Polish Translation and Validation of the Mini Sarcopenia Risk Assessment (MSRA) Questionnaire to Assess Nutritional and Non-Nutritional Risk Factors of Sarcopenia in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Covariates and Data Collection
2.3. Procedure for Translation and Adaption of the Mini Sarcopenia Risk Assessment (MSRA) Questionnaire
2.4. Clinical Validation of the Translation of the Mini Sarcopenia Risk Assessment (MSRA) Questionnaire
2.4.1. Assessment of Sarcopenia Using Six Sets of Different Diagnostic Criteria
2.4.2. Measurements of Muscle Mass, Muscle Strength, and Physical Performance
2.4.3. Assessment of Relationships between PL-MSRA and Other Measurements
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Group
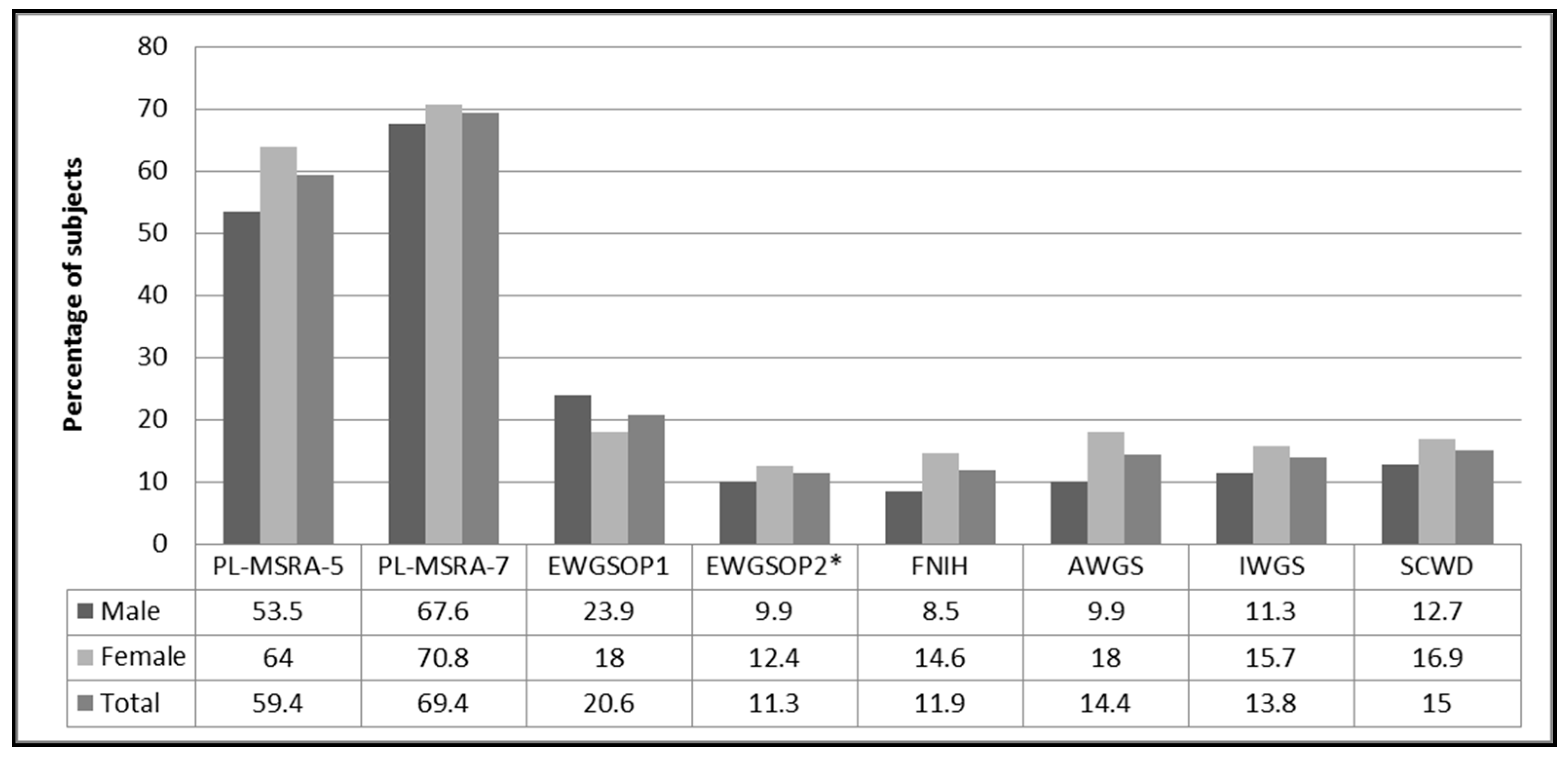
3.2. Prevalence of Sarcopenia
3.3. Inter-Rater Reliability and Test-Retest Reliability
3.4. Clinical Validation of the Polish MSRA Questionnaire Against a Different Reference Standard
3.5. Validity against Other Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Yu, S.C.Y.; Khow, K.S.F.; Jadczak, A.D.; Visvanathan, R. Clinical Screening Tools for Sarcopenia and Its Management. Curr. Gerontol. Geriatr. Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Nawi, S.N.M.; Khow, K.S.; Lim, W.S.; Yu, S.C. Screening Tools for Sarcopenia in Community-Dwellers: A Scoping Review. Ann. Acad. Med. Singap. 2019, 48, 201–216. [Google Scholar]
- Morley, J.E.; Sanford, A.M. Screening for Sarcopenia. J. Nutr. Health Aging 2019, 23, 768–770. [Google Scholar] [CrossRef]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.-Y.; Bruyère, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef]
- Yang, M.; Hu, X.; Xie, L.; Zhang, L.; Zhou, J.; Lin, J.; Wang, Y.; Li, Y.; Han, Z.; Zhang, D.; et al. Validation of the Chinese version of the Mini Sarcopenia Risk Assessment questionnaire in community-dwelling older adults. Medicine 2018, 97, e12426. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A Simple Questionnaire to Rapidly Diagnose Sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef]
- Rossi, A.P.; Micciolo, R.; Rubele, S.; Fantin, F.; Caliari, C.; Zoico, E.; Mazzali, G.; Ferrari, E.; Volpato, S.; Zamboni, M. Assessing the risk of sarcopenia in the elderly: The Mini Sarcopenia Risk Assessment (MSRA) questionnaire. J. Nutr. Health Aging 2017, 21, 743–749. [Google Scholar] [CrossRef]
- Beaudart, C.; Locquet, M.; Bornheim, S.; Reginster, J.-Y.; Bruyère, O. French translation and validation of the sarcopenia screening tool SARC-F. Eur. Geriatr. Med. 2017, 9, 29–37. [Google Scholar] [CrossRef]
- Bahat, G.; Yilmaz, O.; Kiliç, C.; Oren, M.M.; Karan, M.A. Performance of SARC-F in Regard to Sarcopenia Definitions, Muscle Mass and Functional Measures. J. Nutr. Health Aging 2018, 22, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, D.; Marco, E.; Dávalos-Yerovi, V.; López-Escobar, J.; Messaggi-Sartor, M.; Barrera, C.; Ronquillo-Moreno, N.; Vázquez-Ibar, O.; Calle, A.; Inzitari, M.; et al. Translation and Validation of the Spanish Version of the SARC-F Questionnaire to Assess Sarcopenia in Older People. J. Nutr. Health Aging 2019, 23, 518–524. [Google Scholar] [CrossRef]
- Drey, M.; Ferrari, U.; Schraml, M.; Kemmler, W.; Schoene, D.; Franke, A.; Freiberger, E.; Kob, R.; Sieber, C. German Version of SARC-F: Translation, Adaption, and Validation. J. Am. Med. Dir. Assoc. 2020, 21, 747–751.e1. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Leung, J.; Morley, J.E. Validating the SARC-F: A Suitable Community Screening Tool for Sarcopenia? J. Am. Med. Dir. Assoc. 2014, 15, 630–634. [Google Scholar] [CrossRef]
- Kera, T.; Kawai, H.; Hirano, H.; Kojima, M.; Watanabe, Y.; Motokawa, K.; Fujiwara, Y.; Ihara, K.; Kim, H.; Obuchi, S. SARC-F: A validation study with community-dwelling older Japanese adults. Geriatr. Gerontol. Int. 2019, 19, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Parra-Rodríguez, L.; Szlejf, C.; García-González, A.I.; Malmstrom, T.K.; Cruz-Arenas, E.; Rosas-Carrasco, O. Cross-Cultural Adaptation and Validation of the Spanish-Language Version of the SARC-F to Assess Sarcopenia in Mexican Community-Dwelling Older Adults. J. Am. Med. Dir. Assoc. 2016, 17, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, M.; Won, C.W. Validation of the Korean Version of the SARC-F Questionnaire to Assess Sarcopenia: Korean Frailty and Aging Cohort Study. J. Am. Med. Dir. Assoc. 2018, 19, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, K.; Głuszewska, A.; Czesak, J.; Fedyk-Łukasik, M.; Klimek, E.; Sánchez-Rodríguez, D.; Skalska, A.; Gryglewska, B.; Grodzicki, T.; Gąsowski, J. SARC-F as a case-finding tool for sarcopenia according to the EWGSOP2. National validation and comparison with other diagnostic standards. Aging Clin. Exp. Res. 2021, 1–9. [Google Scholar] [CrossRef]
- Barbosa-Silva, T.G.; Menezes, A.M.B.; Bielemann, R.M.; Malmstrom, T.K.; Gonzalez, M.C. Enhancing SARC-F: Improving Sarcopenia Screening in the Clinical Practice. J. Am. Med. Dir. Assoc. 2016, 17, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Murata, K.; Nakadachi, D.; Ishihara, Y.; Imataka, K.; Uchida, A.; Monguchi, K.; Kaneko, R.; Fujiwara, R.; Takahashi, H. Development of a Japanese version of the SARC-F for diabetic patients: An examination of reliability and validity. Aging Clin. Exp. Res. 2017, 29, 935–942. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Nguyen, A.T.; Khuong, L.Q.; Nguyen, T.X.; Nguyen, H.T.T.; Nguyen, T.T.H.; Van Hoang, M.; Pham, T.; Nguyen, T.N.; Vu, H.T.T. Reliability and Validity of SARC-F Questionnaire to Assess Sarcopenia Among Vietnamese Geriatric Patients. Clin. Interv. Aging 2020, 15, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Krzymińska-Siemaszko, R.; Deskur-Śmielecka, E.; Kaluźniak-Szymanowska, A.; Styszyński, A.; Wieczorowska-Tobis, K. Polish version of SARC-F to assess sarcopenia in older adults: An examination of reliability and validity. PLoS ONE 2020, 15, e0244001. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Kaneko, R.; Murata, K. SARC-F for Screening of Sarcopenia Among Older Adults: A Meta-analysis of Screening Test Accuracy. J. Am. Med. Dir. Assoc. 2018, 19, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Hu, X.; Xie, L.; Zhang, L.; Zhou, J.; Lin, J.; Wang, Y.; Li, Y.; Han, Z.; Zhang, D.; et al. Comparing Mini Sarcopenia Risk Assessment With SARC-F for Screening Sarcopenia in Community-Dwelling Older Adults. J. Am. Med. Dir. Assoc. 2019, 20, 53–57. [Google Scholar] [CrossRef]
- Krzymińska-Siemaszko, R.; Tobis, S.; Lewandowicz, M.; Wieczorowska-Tobis, K. Comparison of four sarcopenia screening questionnaires in community-dwelling older adults from Poland using six sets of international diagnostic criteria of sarcopenia. PLoS ONE 2020, 15, e0231847. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Yadigar, S.; Yavuzer, H.; Yavuzer, S.; Cengiz, M.; Yuruyen, M.; Döventaş, A.; Erdinçler, D.S. Primary sarcopenia in older people with normal nutrition. J. Nutr. Health Aging 2015, 20, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Hsu, P.-S.; Krairit, O.; Lee, J.S.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; van Kan, G.A.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.S.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia With Limited Mobility: An International Consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef]
- World Health Organization. Process of Translation and Adaptation of Instruments. Available online: https://www.who.int/substance_abuse/research_tools/translation/en/ (accessed on 10 August 2019).
- Jitapunkul, S.; Pillay, I.; Ebrahim, S. The Abbreviated Mental Test: Its Use and Validity. Age Ageing 1991, 20, 332–336. [Google Scholar] [CrossRef] [PubMed]
- MNA Polish Form. Available online: http://www.mna-elderly.com/forms/MNA_polish.pdf (accessed on 10 August 2019).
- Shelkey, M.; Wallace, M. Katz Index of Independence in Activities of Daily Living (ADL). Director 2000, 8, 72–73. [Google Scholar] [PubMed]
- Graf, C.; Hartford Institute for Geriatric Nursing. The Lawton instrumental activities of daily living (IADL) scale. Med. Surg. Nurs. Off. J. Acad. Med.-Surg. Nurses 2008, 17, 343–344. [Google Scholar]
- Bahat, G.; Yilmaz, O.; Oren, M.M.; Karan, M.A.; Reginster, J.Y.; Bruyère, O.; Beaudart, C. Cross-cultural adaptation and validation of the SARC-F to assess sarcopenia: Methodological report from European Union Geriatric Medicine Society Sarcopenia Special Interest Group. Eur. Geriatr. Med. 2017, 9, 23–28. [Google Scholar] [CrossRef]
- Krzymińska-Siemaszko, R.; Fryzowicz, A.; Czepulis, N.; Kaluźniak-Szymanowska, A.; Dworak, L.B.; Wieczorowska-Tobis, K. The impact of the age range of young healthy reference population on the cut-off points for low muscle mass necessary for the diagnosis of sarcopenia. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4321–4332. [Google Scholar]
- Krzymińska-Siemaszko, R.; Czepulis, N.; Suwalska, A.; Dworak, L.B.; Fryzowicz, A.; Madej-Dziechciarow, B.; Wieczorowska-Tobis, K. The Significance of Body Mass Index in Calculating the Cut-Off Points for Low Muscle Mass in the Elderly: Methodological Issues. BioMed Res. Int. 2014, 2014, 450396. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Linden, A. Measuring diagnostic and predictive accuracy in disease management: An introduction to receiver operating characteristic (ROC) analysis. J. Eval. Clin. Pract. 2006, 12, 132–139. [Google Scholar] [CrossRef]
- Kundel, H.L.; Polansky, M. Measurement of Observer Agreement. Radiology 2003, 228, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.-K.; Fielding, R.A.; Martin, F.C.; Michel, J.-P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Nowson, C.; O’Connell, S. Protein Requirements and Recommendations for Older People: A Review. Nutrients 2015, 7, 6874–6899. [Google Scholar] [CrossRef]
- Visvanathan, R.; Chapman, I. Preventing sarcopaenia in older people. Matur 2010, 66, 383–388. [Google Scholar] [CrossRef]
- Vandewoude, M.F.J.; Alish, C.J.; Sauer, A.C.; Hegazi, R.A. Malnutrition-Sarcopenia Syndrome: Is This the Future of Nutrition Screening and Assessment for Older Adults? J. Aging Res. 2012, 2012, 651570. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Kokkinidis, D.G.; Armstrong, E.J.; Giri, J. Balancing Weight Loss and Sarcopenia in Elderly Patients With Peripheral Artery Disease. J. Am. Hear. Assoc. 2019, 8, e013200. [Google Scholar] [CrossRef] [PubMed]
- Peláez, R.B. Therapeutic Approach to Malnutrition and Sarcopenia. In The Importance of Nutrition as an Integral Part of Disease Management; S Karger AG: New Delhi, India, 2012; Volume 72, pp. 85–99. [Google Scholar]
- Kennedy, R.L.; Malabu, U.; Kazi, M.; Shahsidhar, V. Management of obesity in the elderly: Too much and too late? J. Nutr. Health Aging 2008, 12, 608–621. [Google Scholar] [CrossRef]
- Coker, R.H.; Wolfe, R.R. Weight Loss Strategies in the Elderly: A Clinical Conundrum. Obesity 2017, 26, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Walowski, C.O.; Braun, W.; Maisch, M.J.; Jensen, B.; Peine, S.; Norman, K.; Müller, M.J.; Bosy-Westphal, A. Reference Values for Skeletal Muscle Mass Current Concepts and Methodological Considerations. Nutrients 2020, 12, 755. [Google Scholar] [CrossRef]
- Marra, M.; Sammarco, R.; De Lorenzo, A.; Iellamo, F.; Siervo, M.; Pietrobelli, A.; Donini, L.M.; Santarpia, L.; Cataldi, M.; Pasanisi, F.; et al. Assessment of Body Composition in Health and Disease Using Bioelectrical Impedance Analysis (BIA) and Dual Energy X-Ray Absorptiometry (DXA): A Critical Overview. Contrast Media Mol. Imaging 2019, 2019, 3548284. [Google Scholar] [CrossRef] [PubMed]
| The MSRA-7 and MSRA-5 Questionnaires | ||
|---|---|---|
| MSRA-7 Score | MSRA-5 Score | |
| 1. How old are you? | ||
| ≥70 years | 0 | 0 |
| <70 years | 5 | 5 |
| 2. Were you hospitalized in the last year? | ||
| Yes, and more than 1 hospitalization | 0 | 0 |
| Yes 1 hospitalization, | 5 | 10 |
| No | 10 | 15 |
| 3. What is your activity level? | ||
| I’m able to walk <1000 m | 0 | 0 |
| I’m able to walk more than 1000 m | 5 | 15 |
| 4. Do you eat 3 meals per day regularly? | ||
| No, up to twice per week I skip a meal (e.g., I skip breakfast or I have only milk coffee or soup for dinner) | 0 | - |
| Yes | 5 | - |
| 5. Do you consume any of the following? | ||
| Milk or dairy products (e.g., yogurt, cheese), but not every day | 0 | - |
| Milk or dairy products (e.g., yogurt, cheese), at least once per day | 5 | - |
| 6. Do you consume any of the following? | ||
| Poultry, meat, fish, eggs, legumes, ragout, or ham but not every day | 0 | 0 |
| Poultry, meat, fish, eggs, legumes, ragout, or ham, at least once per day | 5 | 15 |
| 7. Do you lose weight in the last year? | ||
| >2 kg | 0 | 0 |
| no or ≤2 kg | 5 | 10 |
| sum of points | ||
| Low Muscle Strength | Low Muscle Mass | Low Physical Performance | Diagnostic Criteria | |
|---|---|---|---|---|
| Sarcopenia according to EWGSOP1 | HGS < 30 kg for M HGS < 20 kg for W | ALM/height2 ≤ 7.40 kg/m2 for M * ALM/height2 ≤ 5.60 kg/m2 for W * | UGS ≤ 0.8 m/s for both sexes | HGS + LMM and/or UGS + LMM |
| Sarcopenia according to EWGSOP2 | HGS < 27 kg for M HGS < 16 kg for W and/or CST > 15 s for five rises for both sexes | ALM/height2 ≤ 7.00 kg/m2 for M ALM/height2 ≤ 5.50 kg/m2 for W | ─ | HGS and/or CST + LMM |
| Sarcopenia according to AWGS | HGS < 26 kg for M HGS < 18 kg for W | ALM/height2 < 7.00 kg/m2 for M ALM/height2 < 5.40 kg/m2 for W | UGS ≤ 0.8 m/s for both sexes | HGS + LMM and/or UGS + LMM |
| Sarcopenia according to IWGS | ─ | ALM/height2 ≤ 7.23 kg/m2 for M ALM/height2 ≤ 5.67 kg/m2 for W | UGS < 1.0 m/s for both sexes | LMM + UGS |
| Sarcopenia according to FNIH | HGS < 26 kg for M HGS < 16 kg for W | ALM/BMI < 0.798 for M ALM/BMI < 0.512 for W | UGS ≤ 0.8 m/s for both sexes | HGS + LMM + UGS |
| Sarcopenia according to SCWD | ─ | ALM/height2 ≤ 7.29 kg/m2 for M ** ALM/height2 ≤ 5.52 kg/m2 for W ** | UGS ≤ 1.0 m/s for both sexes | LMM + UGS |
| Characteristics | Total (n = 160) | Men (n = 71) | Women (n = 89) | p |
|---|---|---|---|---|
| Age (years) a | 72.6 (7.2) | 71.6 (7.6) | 73.5 (6.7) | 0.0795 |
| Age cohort | ||||
| 65–74 yrs | 101 (63.1) | 48 (67.6) | 53 (59.6) | 0.2408 |
| 75 yrs or more | 59 (36.9) | 23 (32.4) | 36 (40.4) | |
| Level of education b,& | ||||
| no education or primary | 7 (4.4) | 1 (1.4) | 6 (6.9) | 0.2009 |
| higher than primary | 151 (95.6) | 70 (98.6) | 81 (93.1) | |
| Living conditions b,& | ||||
| Living alone | 50 (31.6) | 11 (15.5) | 39 (44.8) | 0.0001 |
| Living with others | 108 (68.4) | 60 (84.5) | 48 (55.2) | |
| Marital status b,& | ||||
| Unmarried | 67 (42.4) | 18 (25.4) | 49 (56.3) | 0.0001 |
| Married | 91 (57.6) | 53 (74.6) | 38 (43.7) | |
| Height (cm) a | 163.6 (9.8) | 172.1 (6.4) | 156.8 (6.0) | 0.0000 |
| Weight (kg) a | 72.4 (15.7) | 79.5 (14.0) | 66.8 (14.7) | 0.0000 |
| BMI (kg/m2) a | 27.0 (5.4) | 26.8 (4.4) | 27.2 (6.1) | 0.6830 |
| MNA score a | 24.9 (3.5) | 25.1 (3.1) | 24.7 (3.8) | 0.8715 |
| MNA status b | ||||
| Malnutrition | 6 (3.8) | 1 (1.4) | 5 (5.6) | 0.3402 |
| Risk of malnutrition | 44 (27.5) | 20 (28.2) | 24 (27.0) | |
| Normal nutritional status | 110 (68.8) | 50 (70.4) | 60 (67.4) | |
| Katz (ADL) score a | 5.8 (0.4) | 5.8 (0.5) | 5.7 (0.4) | 0.0215 |
| Katz (ADL), status b | ||||
| Independent | 158 (98.8) | 70 (98.6) | 88 (98.9) | 0.5789 |
| Partially dependent | 2 (1.3) | 1 (1.4) | 1 (1.1) | |
| Dependent | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Lawton (IADL) score a | 25.5 (2.5) | 25.3 (2.5) | 25.7 (2.4) | 0.2451 |
| AMTS score a | 9.4 (0.6) | 9.4 (0.7) | 9.3 (0.6) | 0.0578 |
| Number of regular drugs a | 5.9 (3.9) | 6.3 (4.0) | 5.6 (3.9) | 0.2651 |
| Number of chronic diseases a,& | 3.3 (1.8) | 2.8 (1.3) | 3.7 (2.0) | 0.0007 |
| Handgrip strength a | 25.2 (9.7) | 32.7 (9.1) | 19.2 (4.9) | 0.0000 |
| Gait speed a | 1.0 (0.3) | 1.0 (0.3) | 0.9 (0.3) | 0.0886 |
| Chair stand test (s) a,* | 12.8 (4.6) | 12.9 (4.8) | 12.8 (4.5) | 0.9769 |
| ALM (kg) a | 19.3 (5.0) | 23.5 (3.6) | 15.8 (2.9) | 0.0000 |
| ALM index (kg/m2) a | 7.1 (1.2) | 7.9 (1.0) | 6.4 (1.0) | 0.0000 |
| Calf circumference a | 35.7 (3.8) | 36.2 (3.4) | 35.3 (4.0) | 0.1043 |
| PL-MSRA-7 score a | 28.4 (7.0) | 28.6 (7.1) | 28.3 (6.9) | 0.6960 |
| PL-MSRA-5 score a | 43.4 (11.9) | 43.7 (12.6) | 43.2 (11.4) | 0.6211 |
| SARC-F score a | 1.8 (2.0) | 1.4 (1.9) | 2.1 (2.0) | 0.0107 |
| SARC-CalF score a | 4.3 (5.2) | 3.8 (5.4) | 4.6 (5.0) | 0.0333 |
| MSRA-7 Items | Total (n = 160) | Men (n = 71) | Women (n = 89) | p | |
|---|---|---|---|---|---|
| Q1.Age | |||||
| ≥70 yrs | 99 (61.9) | 40 (56.3) | 59 (66.3) | 0.1978 | |
| <70 yrs | 61 (38.1) | 31 (43.7) | 30 (33.7) | ||
| Q2. Number of hospital treatment in the last year | |||||
| Yes, more than once | 24 (15.0) | 11 (15.5) | 13 (14.6) | 0.5764 | |
| Yes, once | 37 (23.1) | 19 (26.8) | 18 (20.2) | ||
| No | 99 (61.9) | 41 (57.7) | 58 (65.2) | ||
| Q3. Level of physical activity | |||||
| Able to walk less than 1000 m | 36 (22.5) | 18 (25.4) | 18 (20.2) | 0.4403 | |
| Able to walk more than 1000 m | 124 (77.5) | 53 (74.6) | 71 (79.8) | ||
| Q4. Regular consumption three meals a day | |||||
| No, up to twice a week, I skip a meal | 25 (15.6) | 8 (11.3) | 17 (19.1) | 0.1752 | |
| Yes | 135 (84.4) | 63 (88.7) | 72 (80.9) | ||
| Q5. Consumption of dairy products | |||||
| Yes, but not every day | 38 (23.8) | 20 (28.2) | 18 (20.2) | 0.2407 | |
| Yes, at least once a day | 122 (76.3) | 51 (71.8) | 71 (79.8) | ||
| Q6. Consumption of proteins | |||||
| Yes, but not every day | 35 (21.9) | 14 (19.7) | 21 (23.6) | 0.5556 | |
| Yes, at least once a day | 125 (78.1) | 57 (80.3) | 68 (76.4) | ||
| Q7. Weight loss in the last year | |||||
| >2 kg | 54 (33.8) | 21 (29.6) | 33 (37.1) | 0.3188 | |
| no or ≤2 kg | 106 (66.3) | 50 (70.4) | 56 (62.9) |
| PL-MSRA-5 | PL-MSRA-7 | |||||
|---|---|---|---|---|---|---|
| Sarcopenia (n = 95) | No Sarcopenia (n = 65) | p | Sarcopenia (n = 111) | No Sarcopenia (n = 49) | p | |
| EWGSOP1 | ||||||
| Sarcopenia | 28 (29.5) | 5 (7.7) | 0.0008 | 28 (25.2) | 5 (10.2) | 0.0304 |
| No sarcopenia | 67 (70.5) | 60 (92.3) | 83 (74.8) | 44 (89.8) | ||
| EWGSOP2 | ||||||
| Sarcopenia | 17 (17.9) | 1 (1.5) | 0.0013 | 16 (14.4) | 2 (4.1) | 0.0566 |
| No sarcopenia | 78 (82.1) | 64 (98.5) | 95 (85.6) | 47 (95.9) | ||
| FNIH | ||||||
| Sarcopenia | 17 (17.9) | 2 (3.1) | 0.0044 | 17 (15.3) | 2 (4.1) | 0.0429 |
| No sarcopenia | 78 (82.1) | 63 (96.9) | 94 (84.7) | 47 (95.9) | ||
| AWGS | ||||||
| Sarcopenia | 22 (23.2) | 1 (1.5) | 0.0001 | 21 (18.9) | 2 (4.1) | 0.0137 |
| No sarcopenia | 73 (76.8) | 64 (98.5) | 90 (81.1) | 47 (95.9) | ||
| IWGS | ||||||
| Sarcopenia | 20 (21.1) | 2 (3.1) | 0.0012 | 20 (18.0) | 2 (4.1) | 0.0183 |
| No sarcopenia | 75 (78.9) | 63 (96.9) | 91 (82.0) | 47(95.9) | ||
| SCWD | ||||||
| Sarcopenia | 22 (23.2) | 2 (3.1) | 0.0005 | 22 (19.8) | 2 (4.1) | 0.0102 |
| No sarcopenia | 73 (76.8) | 63 (96.9) | 89 (80.2) | 47 (95.9) | ||
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy | AUC | |
|---|---|---|---|---|---|---|
| EWGSOP1 | ||||||
| PL-MSRA-5 | 84.9 (68.1–94.9) | 47.2 (38.3–56.3) | 29.5 (25.1–34.2) | 92.3 (84.0–96.5) | 55.0 (47.0–62.9) | 0.711 (0.614–0.807) * |
| PL-MSRA-7 | 84.9 (68.1–94.9) | 34.7 (26.4–43.6) | 25.2 (21.8–29.0) | 89.8 (79.1–95.3) | 45.0 (37.1–53.1) | 0.649 (0.543–0.755) |
| EWGSOP2 | ||||||
| PL-MSRA-5 | 94.4 (72.7–99.9) | 45.1 (36.7–53.6) | 17.9 (15.3–20.8) | 98.5 (90.4–99.8) | 50.6 (42.6–58.6) | 0.739 (0.643–0.836)* |
| PL-MSRA-7 | 88.9 (65.3–98.6) | 33.1 (25.4–41.5) | 14.4 (12.1–17.1) | 95.9 (86.2–98.9) | 39.4 (31.8–47.4) | 0.655 (0.527–0.783) |
| FNIH | ||||||
| PL-MSRA-5 | 89.5 (66.9–98.7) | 44.7 (36.3–53.3) | 17.9 (15.0–21.3) | 96.9 (89.3–99.2) | 50.0 (42.0–58.0) | 0.717 (0.614–0.820)* |
| PL-MSRA-7 | 89.5 (66.9–98.7) | 33.3 (25.6–41.8) | 15.3 (13.0–18.0) | 95.9 (86.1–98.9) | 40.0 (32.4–48.0) | 0.596 (0.486–0.707) |
| AWGS | ||||||
| PL-MSRA-5 | 95.7 (78.1–99.9) | 46.7 (38.2–55.4) | 23.2 (20.1–26.5) | 98.5 (90.3–99.8) | 53.8 (45.7–61.7) | 0.759 (0.674–0.845) * |
| PL-MSRA-7 | 91.3 (72.0–98.9) | 34.3 (26.4–42.9) | 18.9 (16.4–21.8) | 95.9 (86.0–98.9) | 42.5 (34.7–50.6) | 0.685 (0.575–0.795) |
| IWGS | ||||||
| PL-MSRA-5 | 90.9 (70.8–98.9) | 45.7 (37.2–54.3) | 21.1 (17.9–24.6) | 96.9 (89.3–99.2) | 51.9 (43.9–59.8) | 0.747 (0.654–0.839) * |
| PL-MSRA-7 | 90.9 (70.8–98.9) | 34.1 (26.2–42.6) | 18.0 (15.5–20.8) | 95.9 (86.0–98.9) | 41.9 (34.1–49.9) | 0.684 (0.574–0.795) |
| SCWD | ||||||
| PL-MSRA-5 | 91.7 (73.0–99.0) | 46.3 (37.7–55.1) | 23.2 (19.8–26.9) | 96.9 (89.2–99.2) | 53.1 (45.1–61.1) | 0.735 (0.645–0.824) * |
| PL-MSRA-7 | 91.7 (73.0–99.0) | 34.6 (26.6–43.2) | 19.8 (17.2–22.7) | 95.9 (85.9–98.9) | 43.1 (35.3–51.2) | 0.667(0.560–0.774) |
| MSRA Q1 | MSRA Q2 | MSRA Q3 | MSRA Q4 | MSRA Q5 | MSRA Q6 | MSRA Q7 | MSRA-7 Total Score | MSRA-5 Total Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | p | C | p | C | p | C | p | C | p | C | p | C | p | C | p | C | p | |
| Age | −0.837 | 0.0000 | 0.077 | 0.3337 | −0.101 | 0.2021 | 0.037 | 0.6412 | 0.119 | 0.1353 | −0.163 | 0.0398 | 0.078 | 0.3247 | −0.241 | 0.0021 | −0.159 | 0.0451 |
| HGS | 0.214 | 0.0066 | −0.055 | 0.4914 | 0.185 | 0.0194 | 0.019 | 0.8078 | −0.048 | 0.5492 | 0.112 | 0.1575 | 0.076 | 0.3387 | 0.157 | 0.0479 | 0.176 | 0.0264 |
| CST | −0.143 | 0.0765 | −0.114 | 0.1579 | −0.361 | 0.0000 | 0.124 | 0.1268 | 0.060 | 0.4607 | 0.148 | 0.0662 | −0.161 | 0.0464 | −0.157 | 0.0526 | −0.264 | 0.0009 |
| USG | 0.146 | 0.0651 | 0.147 | 0.0635 | 0.376 | 0.0000 | −0.071 | 0.3710 | −0.036 | 0.6519 | 0.108 | 0.1739 | 0.184 | 0.0200 | 0.303 | 0.0001 | 0.331 | 0.0000 |
| ALM/BMI | 0.153 | 0.0527 | −0.205 | 0.0093 | 0.038 | 0.6370 | 0.086 | 0.2822 | −0.038 | 0.6376 | 0.131 | 0.0985 | 0.031 | 0.6980 | 0.049 | 0.5414 | 0.070 | 0.3781 |
| ALM | 0.129 | 0.1033 | −0.072 | 0.3688 | 0.065 | 0.4167 | 0.110 | 0.1664 | −0.125 | 0.1158 | 0.103 | 0.1959 | 0.222 | 0.0047 | 0.166 | 0.0361 | 0.206 | 0.0088 |
| ALM index | 0.104 | 0.1919 | 0.039 | 0.6265 | 0.074 | 0.3531 | 0.097 | 0.2238 | −0.127 | 0.1085 | 0.088 | 0.2682 | 0.275 | 0.0004 | 0.211 | 0.0075 | 0.239 | 0.0023 |
| BMI | −0.026 | 0.7455 | 0.179 | 0.0238 | 0.012 | 0.8756 | 0.024 | 0.7664 | −0.059 | 0.4575 | −0.018 | 0.8228 | 0.229 | 0.0035 | 0.158 | 0.0465 | 0.148 | 0.0621 |
| SARC-F | −0.101 | 0.2038 | −0.197 | 0.0127 | −0.379 | 0.0000 | 0.014 | 0.8569 | 0.103 | 0.1965 | 0.061 | 0.4455 | −0.027 | 0.7327 | −0.208 | 0.0085 | −0.297 | 0.0001 |
| SARC-CalF | −0.078 | 0.3263 | −0.242 | 0.0020 | −0.390 | 0.0000 | 0.011 | 0.8885 | 0.112 | 0.1569 | 0.036 | 0.6490 | −0.197 | 0.0126 | −0.310 | 0.0001 | −0.377 | 0.0000 |
| ADL | 0.237 | 0.0026 | 0.038 | 0.6339 | 0.211 | 0.0075 | −0.090 | 0.2593 | −0.092 | 0.2469 | 0.032 | 0.6845 | −0.109 | 0.1683 | 0.063 | 0.4291 | 0.095 | 0.2308 |
| IADL | 0.160 | 0.0429 | 0.140 | 0.0776 | 0.415 | 0.0000 | −0.116 | 0.1457 | 0.058 | 0.4659 | 0.024 | 0.7680 | 0.007 | 0.9267 | 0.251 | 0.0013 | 0.250 | 0.0014 |
| MNA | −0.068 | 0.3936 | 0.327 | 0.0000 | 0.364 | 0.0000 | 0.236 | 0.0027 | 0.053 | 0.5028 | −0.038 | 0.6374 | 0.296 | 0.0001 | 0.433 | 0.0000 | 0.526 | 0.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzymińska-Siemaszko, R.; Deskur-Śmielecka, E.; Styszyński, A.; Wieczorowska-Tobis, K. Polish Translation and Validation of the Mini Sarcopenia Risk Assessment (MSRA) Questionnaire to Assess Nutritional and Non-Nutritional Risk Factors of Sarcopenia in Older Adults. Nutrients 2021, 13, 1061. https://doi.org/10.3390/nu13041061
Krzymińska-Siemaszko R, Deskur-Śmielecka E, Styszyński A, Wieczorowska-Tobis K. Polish Translation and Validation of the Mini Sarcopenia Risk Assessment (MSRA) Questionnaire to Assess Nutritional and Non-Nutritional Risk Factors of Sarcopenia in Older Adults. Nutrients. 2021; 13(4):1061. https://doi.org/10.3390/nu13041061
Chicago/Turabian StyleKrzymińska-Siemaszko, Roma, Ewa Deskur-Śmielecka, Arkadiusz Styszyński, and Katarzyna Wieczorowska-Tobis. 2021. "Polish Translation and Validation of the Mini Sarcopenia Risk Assessment (MSRA) Questionnaire to Assess Nutritional and Non-Nutritional Risk Factors of Sarcopenia in Older Adults" Nutrients 13, no. 4: 1061. https://doi.org/10.3390/nu13041061
APA StyleKrzymińska-Siemaszko, R., Deskur-Śmielecka, E., Styszyński, A., & Wieczorowska-Tobis, K. (2021). Polish Translation and Validation of the Mini Sarcopenia Risk Assessment (MSRA) Questionnaire to Assess Nutritional and Non-Nutritional Risk Factors of Sarcopenia in Older Adults. Nutrients, 13(4), 1061. https://doi.org/10.3390/nu13041061






