRT77 |
| Signaling by MET | 1.1 × 10−9 | 2.0 × 10−8 | FN1; LAMA4; LAMA5; LAMB1; LAMB2; LAMB3; LAMC1 |
| Keratinization | 5.0 × 10−8 | 8.1 × 10−7 | DSP; KRT1; KRT6A; KRT9; KRT10; KRT14; KRT20; KRT77 |
| Integrin cell surface interactions | 6.9 × 10−8 | 1.0 × 10−6 | COL4A2; COL6A1; COL6A2; COL23A1; FN1; HSPG2 |
| Signaling by Receptor Tyrosine Kinases | 3.6 × 10−7 | 5.0 × 10−6 | COL4A2; COL6A1; COL6A2; FN1; LAMA4; LAMA5; LAMB1; LAMB2; LAMB3; LAMC1 |
| Developmental Biology | 1.0 × 10−6 | 1.2 × 10−5 | COL4A2; COL6A1; COL6A2; DSP;KRT1; KRT10; KRT14; KRT20; KRT6A; KRT9; KRT77; LAMB1; LAMC1 |
| Collagen formation | 3.1 × 10−6 | 3.7 × 10−5 | COL23A1; COL4A2; COL6A1; COL6A2; LAMB3 |
| Collagen chain trimerization | 4.8 × 10−6 | 5.3 × 10−4 | COL23A1; COL4A2; COL6A1; COL6A2 |
| Assembly of collagen fibrils and other multimeric structures | 1.7 × 10−5 | 1.7 × 10−4 | COL4A2; COL6A1; COL6A2; LAMB3 |
| Collagen degradation | 2.1 × 10−5 | 1.9 × 10−4 | COL23A1; COL4A2; COL6A1; COL6A2 |
| Collagen biosynthesis and modifying enzymes | 2.5 × 10−5 | 2.2 × 10−4 | COL23A1; COL4A2; COL6A1; COL6A2 |
| Cell junction organization | 8.5 × 10−5 | 0.001 | CLDN3; KRT14; LAMB3; NECTIN1 |
| Fibronectin matrix formation | 1.1 × 10−4 | 0.001 | CEACAM1; FN1 |
| Post-translational protein phosphorylation | 1.5 × 10−4 | 0.001 | FN1; LAMB1; LAMB2; LAMC1 |
| NCAM1 interactions | 1.7 × 10−4 | 0.001 | COL4A2; COL6A1; COL6A2 |
| Regulation of IGF Factor transport and uptake by IGFBPs | 2.6 × 10−4 | 0.002 | FN1; LAMB1; LAMB2; LAMC1 |
| Cell-Cell communication | 3.2 × 10−4 | 0.002 | CLDN3; KRT14; LAMB3; NECTIN1 |
| Type I hemidesmosome assembly | 3.7 × 10−4 | 0.002 | KRT14; LAMB3 |
| Apoptotic cleavage of cell adhesion proteins | 3.7 × 10−4 | 0.002 | DSP; OCLN |
| Signaling by PDGF | 4.8 × 10−4 | 0.003 | COL4A2; COL6A1; COL6A2 |
| NCAM signaling for neurite out-growth | 5.7 × 10−4 | 0.003 | COL4A2; COL6A1; COL6A2 |
| Anchoring fibril formation | 6.8 × 10−4 | 0.003 | COL4A2; LAMB3 |
| Defective GALNT12 causes colorectal cancer 1 | 0.001 | 0.005 | MUC2; MUC4 |
| Termination of O-glycan biosynthesis | 0.002 | 0.01 | MUC2; MUC4 |
| Apoptotic cleavage of cellular proteins | 0.004 | 0.02 | DSP; OCLN |
| Apoptotic execution phase | 0.01 | 0.03 | DSP; OCLN |
| Dectin-2 family | 0.01 | 0.04 | MUC2; MUC4 |
| O-linked glycosylation of mucins | 0.01 | 0.04 | MUC2; MUC4 |
| Cell-cell junction organization | 0.01 | 0.04 | CLDN3; NECTIN1 |
| RUNX1 regulates expression of components of tight junctions | 0.01 | 0.04 | OCLN |
| PTK6 Regulates Cell Cycle | 0.02 | 0.05 | CDKN1B |
| Nectin/Necl trans heterodimerization | 0.02 | 0.05 | NECTIN1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, M.N.; McClintock, S.D.; Jawad-Makki, M.A.H.; Knuver, K.; Ahmad, H.M.; Basrur, V.; Bergin, I.L.; Zick, S.M.; Sen, A.; Turgeon, D.K.; et al. A Multi-Mineral Intervention to Modulate Colonic Mucosal Protein Profile: Results from a 90-Day Trial in Human Subjects. Nutrients 2021, 13, 939. https://doi.org/10.3390/nu13030939
Aslam MN, McClintock SD, Jawad-Makki MAH, Knuver K, Ahmad HM, Basrur V, Bergin IL, Zick SM, Sen A, Turgeon DK, et al. A Multi-Mineral Intervention to Modulate Colonic Mucosal Protein Profile: Results from a 90-Day Trial in Human Subjects. Nutrients. 2021; 13(3):939. https://doi.org/10.3390/nu13030939
Chicago/Turabian StyleAslam, Muhammad N., Shannon D. McClintock, Mohamed Ali H. Jawad-Makki, Karsten Knuver, Haris M. Ahmad, Venkatesha Basrur, Ingrid L. Bergin, Suzanna M. Zick, Ananda Sen, D. Kim Turgeon, and et al. 2021. "A Multi-Mineral Intervention to Modulate Colonic Mucosal Protein Profile: Results from a 90-Day Trial in Human Subjects" Nutrients 13, no. 3: 939. https://doi.org/10.3390/nu13030939
APA StyleAslam, M. N., McClintock, S. D., Jawad-Makki, M. A. H., Knuver, K., Ahmad, H. M., Basrur, V., Bergin, I. L., Zick, S. M., Sen, A., Turgeon, D. K., & Varani, J. (2021). A Multi-Mineral Intervention to Modulate Colonic Mucosal Protein Profile: Results from a 90-Day Trial in Human Subjects. Nutrients, 13(3), 939. https://doi.org/10.3390/nu13030939







