The Role of Nutrition in the COVID-19 Pandemic
Abstract
:1. Introduction
2. Methodology
3. Malnutrition in the COVID-19 Pandemic
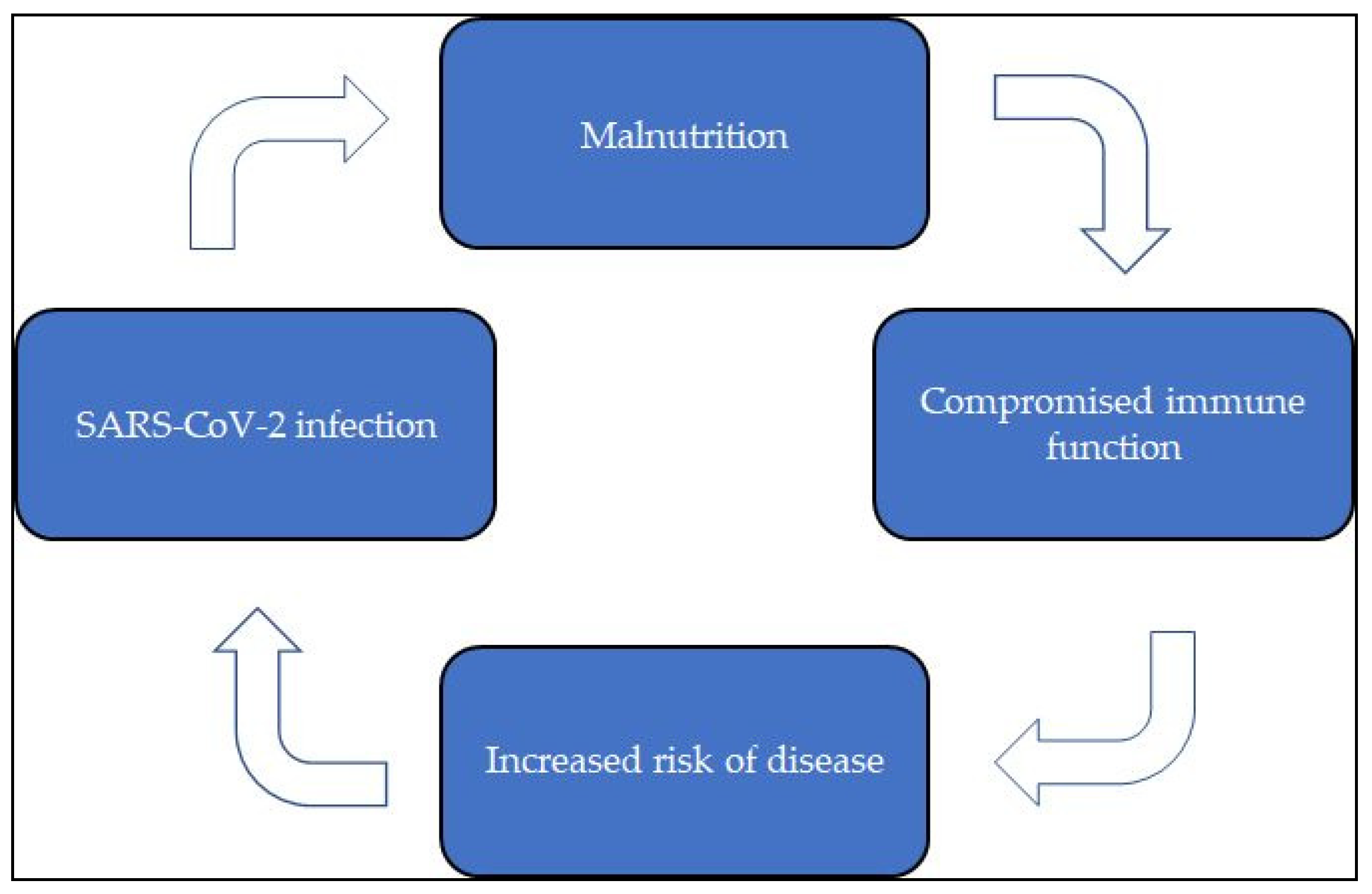
Malnutrition, COVID-19 Infection, and the Immune System
4. COVID-19 and Obesity
5. COVID-19 and Undernutrition
- Breathing difficulties that might limit what patients can eat or drink, such as cough, and shortness of breath; air trapping or early satiety, caused by gulping for air while swallowing and dry mouth due to the impaired nasal breathing, use of inhalers and oxygen therapy;
- Smell or taste loss which can decrease appetite and desire to eat food;
- Increased body temperature which boosts nutritional needs and inflammatory response, reduces appetite, and contributes to muscle loss;
- Feeling of tiredness, which impairs patient’s ability to carry out normal daily activities.
COVID-19, Undernutrition, and Older Adults
- Nutritional status of COVID-19-affected individuals needs to be carefully evaluated;
- Nutritional evaluation performed according to the GLIM criteria needs to be adjusted to the current pandemic scenario;
- To avoid overfeeding, one should consider using indirect calorimetry (IC) only for patients who are unstable and in the ICU for > 10 days, or those on complete parenteral nutrition (PN);
- Propofol administration may cause complications that must be avoided; refeeding syndrome (RS) must be avoided;
- PN should be not preferred to enteral nutrition (EN), and this should begin within two days from admission;
- Generally, gastric EN is feasible. It can also be performed with the patient in a prone position. Pumps with flow regulation should be preferred;
- PN should be considered if EN is either not possible, not indicated or no sufficient, and only after individual case assessment;
- Omega-3 fatty acid-enriched EN is the choice of preference when acute respiratory distress syndrome is present. If PN is necessary, prescription of intravenous fat emulsions enriched with fish oils is advised;
- Nutrition therapy should be maintained as long as necessary to allow the patient regaining sufficient oral intake after extubation;
- Muscle reserves and functionality should be preserved by promoting mobilization.
6. The Benefits of Supplementation in the COVID-19 Pandemic
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- El-Aziz, T.M.A.; Stockand, J.D. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2)—An update on the status. Infect. Genet. Evol. 2020, 83, 104327. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Ji, P.; Pang, J.; Zhong, Z.; Li, H.; He, C.; Zhang, J.; Zhao, C. Clinical characteristics of 3062 COVID-19 patients: A meta-analysis. J. Med. Virol. 2020, 92, 1902–1914. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, E.; Wright, E.; Kushner, B. In Young Adults with COVID-19, Obesity Is Associated with Adverse Outcomes. West. J. Emerg. Med. 2020, 21, 752–755. [Google Scholar] [CrossRef]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef]
- Louie, J.K.; Acosta, M.; Samuel, M.C.; Schechter, R.; Vugia, D.J.; Harriman, K. A Novel Risk Factor for a Novel Virus: Obesity and 2009 Pandemic Influenza A (H1N1). Clin. Infect. Dis. 2011, 52, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Hagau, N.; Slavcovici, A.; Gonganau, D.N.; Oltean, S.; Dirzu, D.S.; Brezoszki, E.S.; Maxim, M.; Ciuce, C.; Mlesnite, M.; Gavrus, R.L.; et al. Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit. Care 2010, 14, R203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Ding, L.; Zou, X.; Shen, Y.; Hu, D.; Hu, X.; Li, Z.; Kamel, I.R. Visceral Adiposity and High Intramuscular Fat Deposition Independently Predict Critical Illness in Patients with SARS-CoV-2. Obesity 2020, 28, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Caruso, D.; Tuccinardi, D.; Risi, R.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Tarallo, M.; Strigari, L.; Manfrini, S.; et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism 2020, 111, 154319. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Y.; Gong, C.; Wang, J.; Liu, B.; Shi, L.; Duan, J. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur. J. Clin. Nutr. 2020, 74, 871–875. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, E.; Ferguson, M.; Banks, M.; Batterham, M.; Bauer, J.; Capra, S.; Isenring, E. Malnutrition and poor food intake are associated with prolonged hospital stay, frequent readmissions, and greater in-hospital mortality: Results from the Nutrition Care Day Survey 2010. Clin. Nutr. 2013, 32, 737–745. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Deng, H.; Wang, Y.; Chen, L.; Gu, X.; Wang, X. Predictive value of the prognostic nutritional index for the severity of coronavirus disease 2019. Nutrition 2021, 84, 111123. [Google Scholar] [CrossRef] [PubMed]
- Anker, M.S.; Landmesser, U.; von Haehling, S.; Butler, J.; Coats, A.J.; Anker, S.D. Weight loss, malnutrition, and cachexia in COVID-19: Facts and numbers. J. Cachex-Sarcopenia Muscle 2021, 12, 9–13. [Google Scholar] [CrossRef]
- Bedock, D.; Lassen, P.B.; Mathian, A.; Moreau, P.; Couffignal, J.; Ciangura, C.; Poitou-Bernert, C.; Jeannin, A.-C.; Mosbah, H.; Fadlallah, J.; et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin. Nutr. ESPEN 2020, 40, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Rouget, A.; Vardon-Bounes, F.; Lorber, P.; Vavasseur, A.; Marion, O.; Marcheix, B.; Lairez, O.; Balardy, L.; Fourcade, O.; Conil, J.-M.; et al. Prevalence of malnutrition in coronavirus disease 19: The NUTRICOV study. Br. J. Nutr. 2020, 1–8. [Google Scholar] [CrossRef]
- Im, J.H.; Je, Y.S.; Baek, J.; Chung, M.-H.; Kwon, H.Y.; Lee, J.-S. Nutritional status of patients with COVID-19. Int. J. Infect. Dis. 2020, 100, 390–393. [Google Scholar] [CrossRef]
- Du, X.; Liu, Y.; Chen, J.; Peng, L.; Jin, Y.; Cheng, Z.; Wang, H.H.X.; Luo, M.; Chen, L.; Zhao, Y. Comparison of the Clinical Implications among Two Different Nutritional Indices in Hospitalized Patients with COVID-19. medRxiv 2000. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Recinella, G.; Marasco, G.; Serafini, G.; Maestri, L.; Bianchi, G.; Forti, P.; Zoli, M. Prognostic role of nutritional status in elderly patients hospitalized for COVID-19: A monocentric study. Aging Clin. Exp. Res. 2020, 32, 2695–2701. [Google Scholar] [CrossRef]
- Pironi, L.; Sasdelli, A.S.; Ravaioli, F.; Baracco, B.; Battaiola, C.; Bocedi, G.; Brodosi, L.; Leoni, L.; Mari, G.A.; Musio, A. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin. Nutr. 2021, 40, 1330–1337. [Google Scholar] [CrossRef]
- Haraj, N.E.; El Aziz, S.; Chadli, A.; Dafir, A.; Mjabber, A.; Aissaoui, O.; Barrou, L.; Hamidi, C.E.K.E.; Nsiri, A.; AL Harrar, R.; et al. Nutritional status assessment in patients with Covid-19 after discharge from the intensive care unit. Clin. Nutr. ESPEN 2021, 41, 423–428. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, S.; Mao, Z.; Wang, W.; Hu, H. Clinical significance of nutritional risk screening for older adult patients with COVID-19. Eur. J. Clin. Nutr. 2020, 74, 876–883. [Google Scholar] [CrossRef]
- Lin, J.-S.; Chiu, Y.-H.; Lin, N.-T.; Chu, C.-H.; Huang, K.-C.; Liao, K.-W.; Peng, K.-C. Different effects of probiotic species/strains on infections in preschool children: A double-blind, randomized, controlled study. Vaccine 2009, 27, 1073–1079. [Google Scholar] [CrossRef]
- Berggren, A.; Ahrén, I.L.; Larsson, N.; Önning, G. Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections. Eur. J. Nutr. 2010, 50, 203–210. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.; Yeoh, Y.K.; Li, A.Y.; Zhan, H.; Wan, Y.; Chung, A.C.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef] [PubMed]
- Miryan, M.; Bagherniya, M.; Sahebkar, A.; Soleimani, D.; Rouhani, M.H.; Iraj, B.; Askari, G. Effects of curcumin-piperine co-supplementation on clinical signs, duration, severity, and inflammatory factors in patients with COVID-19: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 1–2. [Google Scholar] [CrossRef]
- Miryan, M.; Soleimani, D.; Dehghani, L.; Sohrabi, K.; Khorvash, F.; Bagherniya, M.; Sayedi, S.M.; Askari, G. The effect of propolis supplementation on clinical symptoms in patients with coronavirus (COVID-19): A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 1–2. [Google Scholar] [CrossRef]
- Short, K.R.; Kedzierska, K.; Van De Sandt, C.E. Back to the Future: Lessons Learned From the 1918 Influenza Pandemic. Front. Cell. Infect. Microbiol. 2018, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Bold, J.; Harris, M.; Fellows, L.; Chouchane, M. Nutrition, the digestive system and immunity in COVID-19 infection. Gastroenterol. Hepatol. Bed Bench 2020, 13, 331–340. [Google Scholar] [PubMed]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef]
- Bhaskaram, P. Immunobiology of mild micronutrient deficiencies. Br. J. Nutr. 2001, 85, S75–S80. [Google Scholar] [CrossRef] [Green Version]
- Galmés, S.; Serra, F.; Palou, A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients 2020, 12, 2738. [Google Scholar] [CrossRef] [PubMed]
- Fedele, D.; De Francesco, A.; Riso, S.; Collo, A. Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: An overview. Nutrition 2021, 81, 111016. [Google Scholar] [CrossRef] [PubMed]
- Velly, H.; Britton, R.A.; Preidis, G.A. Mechanisms of cross-talk between the diet, the intestinal microbiome, and the undernourished host. Gut Microbes 2016, 8, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Silverio, R.; Gonçalves, D.C.; Andrade, M.F.; Seelaender, M. Coronavirus Disease 2019 (COVID-19) and Nutritional Status: The Missing Link? Adv. Nutr. 2020. [Google Scholar] [CrossRef]
- Bourke, C.D.; Berkley, J.A.; Prendergast, A.J. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol. 2016, 37, 386–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattar, N.; McInnes, I.B.; McMurray, J.J. Obesity Is a Risk Factor for Severe COVID-19 Infection. Circulation 2020, 142, 4–6. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin. Nutr. 2020, 39, 1631–1638. [Google Scholar] [CrossRef]
- Holdoway, A. Nutritional management of patients during and after COVID-19 illness. Br. J. Community Nurs. 2020, 25, S6–S10. [Google Scholar] [CrossRef]
- Hägg, S.; Jylhävä, J.; Wang, Y.; Xu, H.; Metzner, C.; Annetorp, M.; Garcia-Ptacek, S.; Khedri, M.; Boström, A.-M.; Kadir, A.; et al. Age, Frailty, and Comorbidity as Prognostic Factors for Short-Term Outcomes in Patients with Coronavirus Disease 2019 in Geriatric Care. J. Am. Med. Dir. Assoc. 2020, 21, 1555–1559. [Google Scholar] [CrossRef]
- Rolland, Y.; Benetos, A.; Villars, H.; Braun, H.; Blain, H. A COVID-19 Support Platform for Long Term Care Facilities. J. Nutr. Health Aging 2020, 24, 461–462. [Google Scholar] [CrossRef] [Green Version]
- Krznarić, Ž.; Bender, D.V.; Laviano, A.; Cuerda, C.; Landi, F.; Monteiro, R.; Pirlich, M.; Barazzoni, R. A simple remote nutritional screening tool and practical guidance for nutritional care in primary practice during the COVID-19 pandemic. Clin. Nutr. 2020, 39, 1983–1987. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, R.; Laviano, A.; Lobascio, F.; Montagna, E.; Bruno, R.; Ludovisi, S.; Corsico, A.G.; Di Sabatino, A.; Belliato, M.; Calvi, M.; et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): Rationale and feasibility of a shared pragmatic protocol. Nutrition 2020, 74, 110835. [Google Scholar] [CrossRef]
- Thibault, R.; Seguin, P.; Tamion, F.; Pichard, C.; Singer, P. Nutrition of the COVID-19 patient in the intensive care unit (ICU): A practical guidance. Crit. Care 2020, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; De Rosa, F.; Vitiello, A. The Central Role of Clinical Nutrition in COVID-19 Patients During and After Hospitalization in Intensive Care Unit. SN Compr. Clin. Med. 2020, 2, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Romano, L.; Bilotta, F.; Dauri, M.; Macheda, S.; Pujia, A.; De Santis, G.L.; Tarsitano, M.G.; Merra, G.; Di Renzo, L.; Esposito, E.; et al. Short Report - Medical nutrition therapy for critically ill patients with COVID-19. Eur Rev Med Pharmacol Sci 2020, 24, 4035–4039. [Google Scholar] [PubMed]
- Ivanov, I.I.; Frutos, R.D.L.; Manel, N.; Yoshinaga, K.; Rifkin, D.B.; Sartor, R.B.; Finlay, B.B.; Littman, D.R. Specific Microbiota Direct the Differentiation of IL-17-Producing T-Helper Cells in the Mucosa of the Small Intestine. Cell Host Microbe 2008, 4, 337–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, F.; Polk, D. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011, 27, 496–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baindara, P.; Chakraborty, R.; Holliday, Z.; Mandal, S.; Schrum, A. Oral probiotics in coronavirus disease 2019: Connecting the gut–lung axis to viral pathogenesis, inflammation, secondary infection and clinical trials. New Microbes New Infect. 2021, 40, 100837. [Google Scholar] [CrossRef] [PubMed]
- Pecora, F.; Persico, F.; Argentiero, A.; Neglia, C.; Esposito, S. The Role of Micronutrients in Support of the Immune Response against Viral Infections. Nutrients 2020, 12, 3198. [Google Scholar] [CrossRef] [PubMed]
- Vivar-Sierra, A.; Araiza-Macías, M.J.; Hernández-Contreras, J.P.; Vergara-Castañeda, A.; Ramírez-Vélez, G.; Pinto-Almazán, R.; Salazar, J.R.; Loza-Mejía, M.A. In Silico Study of Polyunsaturated Fatty Acids as Potential SARS-CoV-2 Spike Protein Closed Conformation Stabilizers: Epidemiological and Computational Approaches. Molecules 2021, 26, 711. [Google Scholar] [CrossRef]
- Hathaway, D.; Pandav, K.; Patel, M.; Riva-Moscoso, A.; Singh, B.M.; Patel, A.; Min, Z.C.; Singh-Makkar, S.; Sana, M.K.; Sanchez-Dopazo, R.; et al. Omega 3 Fatty Acids and COVID-19: A Comprehensive Review. Infect. Chemother. 2020, 52, 478–495. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
| Publication | Country | Temporary Frame (Beginning of the Pandemic, Middle, Second Wave) | Type of Study | Number of Centers | Number of Patients | Type of Patients (Asymptomatic, Infected, Hospitalized, Admitted in Intensive Care Unit) | Exposure of Interest (BMI/Energy Shortage, Micronutrients Deficiency) | Key Findings of the Study | Study Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Chen et al., Lancet, 2020 [3] | China | Beginning of the pandemic | Retrospective study | Monocentric | 99 | Infected | NA | COVID-19 patients present clinical manifestations of fever, cough, shortness of breath, muscle ache, confusion, headache, sore throat, rhinorrhea, chest pain, diarrhea, and nausea and vomiting | The infection of 2019-nCoV was of clustering onset, is more likely to infect older men with comorbidities, and can result in severe and even fatal respiratory |
| Steinberg et al., BRR, 2020 [5] | U.S.A. | Beginning of the pandemic | Retrospective cohort study | 2 | 210 | Infected | Obesity (BMI > 30 kg/m2) | Association between primary outcomes (in-hospital mortality, need for IMV, and admission to hospital) and obesity | Obesity appears to be an independent risk factor for poor outcomes in young patients with COVID-19 |
| Simmonet et al., Obesity, 2020 [6] | France | Beginning of the pandemic | Retrospective cohort study | Monocentric | 124 | Admitted to ICU | Obesity and severe obesity (BMI > 35 kg/m2) | The proportion of patients who required IMV increased with BMI categories | Obesity is considered a risk factor for SARS-CoV-2 severity |
| Louie et al., CID, 2011 [7] | U.S.A. | NA | - | Monocentric | 534 | Hospitalized | Obesity (BMI ≥ 30 kg/m2) and extreme obesity (BMI ≥ 40 kg/m2) | BMI > 40 kg/m2 is an independent risk factor in hospitalized adults for death from 2009 H1N1 infection | Extreme obesity was associated with increased odds of death |
| Hagau et al., CC, 2010 [8] | Romania | NA | Prospective study | Monocentric | 32 | Hospitalized | Obesity | In obese patients with influenza disease, a significantly increased level of IL-8 was found | Ill patients with nvA (H1N1) virus infection have increased levels of some cytokines |
| Yang et al., Obesity, 2020 [9] | China | Beginning of the pandemic | Retrospective study | Monocentric | 143 | Hospitalized | - | Association between adipose tissue distribution and severity of clinical course | COVID-19 patients with visceral adiposity or high IMF deposition have higher risk for critical illness |
| Watanabe et al., Metabolism, 2020 [10] | Italy | Beginning of the pandemic | Retrospective study | Monocentric | 150 | Infected | Obesity | Visceral fat (VAT) was significantly higher in patients requiring intensive care | VAT is a marker of worse clinical outcomes in patients with COVID-19 |
| Li et al., EJCN, 2020 [11] | China | Beginning of the pandemic | Cross-sectional study | Monocentric | 182 | Hospitalized | Risk of malnutrition and malnutrition | The prevalence of malnutrition in elderly patients with COVID-19 was high | Malnutrition has been identified as a negative prognostic factor |
| Agarwal et al., CN, 2013 [12] | Australia | NA | Prospective cohort study | 56 | 3122 | Hospitalized | Malnutrition | Malnourished patients had greater median LOS, readmissions rates and in-hospital mortality | Malnutrition and poor food intake are independently associated with in-hospital mortality |
| Hu et al., Nutrition, 2020 [13] | China | Beginning of the pandemic | Retrospective trial | Monocentric | 122 | Hospitalized | Undernutrition | Association between the prognostic nutritional index (PNI) score and the severity of COVID-19. | Poorer nutritional status predisposed patients infected with COVID-19 to its severe form |
| Anker et al., JCSM, 2020 [14] | Italy, France | Middle | Retrospective studies | - | 589 | Hospitalized | Risk of malnutrition and malnutrition | Patients with COVID-19 disease are prone to develop significant weight loss and clinical cachexia | Many metabolic and nutritional factors can contribute to body wasting in COVID-19 |
| Bedock et al., CN, 2020 [15] | France | Beginning of the pandemic | Longitudinal study | Monocentric | 114 | Hospitalized | Moderate and severe malnutrition | Association between malnutrition and unfavorable outcomes (transfer to ICU or death) | Low albumin levels at admission are a predictive marker of more severe outcome of the disease |
| Rouget et al., BRJN, 2020 [16] | France | Beginning of the pandemic | Prospective observational cohort study | Monocentric | 80 | Hospitalized | Malnutrition | High prevalence of malnutrition in a general cohort of COVID-19 inpatients according to GLIM criteria | Nutritional support in COVID-19 care is an essential element |
| Im et al., IJID, 2020 [17] | South Korea | Middle | - | Monocentric | 50 | Hospitalized | Micronutrient deficiency | Vitamin D and selenium deficiencies were the most prevalent among COVID-19 patients | Deficiency of vitamin D or selenium may decrease the immune defenses against COVID-19 and cause progression to severe disease |
| Du et al., Medxiv, 2020 [18] | China | Beginning of the pandemic | Retrospective cohort study | Monocentric | 245 | Hospitalized | Malnutrition | In-hospital mortality was significantly higher in the severe group of PNI and in the severe-CONUT group | The CONUT score and PNI could be a reliable prognostic marker of all-cause death in patients with COVID-19. |
| Wu et al., JAMA, 2020 [19] | China | Beginning of the pandemic | Retrospective cohort study | Monocentric | 201 | Hospitalized | Malnutrition | The risk factors related to the development of ARDS and progression from ARDS to death included older age, neutrophilia, and organ and coagulation dysfunction | Older age was associated with greater risk of development of ARDS and death likely owing to less rigorous immune response |
| Recinella et al., ACER, 2020 [20] | Italy | Beginning of the pandemic | - | Monocentric | 109 | Hospitalized | Age > 65, malnutrition | Lower values of body weight, BMI, GNRI and albumin were found in patients experiencing in-hospital death. Higher values of GNRI were found in surviving patients | Nutritional status assessed by GNRI is a significative predictor of survival in elderly patients hospitalized for COVID-19 |
| Pironi et al., CN, 2020 [21] | Italy | Beginning of the pandemic | Cross-sectional study | Monocentric | 268 | Hospitalized | Age, malnutrition | Very high prevalence of nutritional risk and malnutrition in adult patients hospitalized for COVID-19 | The patient energy and protein intake were at the lowest limit or below the recommended amounts, indicating the need for actions to improve the nutritional care practice |
| Haraj et al., CN_ESPEN, 2020 [22] | Morocco | Middle | Descriptive observational study | Monocentric | 41 | Admitted in intensive care unit | Risk of malnutrition and malnutrition | Most COVID-19 patients were at risk of undernutrition | The nutritional diagnosis and the early nutritional management of COVID-19 patients must be integrated into the overall therapeutic strategy |
| Liu et al., EJCN, 2020 [23] | China | Beginning of the pandemic | Retrospective cohort study | Monocentric | 141 | Hospitalized | Age > 65, risk of malnutrition | The most of COVID-19 patients were at risk of malnutrition with longer LOS, higher hospital expense and worse disease severity | The NRS 2002, MNA-sf, and NRI are useful and practical tools with respect to screening for patients with COVID-19 who are at nutritional risk |
| Lin et al., Vaccine, 2009 [24] | Taiwan | NA | Double-blind, randomized, controlled study | 4 | 1062 | NA | Age < 5 | L. casei rhamnosus can control bacterial, viral and respiratory infections | Bio-therapeutic agents may be useful in preventing viral and bacterial infectious disease |
| Berggren et al., EJN, 2010 [25] | Sweden | NA | Randomized, parallel, double-blind placebo-controlled study | 2 | 272 | NA | - | Treatments with probiotics mixtures shorten the duration, reduce the incidence of infection and/or lessen the severity of symptoms | Intake of probiotic mixture contributes to the body’s defense against common cold infections |
| Zuo et al., Gastroenterology, 2020 [26] | China | Beginning of the pandemic | Prospective study | Monocentric | 36 | Hospitalized | Altered intestinal microbiota | Patients with COVID-19 had significant alterations in fecal microbiomes compared with controls (enrichment of opportunistic pathogens and depletion of beneficial commensals) | Fecal microbiota alterations were associated with fecal levels of SARS-CoV-2 and COVID-19 severity. Strategies to alter the intestinal microbiota might reduce disease severity |
| Miryan et al., Trial_pepper_curcumin, 2020 [27] | Iran | Middle-ongoing (ending April 2021) | Randomized, placebo-controlled, double-blind, parallel arm clinical trial | Monocentric | 100 | Hospitalized | NA | Curcumin–piperine could alleviate coronavirus disease’s clinical symptoms, duration, severity, and inflammatory mediators | NA |
| Miryan et al., Trail_propolis, 2020 [28] | Iran | Second wave-ongoing (ending March 2021) | Double-blind, Placebo-controlled, parallel arm, randomized phase II clinical trial | Monocentric | 80 | Hospitalized | NA | Propolis supplementation changes coronavirus disease’s clinical symptoms, duration, and severity | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mentella, M.C.; Scaldaferri, F.; Gasbarrini, A.; Miggiano, G.A.D. The Role of Nutrition in the COVID-19 Pandemic. Nutrients 2021, 13, 1093. https://doi.org/10.3390/nu13041093
Mentella MC, Scaldaferri F, Gasbarrini A, Miggiano GAD. The Role of Nutrition in the COVID-19 Pandemic. Nutrients. 2021; 13(4):1093. https://doi.org/10.3390/nu13041093
Chicago/Turabian StyleMentella, Maria Chiara, Franco Scaldaferri, Antonio Gasbarrini, and Giacinto Abele Donato Miggiano. 2021. "The Role of Nutrition in the COVID-19 Pandemic" Nutrients 13, no. 4: 1093. https://doi.org/10.3390/nu13041093
APA StyleMentella, M. C., Scaldaferri, F., Gasbarrini, A., & Miggiano, G. A. D. (2021). The Role of Nutrition in the COVID-19 Pandemic. Nutrients, 13(4), 1093. https://doi.org/10.3390/nu13041093






