Fortifying Butterfat with Soybean Oil Attenuates the Onset of Diet-Induced Non-Alcoholic Steatohepatitis and Glucose Intolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Cell Culture
2.3. Evaluation of Liver Damage and Inflammation
2.4. Endotoxin and Free Fatty Acid (FFA) Measurements, Tumor Necrosis Factor α (TNF-α) ELISA, and Peroxisome Proliferator-Activated Receptor γ (PPARγ) Transcription Factor Assay
2.5. Western Blot Analysis
2.6. Assessment of Arginase and Myeloperoxidase (MPO) Activity as Well as Griess Assay
2.7. RNA Isolation and Real-Time Polymerase Chain Reaction (RT-PCR)
2.8. Statistical Analysis
3. Results
3.1. Markers of Liver Damage and Inflammation in Mice Fed a Butterfat-Rich FFC ± Soybean Oil
3.2. Glucose Tolerance and Markers of Glucose Metabolism in Liver and Muscle in Mice Fed a Butterfat-Rich FFC ± Soybean Oil
3.3. Markers of Lipogenesis in Liver Tissue of Mice Fed a Butterfat-Rich FFC ± Soybean Oil
3.4. Bacterial Endotoxin Levels in Portal Vein and Markers of Lipid Peroxidation and Endotoxin-Dependent Signaling Cascades in Livers of Mice Fed a Butterfat-Rich FFC ± Soybean Oil
3.5. Effect of Linolenic and Linoleic Acid, in a Ratio Equivalent to That Found in Soybean Oil, on LPS-Dependent Activation of J774A.1 Cells
4. Discussion
4.1. The Protective Effects of Enriching the Diet with Soybean Oil Are Not Associated with Changes of Markers of Lipogenesis
4.2. How Does the Addition of Soybean Oil Attenuate the Development of Diet-Induced NAFLD in Mice?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pagano, G.; Pacini, G.; Musso, G.; Gambino, R.; Mecca, F.; Depetris, N.; Cassader, M.; David, E.; Cavallo-Perin, P.; Rizzetto, M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: Further evidence for an etiologic association. Hepatology 2002, 35, 367–372. [Google Scholar] [CrossRef]
- Katsiki, N.; Perez-Martinez, P.; Anagnostis, P.; Mikhailidis, D.P.; Karagiannis, A. Is nonalcoholic fatty liver disease indeed the hepatic manifestation of metabolic syndrome? Curr. Vasc. Pharmacol. 2018, 16, 219–227. [Google Scholar] [CrossRef]
- Perumpail, B.J.; Khan, M.A.; Yoo, E.R.; Cholankeril, G.; Kim, D.; Ahmed, A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 8263–8276. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis Progression in Nonalcoholic Fatty Liver vs. Nonalcoholic Steatohepatitis: A Systematic Review and Meta-analysis of Paired-Biopsy Studies. Clin. Gastroenterol. Hepatol. 2015, 13, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellmann, C.; Baumann, A.; Brandt, A.; Jin, C.J.; Nier, A.; Bergheim, I. Oral Supplementation of Glutamine Attenuates the Progression of Nonalcoholic Steatohepatitis in C57BL/6J Mice. J. Nutr. 2017, 147, 2041–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellmann, C.; Priebs, J.; Landmann, M.; Degen, C.; Engstler, A.J.; Jin, C.J.; Gärttner, S.; Spruss, A.; Huber, O.; Bergheim, I. Diets rich in fructose, fat or fructose and fat alter intestinal barrier function and lead to the development of nonalcoholic fatty liver disease over time. J. Nutr. Biochem. 2015, 26, 1183–1192. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; De Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Fagà, E.; Silli, B.; Pagano, G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003, 37, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-H.; Guan, B.-J.; Gao, H.-Y.; Peng, X.-E. Omega-3 polyunsaturated fatty acid supplementation and non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e12271. [Google Scholar] [CrossRef]
- Parker, H.M.; Johnson, N.A.; Burdon, C.A.; Cohn, J.S.; O’Connor, H.T.; George, J. Omega-3 supplementation and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 56, 944–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pryde, E. Composition of soybean oil. In Handbook of Soy Oil Processing and Utilization; American Soybean Ass.: St. Louis, MO, USA, 1980; pp. 13–31. [Google Scholar]
- Wang, T. Soybean Oil. Vegetable Oils in Food Technology: Composition, Properties and Uses; CRS Press: Boca Raton, FL, USA, 2011; pp. 18–58. [Google Scholar]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henkel, J.; Alfine, E.; Saín, J.; Jöhrens, K.; Weber, D.; Castro, J.P.; König, J.; Stuhlmann, C.; Vahrenbrink, M.; Jonas, W.; et al. Soybean Oil-Derived Poly-Unsaturated Fatty Acids Enhance Liver Damage in NAFLD Induced by Dietary Cholesterol. Nutrients 2018, 10, 1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henkel, J.; Coleman, C.D.; Schraplau, A.; Jöhrens, K.; Weber, D.; Castro, J.P.; Hugo, M.; Schulz, T.J.; Krämer, S.; Schürmann, A.; et al. Induction of steatohepatitis (NASH) with insulin resistance in wildtype B6 mice by a western-type diet containing soybean oil and cholesterol. Mol. Med. 2017, 23, 70–82. [Google Scholar] [CrossRef]
- Zhao, M.; Zang, B.; Cheng, M.; Ma, Y.; Yang, Y.; Yang, N. Differential responses of hepatic endoplasmic reticulum stress and inflammation in diet-induced obese rats with high-fat diet rich in lard oil or soybean oil. PLoS ONE 2013, 8, e78620. [Google Scholar] [CrossRef]
- Calzadilla Bertot, L.; Adams, L.A. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef] [Green Version]
- Spruss, A.; Henkel, J.; Kanuri, G.; Blank, D.; Püschel, G.P.; Bischoff, S.C.; Bergheim, I. Female mice are more susceptible to nonalcoholic fatty liver disease: Sex-specific regulation of the hepatic AMP-activated protein kinase-plasminogen activator inhibitor 1 cascade, but not the hepatic endotoxin response. Mol. Med. 2012, 18, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Marin, V.; Rosso, N.; Dal Ben, M.; Raseni, A.; Boschelle, M.; Degrassi, C.; Nemeckova, I.; Nachtigal, P.; Avellini, C.; Tiribelli, C.; et al. An Animal Model for the Juvenile Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis. PLoS ONE 2016, 11, e0158817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, C.J.; Sellmann, C.; Engstler, A.J.; Ziegenhardt, D.; Bergheim, I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH). Br. J. Nutr. 2015, 114, 1745–1755. [Google Scholar] [CrossRef] [Green Version]
- Marcher, A.-B.; Bendixen, S.M.; Terkelsen, M.K.; Hohmann, S.S.; Hansen, M.H.; Larsen, B.D.; Mandrup, S.; Dimke, H.; Detlefsen, S.; Ravnskjaer, K. Transcriptional regulation of Hepatic Stellate Cell activation in NASH. Sci. Rep. 2019, 9, 2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cousin, S.P.; Hügl, S.R.; Wrede, C.E.; Kajio, H.; Myers, M.G.; Rhodes, C.J. Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1. Endocrinology 2001, 142, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Wobser, H.; Dorn, C.; Weiss, T.S.; Amann, T.; Bollheimer, C.; Büttner, R.; Schölmerich, J.; Hellerbrand, C. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res. 2009, 19, 996–1005. [Google Scholar] [CrossRef] [Green Version]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Spruss, A.; Kanuri, G.; Stahl, C.; Bischoff, S.C.; Bergheim, I. Metformin protects against the development of fructose-induced steatosis in mice: Role of the intestinal barrier function. Lab. Investig. 2012, 92, 1020–1032. [Google Scholar] [CrossRef] [Green Version]
- Wagnerberger, S.; Spruss, A.; Kanuri, G.; Volynets, V.; Stahl, C.; Bischoff, S.C.; Bergheim, I. Toll-like receptors 1–9 are elevated in livers with fructose-induced hepatic steatosis. Br. J. Nutr. 2012, 107, 1727–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nier, A.; Engstler, A.J.; Maier, I.B.; Bergheim, I. Markers of intestinal permeability are already altered in early stages of non-alcoholic fatty liver disease: Studies in children. PLoS ONE 2017, 12, e0183282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engstler, A.J.; Sellmann, C.; Jin, C.J.; Brandt, A.; Herz, K.; Priebs, J.; Bergheim, I. Treatment with alpha-galactosylceramide protects mice from early onset of nonalcoholic steatohepatitis: Role of intestinal barrier function. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Brandt, A.; Jin, C.J.; Nolte, K.; Sellmann, C.; Engstler, A.J.; Bergheim, I. Short-Term Intake of a Fructose-, Fat- and Cholesterol-Rich Diet Causes Hepatic Steatosis in Mice: Effect of Antibiotic Treatment. Nutrients 2017, 9, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corraliza, I.; Campo, M.; Soler, G.; Modolell, M. Determination of arginase activity in macrophages: A micromethod. J. Immunol. Methods 1994, 174, 231–235. [Google Scholar] [CrossRef]
- Brandt, A.; Rajcic, D.; Jin, C.J.; Sánchez, V.; Engstler, A.J.; Jung, F.; Nier, A.; Baumann, A.; Bergheim, I. Fortifying diet with rapeseed oil instead of butterfat attenuates the progression of diet-induced non-alcoholic fatty liver disease (NAFLD) and impairment of glucose tolerance. Metabolism 2020, 109, 154283. [Google Scholar] [CrossRef]
- Lima, C.X.; Sanches, M.D.; de Rezende Neto, J.B.; Silva, R.C.d.O.; Teixeira, M.M.; Souza, D.d.G.d.; Santos, G.d.C.; Melo, J.R.d.C. Hyperbaric oxygen therapy aggravates liver reperfusion injury in rats. Acta Cir. Bras. 2008, 23, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Spruss, A.; Kanuri, G.; Wagnerberger, S.; Haub, S.; Bischoff, S.C.; Bergheim, I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 2009, 50, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.R.; Rosso, N.; Bedogni, G.; Tiribelli, C.; Bellentani, S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018, 38, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Loria, P.; Adinolfi, L.E.; Bellentani, S.; Bugianesi, E.; Grieco, A.; Fargion, S.; Gasbarrini, A.; Loguercio, C.; Lonardo, A.; Marchesini, G.; et al. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig. Liver Dis. 2010, 42, 272–282. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Salomone, F.; Mlynarsky, L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017, 37, 936–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Honma, M.; Sawada, S.; Ueno, Y.; Murakami, K.; Yamada, T.; Gao, J.; Kodama, S.; Izumi, T.; Takahashi, K.; Tsukita, S.; et al. Selective insulin resistance with differential expressions of IRS-1 and IRS-2 in human NAFLD livers. Int. J. Obes. 2018, 42, 1544–1555. [Google Scholar] [CrossRef] [Green Version]
- Son, C.G.; Wei, Z.; Raghavendran, H.B.; Wang, J.-H.; Janda, E. Medicinal Herbs and Their Active Compounds for Fatty Liver Diseases. Evid. Based Complementary Altern. Med. 2017, 2017, 3612478. [Google Scholar] [CrossRef] [Green Version]
- Kerouz, N.J.; Hörsch, D.; Pons, S.; Kahn, C.R. Differential regulation of insulin receptor substrates-1 and -2 (IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in liver and muscle of the obese diabetic (ob/ob) mouse. J. Clin. Investig. 1997, 100, 3164–3172. [Google Scholar] [CrossRef]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Holub, B.J. Clinical nutrition: 4. Omega-3 fatty acids in cardiovascular care. Can. Med. Assoc. J. 2002, 166, 608–615. [Google Scholar]
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003, 8, 164–172. [Google Scholar]
- Brown, J.E.; Wahle, K.W. Effect of fish-oil and vitamin E supplementation on lipid peroxidation and whole-blood aggregation in man. Clin. Chim. Acta 1990, 193, 147–156. [Google Scholar] [CrossRef]
- Nair, P.P.; Judd, J.T.; Berlin, E.; Taylor, P.R.; Shami, S.; Sáinz, E.; Bhagavan, H.N. Dietary fish oil-induced changes in the distribution of alpha-tocopherol, retinol, and beta-carotene in plasma, red blood cells, and platelets: Modulation by vitamin E. Am. J. Clin. Nutr. 1993, 58, 98–102. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Z.; Liang, X.; Matulic, D.; Hussein, M.; Gao, J. Growth performance, fatty-acid composition, lipid deposition and hepatic-lipid metabolism-related gene expression in juvenile pond loach Misgurnus anguillicaudatus fed diets with different dietary soybean oil levels. J. Fish Biol. 2018, 92, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, M.; Zhao, M.; Ge, A.; Guo, F.; Zhang, M.; Yang, Y.; Liu, L.; Yang, N. Differential effects of high-fat-diet rich in lard oil or soybean oil on osteopontin expression and inflammation of adipose tissue in diet-induced obese rats. Eur. J. Nutr. 2013, 52, 1181–1189. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Wang, D.; Sul, H.S. Upstream stimulatory factor binding to the E-box at− 65 is required for insulin regulation of the fatty acid synthase promoter. J. Biol. Chem. 1997, 272, 26367–26374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohjima, M.; Enjoji, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; et al. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2007, 20, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, K.; Shimada, K.; Harada, S.; Morimoto, Y.; Hirasawa, A.; Tokuyama, S. DHA supplementation prevent the progression of NASH via GPR120 signaling. Eur. J. Pharmacol. 2018, 820, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Dai, Y.; Zhang, L.; Tian, Y.; Li, Z.; Wang, J. Effects of Edible Oils with Different n-6/n-3 PUFA Ratios on Articular Cartilage Degeneration via Regulating the NF-κB Signaling Pathway. J. Agric. Food Chem. 2020, 68, 12641–12650. [Google Scholar] [CrossRef] [PubMed]
- Diehl, K.L.; Vorac, J.; Hofmann, K.; Meiser, P.; Unterweger, I.; Kuerschner, L.; Weighardt, H.; Förster, I.; Thiele, C. Kupffer Cells Sense Free Fatty Acids and Regulate Hepatic Lipid Metabolism in High-Fat Diet and Inflammation. Cells 2020, 9, 2258. [Google Scholar] [CrossRef]
- Heinonen, I.; Rinne, P.; Ruohonen, S.T.; Ruohonen, S.; Ahotupa, M.; Savontaus, E. The effects of equal caloric high fat and western diet on metabolic syndrome, oxidative stress and vascular endothelial function in mice. Acta Physiol. 2014, 211, 515–527. [Google Scholar] [CrossRef]
- Sellmann, C.; Jin, C.J.; Engstler, A.J.; De Bandt, J.P.; Bergheim, I. Oral citrulline supplementation protects female mice from the development of non-alcoholic fatty liver disease (NAFLD). Eur. J. Nutr. 2017, 56, 2519–2527. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, H.; Zhao, Q.; Wang, X.; Zhang, J.; Zhao, X. Polyphenol-Rich Loquat Fruit Extract Prevents Fructose-Induced Nonalcoholic Fatty Liver Disease by Modulating Glycometabolism, Lipometabolism, Oxidative Stress, Inflammation, Intestinal Barrier, and Gut Microbiota in Mice. J. Agric. Food Chem. 2019, 67, 7726–7737. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Fan, G.; Lukens, J.R.; Vogel, P.; Tonks, N.K.; Kanneganti, T.D. Tyrosine Kinase SYK Licenses MyD88 Adaptor Protein to Instigate IL-1α-Mediated Inflammatory Disease. Immunity 2017, 46, 635–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, J.P.; Jung, T.; Grune, T.; Siems, W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017, 111, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellmann, C.; Degen, C.; Jin, C.J.; Nier, A.; Engstler, A.J.; Hasan, D.; De Bandt, A.J.P.; Bergheim, I. Oral arginine supplementation protects female mice from the onset of non-alcoholic steatohepatitis. Amino Acids 2017, 49, 1215–1225. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, A.; Ponziani, F.R.; Biolato, M.; Valenza, V.; Marrone, G.; Sganga, G.; Gasbarrini, A.; Miele, L.; Grieco, A. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation. World J. Gastroenterol. 2019, 25, 4814–4834. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Xu, Q.; Wang, Q.; Wu, H.; Hua, J. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci. Rep. 2017, 7, 44612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrozova, G.; Pekarova, M.; Lojek, A. Effect of polyunsaturated fatty acids on the reactive oxygen and nitrogen species production by raw 264.7 macrophages. Eur. J. Nutr. 2010, 49, 133–139. [Google Scholar] [CrossRef]
- Saiki, P.; Kawano, Y.; Van Griensven, L.; Miyazaki, K. The anti-inflammatory effect of Agaricus brasiliensis is partly due to its linoleic acid content. Food Funct. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
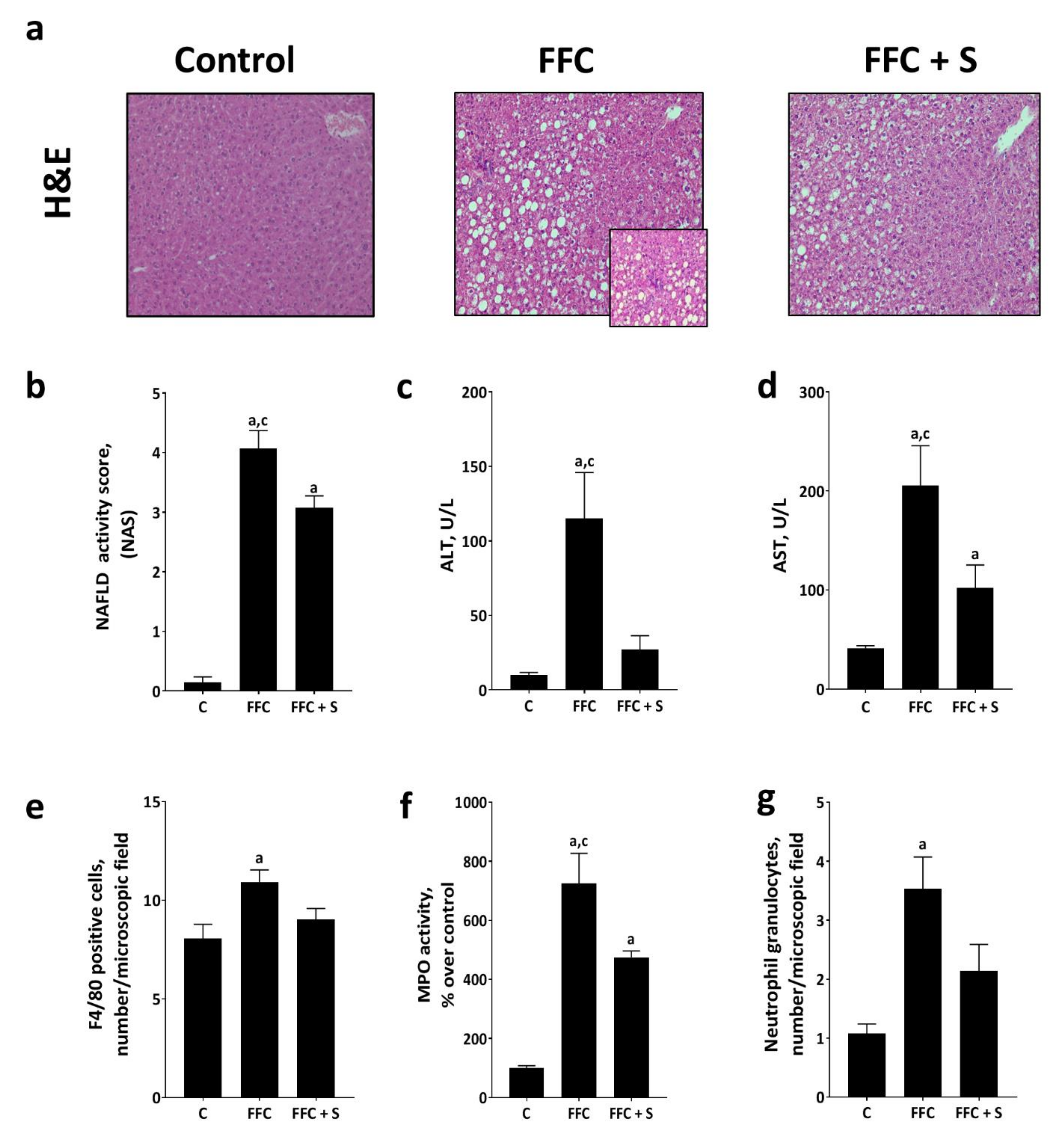
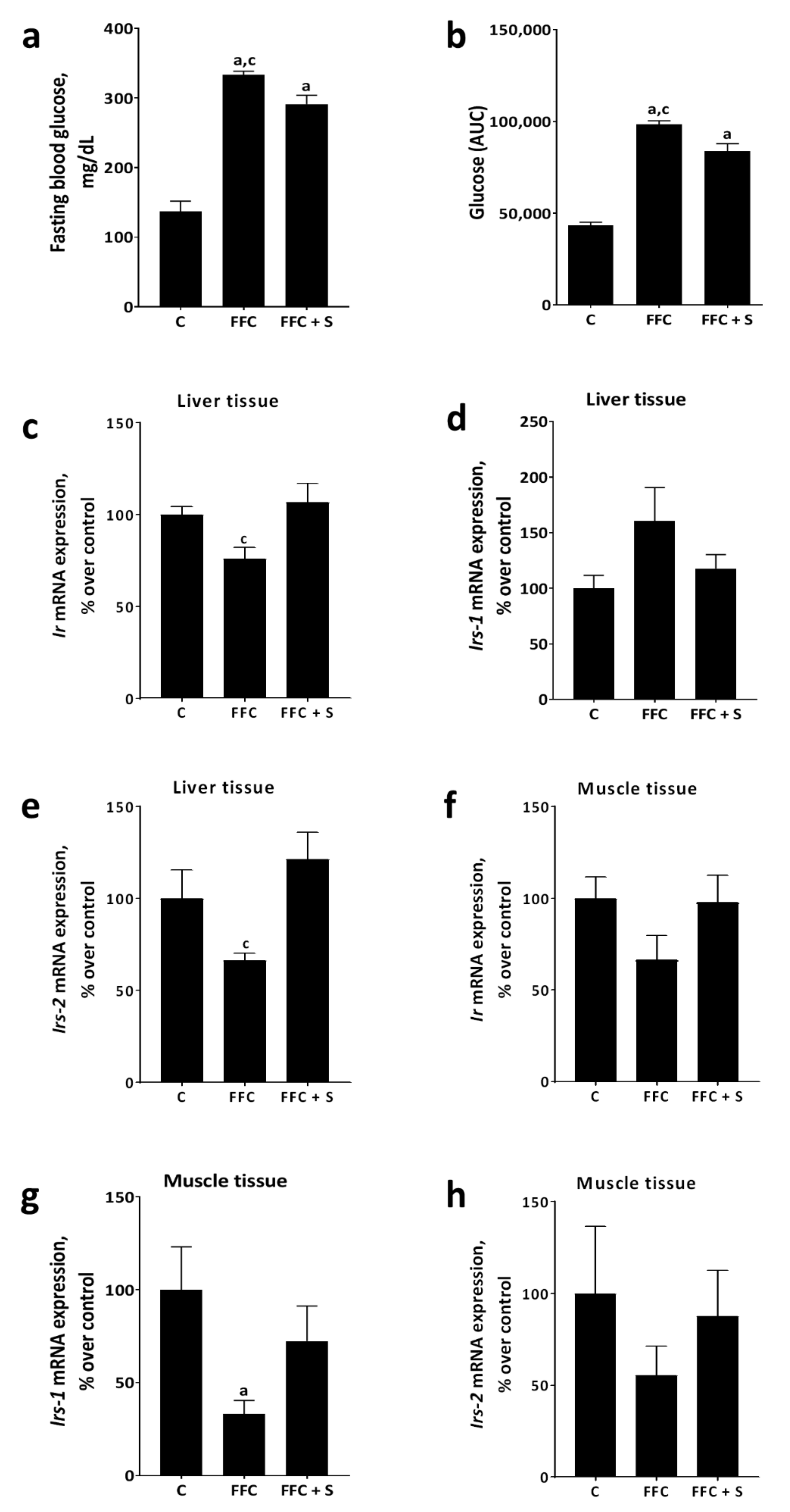
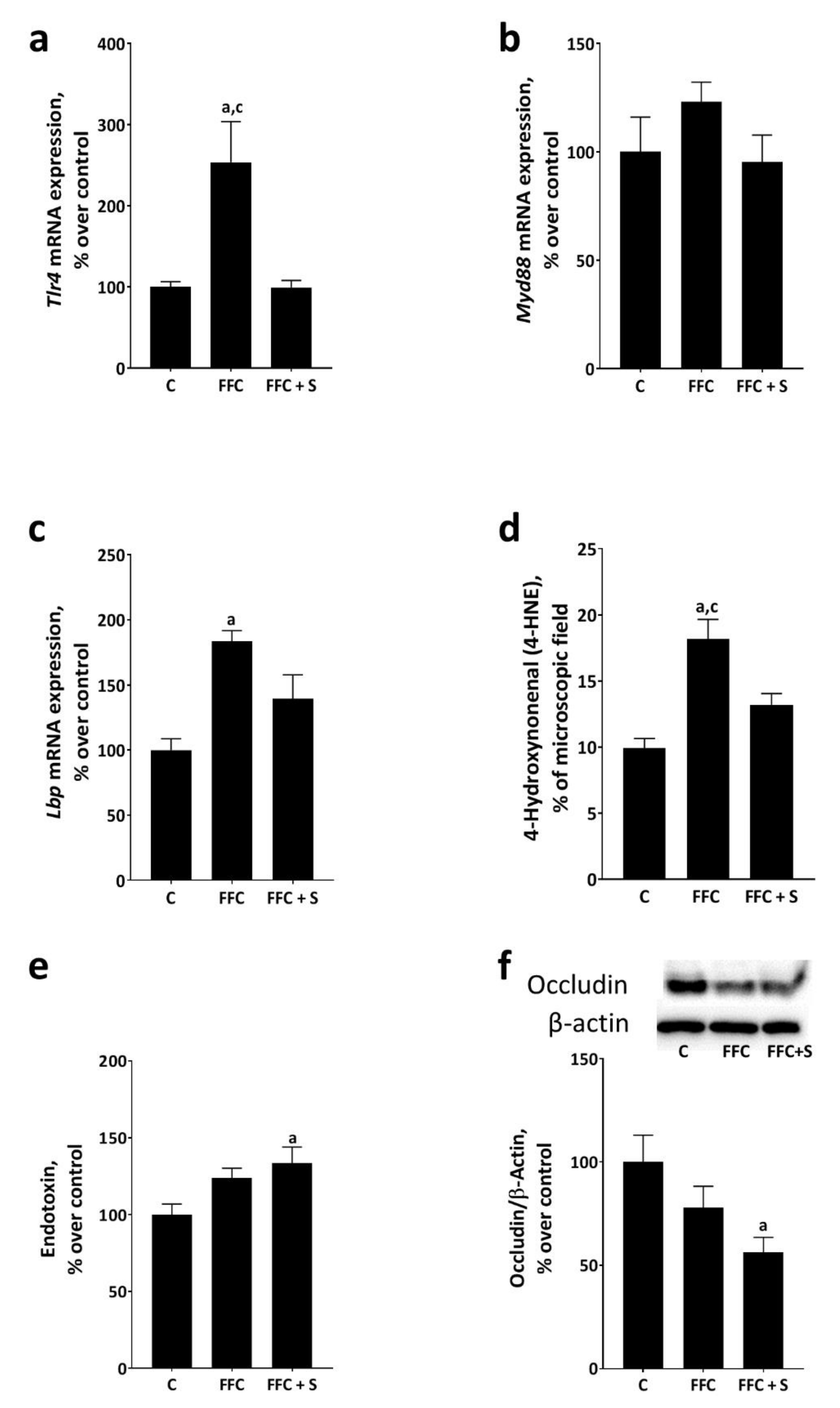

| Parameter | Diet Groups | ||
|---|---|---|---|
| C | FFC | FFC + S | |
| Caloric intake, kcal/g body weight/day | 0.39 ± 0.01 | 0.44 ± 0.01 a | 0.43 ± 0.01 a |
| Body weight, g | 21.6 ± 0.5 | 23.9 ± 0.6 a | 23.8 ± 0.7 a |
| Weight gain, g | 3.6 ± 0.3 | 5.8 ± 0.4 a | 5.5 ± 0.5 a |
| Liver weight, g * | 0.9 ± 0.03 | 1.7 ± 0.13 a,c | 1.3 ± 0.05 a |
| Liver to body weight ratio, % * | 4.1 ± 0.09 | 7.1 ± 0.36 a,c | 5.7 ± 0.08 a |
| Steatosis, NAS | 0.3 ± 0.1 | 2.4 ± 0.2 a,c | 1.9 ± 0.1 a |
| Inflammation, NAS | 0 ± 0 | 1.6 ± 0.1 a,c | 1.0 ± 0.1 a |
| Ballooning | 0 ± 0 | 0.04 ± 0.02 | 0.04 ± 0.02 |
| TNF-α, pg/mg protein * | 28.8 ± 3.7 | 39.5 ± 4.0 | 33.6 ± 0.9 |
| Arginase activity, % over control * | 100 ± 14 | 37.4 ± 2.3 a | 50.3 ± 7.1 a |
| Parameter | Diet Groups | ||
|---|---|---|---|
| C | FFC | FFC + S | |
| Srebp-1c mRNA expression, % of control | 100 ± 9.5 | 130 ± 17 | 117 ± 12 |
| Acc mRNA expression, % of control * | 100 ± 18 | 446 ± 75 a | 428 ± 78 a |
| Scd-1 mRNA expression, % of control * | 100 ± 9.8 | 393 ± 67 a | 355 ± 80 a |
| Acox1 mRNA expression, % of control | 100 ± 12 | 135 ± 7.8 c | 91.5 ± 9.0 |
| Cpt1 mRNA expression, % of control | 100 ± 14 | 148 ± 9.1 a | 130 ± 11 |
| Fas mRNA expression, % of control * | 100 ± 15 | 617 ± 65 a | 386 ± 63 a |
| PPARγ activity, % of control * | 100 ± 2.0 | 150 ± 19 a | 123 ± 9.3 |
| Gpr120 mRNA expression, % of control | 100 ± 14 | 242 ± 11 a,c | 180 ± 23 a |
| FFA, nmol/µL | 0.47 ± 0.06 | 0.33 ± 0.03 | 0.42 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, V.; Brandt, A.; Jin, C.J.; Rajcic, D.; Engstler, A.J.; Jung, F.; Nier, A.; Baumann, A.; Bergheim, I. Fortifying Butterfat with Soybean Oil Attenuates the Onset of Diet-Induced Non-Alcoholic Steatohepatitis and Glucose Intolerance. Nutrients 2021, 13, 959. https://doi.org/10.3390/nu13030959
Sánchez V, Brandt A, Jin CJ, Rajcic D, Engstler AJ, Jung F, Nier A, Baumann A, Bergheim I. Fortifying Butterfat with Soybean Oil Attenuates the Onset of Diet-Induced Non-Alcoholic Steatohepatitis and Glucose Intolerance. Nutrients. 2021; 13(3):959. https://doi.org/10.3390/nu13030959
Chicago/Turabian StyleSánchez, Victor, Annette Brandt, Cheng Jun Jin, Dragana Rajcic, Anna Janina Engstler, Finn Jung, Anika Nier, Anja Baumann, and Ina Bergheim. 2021. "Fortifying Butterfat with Soybean Oil Attenuates the Onset of Diet-Induced Non-Alcoholic Steatohepatitis and Glucose Intolerance" Nutrients 13, no. 3: 959. https://doi.org/10.3390/nu13030959






