Iron Protein Succinylate in the Management of Iron Deficiency Anemia: A Comparative Study with Ferrous Sulphate at Low and High Therapeutic Doses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
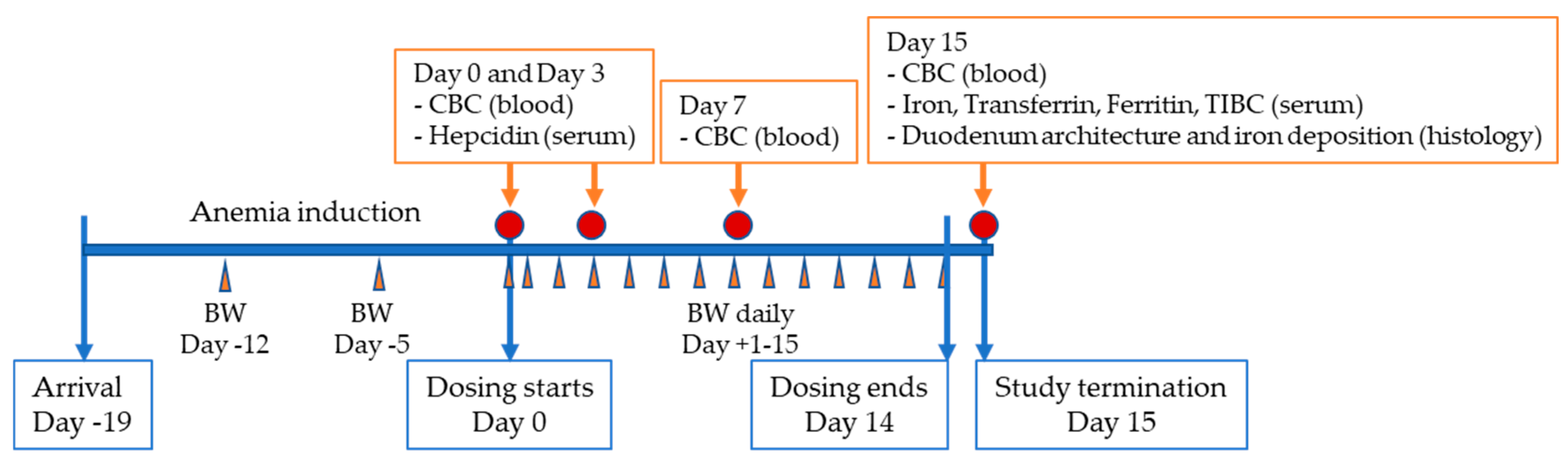
2.2. Procedures
3. Results
3.1. Treatment with Ferplex® and FeSO4 Reverses Diet-Induced Anemia
3.2. The Effect of High-Dose Ferplex® and FeSO4 on Intestinal Mucosa
3.3. Iron Supplementation and Serum Hepcidin Induction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef] [Green Version]
- Camaschella, C. Iron deficiency: New insights into diagnosis and treatment. Hematol. Am. Soc. Hematol. Educ. Program. 2015, 2015, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [Green Version]
- Daru, J.; Zamora, J.; Fernández-Félix, B.M.; Vogel, J.; Oladapo, O.T.; Morisaki, N.; Tunçalp, Ö.; Torloni, M.R.; Mittal, S.; Jayaratne, K.; et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: A multilevel analysis. Lancet Glob. Health 2018, 6, e548–e554. [Google Scholar] [CrossRef] [Green Version]
- The Gastroenterological Society of Australia. Iron Deficiency; Clinical Update; Gastroenterological Society of Australia: Mulgrave, Australia, 2015. [Google Scholar]
- Smith, G.A.; Fisher, S.A.; Doree, C.; Angelantonio, E.D.; Roberts, D.J. Oral or parenteral iron supplementation to reduce deferral, iron deficiency and/or anaemia in blood donors. Cochrane Database Syst. Rev. 2014, 7, CD009532. [Google Scholar] [CrossRef]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.A.; Powell, J.J. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: A systematic review and meta-analysis. PLoS ONE 2015, 10, e01173383. [Google Scholar] [CrossRef] [Green Version]
- Santiago, P. Ferrous versus ferric oral iron formulations for the treatment of iron deficienc: A clinical overview. Sci. World J. 2012, 2012, 846824. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Francés, A.; Martínez-Bujanda, J.L. Efficacy and tolerability of oral iron protein succinylat: A systematic review of three decades of research. Curr. Med. Res. Opin. 2020, 36, 613–623. [Google Scholar] [CrossRef] [Green Version]
- Liguori, L. Iron protein succinylate in the treatment of iron deficiency: Controlled, double-blind, multicenter clinical trial on over 1000 patients. Int. J. Clin. Pharmacol. Ther. Toxicol. 1993, 31, 103–123. [Google Scholar]
- Cremonesi, P.; Acebron, A.; Raja, K.B.; Simpson, R.J. Iron absorption: Biochemical and molecular insights into the importance of iron species for intestinal uptake. Pharmacol. Toxicol. 2002, 91. [Google Scholar] [CrossRef]
- Cremonesi, P.; Strada, D.; Galimberti, G.; Sportoletti, G. Iron derivatives of modified milk protein. Arzneimittelforschung 1984, 34, 948–952. [Google Scholar]
- Cremonesi, P.; Caramazza, I. Chemical and biological characterization of iron-protein succinylate (ITF 282). Int. J. Clin. Pharmacol. Ther. Toxicol. 1993, 31, 40–51. [Google Scholar]
- Pagella, P.; Bellavite, O.; Agozzino, S.; Donà, G. Pharmacological and toxicological studies on an iron succinyl-protein complex (ITF282) for oral treatment of iron deficiency anemia. Arzneimittelforschung 1984, 34, 952–958. [Google Scholar]
- Nemeth, E.; Ganz, T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006, 26, 323–342. [Google Scholar] [CrossRef]
- Arezes, J.; Nemeth, E. Hepcidin and iron disorders: New biology and clinical approaches. Int. Jnl. Lab. Hem. 2015, 37, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Piperno, A.; Girelli, D.; Nemeth, E.; Trombini, P.; Bozzini, C.; Poggiali, E.; Phung, Y.; Ganz, T.; Camaschella, C. Blunted hepcidin response to oral iron challenge in HFE-related hemochromatosis. Blood 2007, 110, 4096–4100. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, N.U.; Cercamondi, C.I.; Brittenham, G.; Zeder, C.; Geurts-Moespot, A.J.; Swinkels, D.W.; Moretti, D.; Zimmermann, M.B. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: Two open-label, randomised controlled trials. Lancet Haematol. 2017, 4, e524–e533. [Google Scholar] [CrossRef]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; De-Regil, L.M.; Malave, H.G.; Flores-Urrutia, M.C.; Dowswell, T. Intermittent oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2015, 2015, CD009997. [Google Scholar] [CrossRef]
- Kajarabille, N.; Brown, C.; Cucliciu, A.; Thapaliya, G.; Latunde-Dada, G.O. Bioavailability of iron multi-amino acid chelate preparation in mice and human duodenal HuTu 80 cells. Br. J. Nutr. 2017, 117, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Asperti, M.; Gryzik, M.; Brilli, E.; Castagna, A.; Corbella, M.; Gottardo, R.; Girelli, D.; Tarantino, G.; Arosio, P.; Poli, M. Sucrosomial® Iron Supplementation in Mice, Effects on Blood Parameters, Hepcidin, and Inflammation. Nutrients 2018, 10, 1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, M.K.; Kulkarni, S.S.; Mohanty, N.; Kadam, N.N.; Swain, N.S. Standardization and Development of Rat Model with Iron Deficiency Anaemia Utilising Commercial Available Iron Deficient Food. Biosci. Biotech. Res. Asia. 2019, 16, 71–77. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Huang, Q.; Liu, C.; Jia, S.; Wang, Y.; An, F.; Song, H. Effectiveness of AOS–iron on iron deficiency anemia in rats. RSC Adv. 2019, 9, 5053–5063. [Google Scholar] [CrossRef] [Green Version]
- Evstatiev, R.; Bukaty, A.; Jimenez, K.; Kulnigg-Dabsch, S.; Surman, L.; Schmid, W.; Eferl, R.; Lippert, K.; Scheiber-Mojdehkar, B.; Kvasnicka, H.M.; et al. Iron deficiency alters megakaryopoiesis and platelet phenotype independent of thrombopoietin. Am. J. Hematol. 2014, 89, 524–529. [Google Scholar] [CrossRef] [Green Version]
- Parodi, E.; Giraudo, M.T.; Davitto, M.; Ansaldi, G.; Mondino, A.; Garbarini, L.; Franzil, A.; Mazzone, R.; Russo, G.; Ramenghi, U. Reticulocyte parameters: Markers of early response to oral treatment in children with severe iron-deficiency anemia. J. Pediatr. Hematol. Oncol. 2012, 34, e249–e252. [Google Scholar] [CrossRef] [Green Version]
- Gelaw, Y.; Woldu, B.; Melku, M. The Role of Reticulocyte Hemoglobin Content for Diagnosis of Iron Deficiency and Iron Deficiency Anemia, and Monitoring of Iron Therapy: A Literature Review. Clin. Lab. 2019, 65. [Google Scholar] [CrossRef]
- Fang, S.; Zhuo, Z.; Yu, X.; Wang, H.; Feng, J. Oral administration of liquid iron preparation containing excess iron induces intestine and liver injury, impairs intestinal barrier function and alters the gut microbiota in rats. J. Trace. Elem. Med. Biol. 2018, 47, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Naiman, J.L.; Oski, F.A.; Diamond, K.L.K.; Vawter, G.F.; Shwachman, H. The gastrointestinal effects of iron-deficiency anemia. Pediatrics 1964, 33, 83–99. [Google Scholar] [PubMed]
- Guha, D.K.; Walia, B.N.; Tandon, B.N.; Deo, M.G.; Ghai, O.P. Small bowel changes in iron-deficiency anaemia of childhood. Arch. Dis. Child. 1968, 43, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Köpcke, W.; Sauerland, M.C. Meta-analysis of efficacy and tolerability data on iron proteinsuccinylate in patients with iron deficiency anemia of different severity. Arzneimittelforschung 1995, 45, 1211–1216. [Google Scholar]
- Dogan, A.; Alioglu, B.; Dindar, N.; Dallar, Y. Increased serum hepcidin and ghrelin levels in children treated for iron deficiency anemia. J. Clin. Lab. Anal. 2013, 27, 81–85. [Google Scholar] [CrossRef]
- Mehta, S.; Sharma, B.S.; Gulati, S.; Sharma, N.; Goyal, L.K.; Mehta, S. A prospective, randomized, interventional study of oral iron supplementation comparing daily dose with alternate day regimen using hepcidin as a biomarker in iron deficiency anemia. J. Assoc. Phys. India 2020, 68, 39–41. [Google Scholar]
- Means, R.T. Pathophysiology in Medicine, Hepcidin and iron regulation in health and disease. Am. J. Med. Sci. 2013, 345, 57–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laftah, A.H.; Ramesh, B.; Simpson, R.J.; Solanky, N.; Bahram, S.; Schümann, K.; Debnam, E.S.; Srai, S.K.S. Effect of hepcidin on intestinal iron absorption in mice. Blood 2004, 103, 3940–3944. [Google Scholar] [CrossRef] [Green Version]
- Yamaji, S.; Sharp, P.; Ramesh, B.; Srai, S.K. Inhibition of iron transport across human intestinal epithelial cells by hepcidin. Blood 2004, 104, 2178–2180. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.N.; Niu, Q.M.; Ge, L.; Zhang, N.; Yan, S.F.; Chen, W.B.; Chang, Y.Z.; Zhao, S.E. Sex Differences in Iron Status and Hepcidin Expression in Rats. Biol. Trace Elem. Res. 2014, 160, 258–267. [Google Scholar] [CrossRef]







| Group Name | Diet | Treatment |
|---|---|---|
| Control | Standard chow | Vehicle (water) |
| Anemic-vehicle | Fe-restricted diet | Vehicle (water) |
| Monitor group | Fe-restricted diet | None |
| Ferplex® 80 mg | Fe-restricted diet | Elemental Fe 7.1 mg/kg |
| Ferplex® 200 mg | Fe-restricted diet | Elemental Fe 17.1 mg/kg |
| FeSO4 80 mg | Fe-restricted diet | Elemental Fe 7.1 mg/kg |
| FeSO4 200 mg | Fe-restricted diet | Elemental Fe 17.1 mg/kg |
| Group Name (n) | Ferritin ng/mL (Mean ± SEM) | TIBC µmol/L (Mean ± SEM) |
|---|---|---|
| Control (8) | 863 ± 131 | 81.6 ± 1.3 |
| Anemic-vehicle (6) | 894 ± 83 | 77.2 ± 3.4 |
| Ferplex® 80 mg (8) | 1048 ± 90 | 83.2 ± 1.9 |
| Ferplex® 200 mg (6) | 1483 ± 156 | 81.3 ± 1.5 |
| FeSO4 80 mg (7) | 1330 ± 171 | 87.9 ± 2.2 |
| FeSO4 200 mg (7) | 1274 ± 175 | 80.2 ± 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urso, K.; Leal Martínez-Bujanda, J.; del Prado, J.M. Iron Protein Succinylate in the Management of Iron Deficiency Anemia: A Comparative Study with Ferrous Sulphate at Low and High Therapeutic Doses. Nutrients 2021, 13, 968. https://doi.org/10.3390/nu13030968
Urso K, Leal Martínez-Bujanda J, del Prado JM. Iron Protein Succinylate in the Management of Iron Deficiency Anemia: A Comparative Study with Ferrous Sulphate at Low and High Therapeutic Doses. Nutrients. 2021; 13(3):968. https://doi.org/10.3390/nu13030968
Chicago/Turabian StyleUrso, Katia, Javier Leal Martínez-Bujanda, and Jaime Moscoso del Prado. 2021. "Iron Protein Succinylate in the Management of Iron Deficiency Anemia: A Comparative Study with Ferrous Sulphate at Low and High Therapeutic Doses" Nutrients 13, no. 3: 968. https://doi.org/10.3390/nu13030968






