Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency
Abstract
:1. Introduction
2. Biochemistry of Magnesium to Understand the Consequences of Its Deficiencies
- −
- The complex MgATP2- is required for the activity of many enzymes. In general, Mg2+ acts as a cofactor in all reactions involving the utilization and transfer of ATP, including cellular responses to growth factors and cell proliferation, being thus implicated in virtually every process in the cells. Mg2+ availability is a critical issue for carbohydrate metabolism, which may explain its role in diabetes mellitus type 2 [40];
- −
- Mg2+ is necessary for the correct structure and activity of DNA and RNA polymerases [41,42]. In addition, topoisomerases, helicases, exonucleases, and large groups of ATPases require Mg2+ for their activity, therefore Mg2+ is essential in DNA replication, RNA transcription, and protein formation, being thus involved in the control of cell proliferation. Moreover, Mg2+ is crucial to maintain genomic and genetic stability, stabilizing the natural DNA conformation and acting as a cofactor for almost every enzyme involved in nucleotide and base excision repair and mismatch repair. Given these effects, low Mg2+ availability can be involved in the development of cancer [2];
- −
- Serum Mg2+ concentrations are strongly related to bone metabolism; bone surface Mg2+ is constantly exchanged with blood Mg2+ [43,44]. Furthermore, Mg2+ induces osteoblast proliferation [45] therefore, the consequences of Mg2+ deficiency are accelerated bone loss and a decline in bone formation [46];
- −
- Mg2+ participates in controlling the activity of some ionic channels in many tissues. Its mechanism of action relies on either direct interaction with the channel, or an indirect modification of channel function through other proteins (e.g., enzymes or G proteins), or via membrane surface charges and phospholipids [47]. Furthermore, Mg2+ acts as a physiological Ca2+ antagonist within cells, since it can compete with Ca2+ for binding sites in proteins and Ca2+ transporters [48]. These abilities are involved in the observed effect of magnesium on the cardiovascular system, muscle, and brain;
- −
- Neuronal magnesium concentrations downregulate the excitability of the N-methyl-D-aspartate (NMDA) receptor, which is essential for excitatory synaptic transmission and neuronal plasticity in learning and memory [49]. Magnesium blocks the calcium channel in the NMDA receptor and must be removed for glutamatergic excitatory signaling. Low serum Mg2+ levels increase NMDA receptor activity thus enhancing Ca2+ and Na+ influx and neuronal excitability. For these reasons, a deficiency of Mg2+ has been hypothesized in many neurological disorders, such as migraine, chronic pain, epilepsy, Alzheimer’s, Parkinson’s, and stroke, as well as anxiety and depression [50].
2.1. Magnesium as Enzymatic Cofactor
2.2. Magnesium and Nucleic Acids
2.3. Magnesium and Bone Metabolism
2.4. Magnesium, Calcium, and Cardiovascular System
2.5. Magnesium, Calcium, and Brain
3. Nutritional Strategies to Avoid Magnesium Deficiencies
3.1. Recommended Intake and Categories of People That Risk Inadequate Magnesium Intake
- −
- The population reference intakes (PRIs), which refer to the level of nutrient intake that is adequate for the majority of people in a population group;
- −
- The average requirements (ARs), which refer to the intake level that is adequate to meet the physiological requirements of 50% of healthy individuals. This parameter is usually taken into consideration not only to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them but also to assess the nutrient intakes of individuals.
- −
- Adequate intake (AI), therefore, refers to the intake assumed to ensure nutritional adequacy;
- −
- Tolerable upper intake level (UL): Maximum daily intake which is considered to be safe/without adverse health effects on the totality of the considered population.
- −
- Older people absorb less magnesium from the gut and lose more magnesium because of an increased renal excretion. Chronic magnesium deficiency is indeed common in the elderly, usually due to a decrease both in diet assumption and intestinal absorption, and it is probably exacerbated by estrogen deficit, which occurs in aging women and men and cause hypermagnesuria [132]. In a very recent and comprehensive review [34], Lo Piano and colleagues highlight the risk and consequences of the reduce intake and absorption of magnesium by elderly people;
- −
- People affected by gastrointestinal diseases with consequent general malabsorption, such as Crohn’s disease [117,133,134,135,136,137,138,139,140], inflammatory bowel diseases [135,138,140,141], and celiac disease [142,143,144,145,146,147,148,149,150]. In particular, besides the absorption inefficiency due to celiac disease, a gluten free-diet was found to be poor in fiber and micronutrients, such as magnesium [151,152]. Therefore, people suffering from celiac disease are a typical example of subjects particularly susceptible to magnesium deficiency as they are simultaneously exposed to two risk factors;
- −
- −
- People who used to drink alcohol/alcoholics or are affected by long-term alcoholism [3,157,158,159,160] and are therefore affected by intestinal malabsorption. Spirits (such as brandy, cognac, gin, rum, vodka, and whisky) do not contain significant traces of magnesium. Moderate alcohol consumption, such as wine and beer during meals, is acceptable and is also included in the Mediterranean food pyramid (2–4 units/day), however, despite beer and wine having magnesium levels that range from 30–250 mg/L and fermented apple ciders ranging from 10–50 mg/L, such beverages cannot be considered as a reliable source of magnesium because they cause magnesiuresis and can have a laxative effect, with consequent problems on bioavailability and absorption. Ethanol is indeed magnesiuretic by causing proximal tubular dysfunction and increasing urinary magnesium loss, and its effect is rapid and common in people with an already negative magnesium balance [6,161];
- −
- People under treatment with drugs (e.g., diuretics, proton pump inhibitors, tacrolimus, an immunosuppressor, chemotherapeutic agents, and some phosphate-based drugs) [6].
3.2. Magnesium Food Content and Bioavailability
- −
- Phytates and oxalates present in foods rich in fiber can decrease the absorption of magnesium because of metal chelation. Nevertheless, the decrease of magnesium absorption caused by phytate and cellulose is usually compensated by an increased magnesium intake due to high magnesium concentrations in phytate- and cellulose-rich products [4,174,175,176,177];
- −
- Phosphorus: high luminal concentrations of phosphates can reduce magnesium absorption, mainly because of salt formation [178]. A major source of phosphorus is represented by soft drinks: the consumption of these beverages, typically rich in phosphoric acid, has been significantly rising in the last quarter of a century. The increase in dietary phosphate is also linked to phosphate additives, present in many food items but mainly processed meats [18]. Dairy and in particular cheese have a very high phosphorus/magnesium ratio. For example, cheddar cheese has a phosphorus/magnesium ratio of ~18 and a calcium/magnesium ratio of ~26.66. On the contrary, pumpkin seeds have a phosphorus/magnesium ratio of 0.35 and a calcium/magnesium ratio of 0.21 [18];
- −
- Very high calcium intakes can reduce the absorption of magnesium, in particular, magnesium bioavailability decreases when calcium intake is over 10 mg/kg/day [18]. Increasing evidence suggests that the optimal serum magnesium/calcium ratio is 0.4 and if it is in the range 0.36–0.28, it is considered too low. This ratio is a more practical and sensitive of magnesium status and/or turnover, than the serum magnesium level alone [12];
- −
- Dietary aluminum may contribute to a magnesium deficit by means of an approximately 5-fold reduction of its absorption, of 41% of its retention, and by causing a reduction of magnesium in the bone. Since aluminum is widespread in modern day society (such as in cookware, deodorants, over the counter and prescription drugs, powder, baked products, and others), this could represent an important contributor to magnesium deficiency [18];
- −
- Peptides from casein or whey could bind magnesium, which may promote absorption, analogously to other divalent cations [179]. A low protein intake (<30 g/die) could negatively influence the absorption of magnesium, however, other studies showed that magnesium use was not affected by the level of protein intake [180];
- −
- −
- −
- High doses of zinc can interfere with magnesium. Nielsen et al. reported that an intake of 53 mg zinc/day (4-fold higher than LARN) over 90 days can decrease magnesium balance [185];
- −
- As for beverages, magnesium levels are decreased by excess ethanol, soft drinks, and coffee intake [186];
- −
- Some drugs negatively affect the state of magnesium, in particular diuretics, insulin, and digitalis [23].
3.3. Nutritional and Health Claims for Magnesium
- −
- “Magnesium contributes to a reduction of tiredness and fatigue”;
- −
- “Magnesium contributes to electrolyte balance”;
- −
- “Magnesium contributes to normal energy-yielding metabolism”;
- −
- “Magnesium contributes to normal functioning of the nervous system”;
- −
- “Magnesium contributes to normal muscle function”;
- −
- “Magnesium contributes to normal protein synthesis”;
- −
- “Magnesium contributes to normal psychological function”;
- −
- “Magnesium contributes to the maintenance of normal bones”;
- −
- “Magnesium contributes to the maintenance of normal teeth”;
- −
- “Magnesium has a role in the process of cell division”.
3.4. Dietary Supplements of Magnesium
4. Methods to Evaluate Magnesium Status
4.1. Atomic Absorption Spectroscopy
4.2. Ion Selective Electrodes
4.3. Optical Sensors
4.3.1. Colorimetric or Enzymatic Assay
4.3.2. Fluorescent Chemosensors
4.4. Element Bioimaging
5. Magnesium Deficiency and High Social Impact Diseases
5.1. Diabetes Mellitus
5.2. Osteoporosis
5.3. Cardiovascular Diseases
5.4. Cancer
5.5. Neurological Diseases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romani, A.M. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011, 512, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Saris, N.E.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Intestinal Absorption and Factors Influencing Bioavailability of Magnesium-An Update. Curr. Nutr. Food Sci. 2017, 13, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Konrad, M.; Schlingmann, K.P.; Gudermann, T. Insights into the molecular nature of magnesium homeostasis. Am. J. Physiol. Physiol. 2004, 286, F599–F605. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.A.A.; Ismail, Y.; Ismail, A.A. Chronic magnesium deficiency and human disease; time for reappraisal? QJM 2018, 111, 759–763. [Google Scholar] [CrossRef]
- Elin, R.J. Assessment of magnesium status for diagnosis and therapy. Magnes. Res. 2010, 23, 194–198. [Google Scholar]
- Reddi, A.S.; Reddi, A.S. Disorders of Magnesium: Hypomagnesemia. In Fluid, Electrolyte and Acid-Base Disorders; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Witkowski, M.; Hubert, J.; Mazur, A. Methods of assessment of magnesium status in humans: A systematic review. Magnes. Res. 2011, 24, 163–180. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, F.H. Guidance for the determination of status indicators and dietary requirements for magnesium. Magnes. Res. 2016, 29, 154–160. [Google Scholar] [CrossRef]
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.; Song, Y.; et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv. Nutr. 2016, 7, 977–993. [Google Scholar] [CrossRef]
- Razzaque, M.S. Magnesium: Are we consuming enough? Nutrients 2018, 10, 1863. [Google Scholar] [CrossRef] [Green Version]
- Costello, R.; Wallace, T.; Rosanoff, A. Nutrient Information: Magnesium. Adv. Nutr. Int. Rev. J. 2016, 7, 199–201. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E. Magnesium deficiency and osteoporosis: Animal and human observations. J. Nutr. Biochem. 2004, 15, 710–716. [Google Scholar] [CrossRef]
- Whang, R. Frequency of hypomagnesemia and hypermagnesemia. Requested vs routine. JAMA 1990, 263, 3063–3064. [Google Scholar] [CrossRef]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef]
- Khalil, S.I. Magnesium the forgotten cation. Int. J. Cardiol. 1999, 68, 133–135. [Google Scholar]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.J.; González, E.A.; Slatopolsky, E. Clinical Consequences and Management of Hypomagnesemia. J. Am. Soc. Nephrol. 2008, 20, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Van Laecke, S. Hypomagnesemia and hypermagnesemia. Acta Clin. Belgica Int. J. Clin. Lab. Med. 2019, 74, 41–47. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [Green Version]
- Gröber, U. Magnesium and Drugs. Int. J. Mol. Sci. 2019, 20, 2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäffers, O.J.M.; Hoenderop, J.G.J.; Bindels, R.J.M.; De Baaij, J.H.F. The rise and fall of novel renal magnesium transporters. Am. J. Physiol. Physiol. 2018, 314, F1027–F1033. [Google Scholar] [CrossRef] [Green Version]
- Auwercx, J.; Rybarczyk, P.; Kischel, P.; Dhennin-Duthille, I.; Chatelain, D.; Sevestre, H.; Van Seuningen, I.; Ouadid-Ahidouch, H.; Jonckheere, N.; Gautier, M. Mg2+ transporters in digestive cancers. Nutrients 2021, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.-G.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. TRPM7, Magnesium, and Signaling. Int. J. Mol. Sci. 2019, 20, 1877. [Google Scholar] [CrossRef] [Green Version]
- Gile, J.; Ruan, G.; Abeykoon, J.; McMahon, M.M.; Witzig, T. Magnesium: The overlooked electrolyte in blood cancers? Blood Rev. 2020, 44, 100676. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jin, F.; Funato, Y.; Xu, Z.; Zhu, W.; Wang, J.; Sun, M.; Zhao, Y.; Yu, Y.; Miki, H.; et al. Structural basis for the Mg2+ recognition and regulation of the CorC Mg2+ transporter. Sci. Adv. 2021, 7, eabe6140. [Google Scholar] [CrossRef]
- Giménez-Mascarell, P.; Schirrmacher, C.E.; Martínez-Cruz, L.A.; Müller, D. Novel aspects of renal magnesium homeostasis. Front. Pediatr. 2018, 6, 77. [Google Scholar] [CrossRef]
- Blaine, J.; Chonchol, M.; Levi, M. Renal Control of Calcium, Phosphate, and Magnesium Homeostasis. Clin. J. Am. Soc. Nephrol. 2014, 10, 1257–1272. [Google Scholar] [CrossRef]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef] [Green Version]
- Dolati, S.; Rikhtegar, R.; Mehdizadeh, A.; Yousefi, M. The Role of Magnesium in Pathophysiology and Migraine Treatment. Biol. Trace Element Res. 2019, 196, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Quamme, G.A. Recent developments in intestinal magnesium absorption. Curr. Opin. Gastroenterol. 2008, 24, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Piano, F.L.; Corsonello, A.; Corica, F. Magnesium and elderly patient: The explored paths and the ones to be explored: A review. Magnes. Res. 2019, 32, 1–15. [Google Scholar]
- Sun, Y.; Sukumaran, P.; Singh, B.B. Magnesium-Induced Cell Survival Is Dependent on TRPM7 Expression and Function. Mol. Neurobiol. 2020, 57, 528–538. [Google Scholar] [CrossRef] [Green Version]
- Ebel, H.; Günther, T.; Günther, H.E.T. Magnesium Metabolism: A Review. Clin. Chem. Lab. Med. 1980, 18, 257–270. [Google Scholar] [CrossRef]
- Seo, J.W.; Park, T.J. Magnesium Metabolism. Electrolytes Blood Press. 2008, 6, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Bairoch, A. The ENZYME database in 2000. Nucleic Acids Res. 2000, 28, 304–305. [Google Scholar] [CrossRef] [Green Version]
- Caspi, R.; Altman, T.; Dreher, K.; Fulcher, C.A.; Subhraveti, P.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2012, 40, D742–D753. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Wang, H.; Jing, Z.; Wang, Y.; Cheng, Y.; Wang, W.; Sun, W. Role of Magnesium in Type 2 Diabetes Mellitus. Biol. Trace Element Res. 2020, 196, 74–85. [Google Scholar] [CrossRef]
- Brautigam, C.A.; Steitz, T.A. Structural and functional insights provided by crystal structures of DNA polymerases and their substrate complexes. Curr. Opin. Struct. Biol. 1998, 8, 54–63. [Google Scholar] [CrossRef]
- Chul Suh, W.; Leirmo, S.; Thomas Record, M. Roles of Mg2+ in the Mechanism of Formation and Dissociation of Open Complexes between Escherichia coli RNA Polymerase and the λPR Promoter: Kinetic Evidence for a Second Open Complex Requiring Mg2+. Biochemistry 1992, 31, 7815–7825. [Google Scholar]
- Alfrey, A.C.; Miller, N.L.; Trow, R. Effect of Age and Magnesium Depletion on Bone Magnesium Pools in Rats. J. Clin. Investig. 1974, 54, 1074–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.A.; Grande, A.; Frassineti, C. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3. Int. J. Mol. Sci. 2019, 20, 385. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.-C.; Pringa, E.; Chou, L. Effect of magnesium on the osteogenesis of normal human osteoblasts. Magnes. Res. 2017, 30, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Zofkova, I.; Davis, M.; Blahos, J. Trace Elements Have Beneficial, as Well as Detrimental Effects on Bone Homeostasis. Physiol. Res. 2017, 66, 391–402. [Google Scholar] [CrossRef]
- Mubagwa, K.; Gwanyanya, A.; Zakharov, S.; Macianskiene, R. Regulation of cation channels in cardiac and smooth muscle cells by intracellular magnesium. Arch. Biochem. Biophys. 2007, 458, 73–89. [Google Scholar] [CrossRef]
- Iseri, L.T.; French, J.H. Magnesium: Nature’s physiologic calcium blocker. Am. Heart J. 1984, 108, 188–193. [Google Scholar] [CrossRef]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef] [Green Version]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The MetaCyc database of metabolic pathways and enzymes—A 2019 update. Nucleic Acids Res. 2020, 48, D445–D453. [Google Scholar] [CrossRef] [Green Version]
- Sanders, G.T.; Huijgen, H.J.; Sanders, R. Magnesium in Disease: A Review with Special Emphasis on the Serum Ionized Magnesium. Clin. Chem. Lab. Med. 1999, 37, 1011–1033. [Google Scholar] [CrossRef]
- Garfinkel, L.; Garfinkel, D. Magnesium regulation of the glycolytic pathway and the enzymes involved. Magnesium 1985, 4, 60–72. [Google Scholar]
- Gomez Puyou, A.; Ayala, G.; Muller, U.; Tuena de Gomez Puyou, M. Regulation of the synthesis and hydrolysis of ATP by mitochondrial ATPase. Role of Mg2+. J. Biol. Chem. 1983, 258, 13680–13684. [Google Scholar] [CrossRef]
- Willson, V.J.C.; Tipton, K.F. The Activation of Ox-Brain NAD+-Dependent Isocitrate Dehydrogenase by Magnesium Ions. JBIC J. Biol. Inorg. Chem. 1981, 113, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Panov, A.; Scarpa, A. Independent Modulation of the Activity of α-Ketoglutarate Dehydrogenase Complex by Ca2+ and Mg2+. Biochemtry 1996, 35, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.P.; Diggle, T.A.; Denton, R.M. Sensitivity of pyruvate dehydrogenase phosphate phosphatase to magnesium ions. Similar effects of spermine and insulin. Biochem. J. 1986, 238, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Galkin, M.A.; Syroeshkin, A.V. Kinetic mechanism of ATP synthesis catalyzed by mitochondrial Fo x F1-ATPase. Biochemtry 1999, 64, 1176–1185. [Google Scholar]
- Barbiroli, B.; Iotti, S.; Cortelli, P.; Martinelli, P.; Lodi, R.; Carelli, V.; Montagna, P. Low Brain Intracellular Free Magnesium in Mitochondrial Cytopathies. Br. J. Pharmacol. 1999, 19, 528–532. [Google Scholar] [CrossRef]
- Shigematsu, M.; Nakagawa, R.; Tomonaga, S.; Funaba, M.; Matsui, T. Fluctuations in metabolite content in the liver of magnesium-deficient rats. Br. J. Nutr. 2016, 116, 1694–1699. [Google Scholar] [CrossRef] [Green Version]
- Mooren, F.C. Magnesium and disturbances in carbohydrate metabolism. Diabetes Obes. Metab. 2015, 17, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J. Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch. Biochem. Biophys. 2007, 458, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K. Effects of Magnesium Deficiency on Mechanisms of Insulin Resistance in Type 2 Diabetes: Focusing on the Processes of Insulin Secretion and Signaling. Int. J. Mol. Sci. 2019, 20, 1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohrabipour, S.; Sharifi, M.R.; Talebi, A.; Soltani, N. Effect of magnesium sulfate administration to improve insulin resistance in type 2 diabetes animal model: Using the hyperinsulinemic-euglycemic clamp technique. Fundam. Clin. Pharmacol. 2018, 32, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Davidsson, L.; Walczyk, T.; Hurrell, R.F. Fractional magnesium absorption is significantly lower in human subjects from a meal served with an oxalate-rich vegetable, spinach, as compared with a meal served with kale, a vegetable with a low oxalate content. Br. J. Nutr. 2004, 91, 601–606. [Google Scholar] [CrossRef]
- Anastassopoulou, J.; Theophanides, T. Magnesium-DNA interactions and the possible relation of magnesium to carcinogenesis. Irradiation and free radicals. Crit. Rev. Oncol. 2002, 42, 79–91. [Google Scholar] [CrossRef]
- Wolf, F.; Maier, J.; Nasulewicz, A.; Feillet-Coudray, C.; Simonacci, M.; Mazur, A.; Cittadini, A. Magnesium and neoplasia: From carcinogenesis to tumor growth and progression or treatment. Arch. Biochem. Biophys. 2007, 458, 24–32. [Google Scholar] [CrossRef]
- Yang, W. An overview of Y-family DNA polymerases and a case study of human DNA polymerase π. Biochemistry 2014, 53, 2793–2803. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, T.; Adams, A.; Fresco, J.R. Renaturation of transfer ribonucleic acids through site binding of magnesium. Proc. Natl. Acad. Sci. USA 1966, 55, 941–948. [Google Scholar] [CrossRef] [Green Version]
- Misra, V.K.; Draper, D.E. The linkage between magnesium binding and RNA folding 1 1Edited by B. Honig. J. Mol. Biol. 2002, 317, 507–521. [Google Scholar] [CrossRef]
- Tan, Z.J.; Chen, S.J. Importance of diffuse metal ion binding to RNA. Met. Ions Life Sci. 2011, 9, 101–124. [Google Scholar] [PubMed]
- Fandilolu, P.M.; Kamble, A.S.; Dound, A.S.; Sonawane, K.D. Role of Wybutosine and Mg2+ Ions in Modulating the Structure and Function of tRNAPhe: A Molecular Dynamics Study. ACS Omega 2019, 4, 21327–21339. [Google Scholar] [CrossRef] [Green Version]
- Strulson, C.A.; Boyer, J.A.; Whitman, E.E.; Bevilacqua, P.C. Molecular crowders and cosolutes promote folding cooperativity of RNA under physiological ionic conditions. RNA 2014, 20, 331–347. [Google Scholar] [CrossRef] [Green Version]
- Yamagami, R.; Bingaman, J.L.; Frankel, E.A.; Bevilacqua, P.C. Cellular conditions of weakly chelated magnesium ions strongly promote RNA stability and catalysis. Nat. Commun. 2018, 9, 2149. [Google Scholar] [CrossRef]
- Yamagami, R.; Huang, R.; Bevilacqua, P.C. Cellular Concentrations of Nucleotide Diphosphate-Chelated Magnesium Ions Accelerate Catalysis by RNA and DNA Enzymes. Biochemtry 2019, 58, 3971–3979. [Google Scholar] [CrossRef] [PubMed]
- Forrest, D. Unusual relatives of the multisubunit RNA polymerase. Biochem. Soc. Trans. 2019, 47, 219–228. [Google Scholar] [CrossRef]
- Rubin, H. The membrane, magnesium, mitosis (MMM) model of cell proliferation control. Magnes. Res. 2005, 18, 268–274. [Google Scholar]
- Castiglioni, S.; Maier, J.A. Magnesium and cancer: A dangerous liason. Magnes. Res. 2011, 24, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Rimnac, C.M.; Bansal, M.; Federman, M.; Lian, J.; Boyan, B.D. Effect of short-term hypomagnesemia on the chemical and mechanical properties of rat bone. J. Orthop. Res. 1992, 10, 774–783. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A.M. Magnesium and Osteoporosis: Current State of Knowledge and Future Research Directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef] [Green Version]
- Salimi, M.H.; Heughebaert, J.C.; Nancollas, G.H. Crystal growth of calcium phosphates in the presence of magnesium ions. Langmuir 1985, 1, 119–122. [Google Scholar] [CrossRef]
- Cohen, L.; Kitzes, R. Infrared spectroscopy and magnesium content of bone mineral in osteoporotic women. ISR J. Med. Sci. 1981, 17, 1123–1125. [Google Scholar] [PubMed]
- Swaminathan, R. Magnesium Metabolism and its Disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar] [PubMed]
- Ozsoylu, S.; Hanioğlu, N. Serum magnesium levels in children with vitamin D deficiency rickets. Turk. J. Pediatr. 1977, 19, 89–96. [Google Scholar]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopat. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.; Sivakumar, B. Magnesium-dependent vitamin-D-resistant rickets. Lancet 1974, 303, 963–965. [Google Scholar] [CrossRef]
- Erem, S.; Atfi, A.; Razzaque, M.S. Anabolic effects of vitamin D and magnesium in aging bone. J. Steroid Biochem. Mol. Biol. 2019, 193, 105400. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Miller, P.D. Skeletal Effects of Primary Hyperparathyroidism: Bone Mineral Density and Fracture Risk. J. Clin. Densitom. 2013, 16, 28–32. [Google Scholar] [CrossRef]
- Vetter, T.; Lohse, M.J. Magnesium and the parathyroid. Curr. Opin. Nephrol. Hypertens. 2002, 11, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ortiz, M.E.; Canalejo, A.; Herencia, C.; Martínez-Moreno, J.M.; Peralta-Ramírez, A.; Perez-Martinez, P.; Navarro-González, J.F.; Rodríguez, M.; Peter, M.; Gundlach, K.; et al. Magnesium modulates parathyroid hormone secretion and upregulates parathyroid receptor expression at moderately low calcium concentration. Nephrol. Dial. Transplant. 2014, 29, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and Hormonal Effects of Magnesium Deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef]
- Mazur, A.; Maier, J.A.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, G.L. The Role of Calcium in Inflammation-Associated Bone Resorption. Biomolecules 2018, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Güzel, A.; Doğan, E.; Türkçü, G.; Kuyumcu, M.; Kaplan, İ.; Çelik, F.; Yıldırım, Z.B. Dexmedetomidine and Magnesium Sulfate: A Good Combination Treatment for Acute Lung Injury? J. Investig. Surg. 2019, 32, 331–342. [Google Scholar] [CrossRef]
- Tang, C.-F.; Ding, H.; Jiao, R.-Q.; Wu, X.-X.; Kong, L.-D. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur. J. Pharmacol. 2020, 886, 173546. [Google Scholar] [CrossRef] [PubMed]
- Iotti, S.; Wolf, F.; Mazur, A.; Maier, J.A. The COVID-19 pandemic: Is there a role for magnesium? Hypotheses and perspectives. Magnes. Res. 2020, 33, 21–27. [Google Scholar] [CrossRef]
- White, R.E.; Hartzell, H.C. Effects of intracellular free magnesium on calcium current in isolated cardiac myocytes. Science 1988, 239, 778–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Tashiro, M.; Berlin, J.R. Regulation of L-type calcium current by intracellular magnesium in rat cardiac myocytes. J. Physiol. 2004, 555, 383–396. [Google Scholar] [CrossRef]
- Chakraborti, S.; Chakraborti, T.; Mandal, M.; Mandal, A.; Das, S.; Ghosh, S. Protective role of magnesium in cardiovascular diseases: A review. Mol. Cell. Biochem. 2002, 238, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.S.; Thomsen, P.E.B. The electrophysiological effects of intravenous magnesium on human sinus node, atrioventricular node, atrium, and ventricle. Clin. Cardiol. 1989, 12, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Netti, L.; Mariani, M.V.; Maraone, A.; D’Amato, A.; Scarpati, R.; Infusino, F.; Pucci, M.; LaValle, C.; Maestrini, V.; et al. Prevention of Cardiovascular Disease: Screening for Magnesium Deficiency. Cardiol. Res. Pract. 2019, 2019, 4874921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Houston, M. The Role of Magnesium in Hypertension and Cardiovascular Disease. J. Clin. Hypertens. 2011, 13, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Bilbey, D.L.; Prabhakaran, V.M. Muscle cramps and magnesium deficiency: Case reports. Can. Fam. Physician Med. Fam. Can. 1996, 42, 1348–1351. [Google Scholar]
- Garrison, S.R.; Korownyk, C.S.; Kolber, M.R.; Allan, G.M.; Musini, V.M.; Sekhon, R.K.; Dugré, N. Magnesium for skeletal muscle cramps. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Stroebel, D.; Casado, M.; Paoletti, P. Triheteromeric NMDA receptors: From structure to synaptic physiology. Curr. Opin. Physiol. 2018, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Möykkynen, T.; Uusi-Oukari, M.; Heikkilä, J.; Lovinger, D.M.; Lüddens, H.; Korpi, E.R. Magnesium potentiation of the function of native and recombinant GABAA receptors. Neuroreport 2001, 12, 2175–2179. [Google Scholar] [CrossRef]
- Olloquequi, J.; Cornejo-Córdova, E.; Verdaguer, E.; Soriano, F.X.; Binvignat, O.; Auladell, C.; Camins, A. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: Therapeutic implications. J. Psychopharmacol. 2018, 32, 265–275. [Google Scholar] [CrossRef]
- Steinert, J.R.; Postlethwaite, M.; Jordan, M.D.; Chernova, T.; Robinson, S.W.; Forsythe, I.D. NMDAR-mediated EPSCs are maintained and accelerate in time course during maturation of mouse and rat auditory brainstem in vitro. J. Physiol. 2010, 588, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Walter, S.; Rapoport, A.M. Calcitonin Gene-Related Peptide (CGRP) and Migraine Current Understanding and State of Development. Headache J. Head Face Pain 2013, 53, 1230–1244. [Google Scholar] [CrossRef]
- Weglicki, W.B. Hypomagnesemia and Inflammation: Clinical and Basic Aspects. Annu. Rev. Nutr. 2012, 32, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.M. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Am. J. Clin. Nutr. 2007, 85, 924. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997. [Google Scholar]
- Price, M.; Preedy, V. Dietary Reference Values. In Metabolism and Pathophysiology of Bariatric Surgery; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 399–417. [Google Scholar]
- EFSA Scientific Panel NDA. Scientific Opinion on Dietary Reference Values for magnesium. EFSA J. 2015, 13, 4186. [Google Scholar]
- Cioffi, I.; Imperatore, N.; Di Vincenzo, O.; Pagano, M.C.; Santarpia, L.; Pellegrini, L.; Testa, A.; Marra, M.; Contaldo, F.; Castiglione, F.; et al. Evaluation of nutritional adequacy in adult patients with Crohn’s disease: A cross-sectional study. Eur. J. Nutr. 2020, 59, 3647–3658. [Google Scholar] [CrossRef] [Green Version]
- Di Riferimento, L.L.D.A. Di Nutrienti ed Energia per la Popolazione Italiana; Doc. di Sintesi per XXXV Congr.; Società Italiana di Nutrizione Umana (SINU): Milano, Italy, 2012. [Google Scholar]
- Dietary Reference Values for nutrients Summary report. EFSA Support. Publ. 2017, 14, e15121. [CrossRef]
- Tedstone, A.; Dunce, N.; Aviles, M.; Shetty, P.; Daniels, L. Effectiveness of interventions to promote healthy feeding in infants under one year of age. Natl. Inst. Heal. Res. 2018, 61, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, L.M.; Dwyer, J.T.; Bailey, R.L.; Reidy, K.C.; Saavedra, J.M. Harmonizing Micronutrient Intake Reference Ranges for Dietary Guidance and Menu Planning in Complementary Feeding. Curr. Dev. Nutr. 2020, 4, nzaa017. [Google Scholar] [CrossRef]
- Shergill-Bonner, R. Micronutrients. Paediatr. Child Health. 2017, 27, 357–362. [Google Scholar] [CrossRef]
- Melby, M.K.; Utsugi, M.; Miyoshi, M.; Watanabe, S. Overview of nutrition reference and dietary recommendations in Japan: Application to nutrition policy in Asian countries. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S2), 394–398. [Google Scholar] [PubMed]
- Durlach, J.; Pagès, N.; Bac, P.; Bara, M.; Guiet-Bara, A. New data on the importance of gestational Mg deficiency. Magnes. Res. 2004, 17, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Crosby, D.D.; Shepherd, E.; Crowther, C.A. Magnesium supplementation in pregnancy. Cochrane Database Syst. Rev. 2014, 2014, CD000937. [Google Scholar] [CrossRef]
- Lukaski, H.C. Magnesium, zinc, and chromium nutriture and physical activity. Am. J. Clin. Nutr. 2000, 72, 585S–593S. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xun, P.; Wang, R.; Mao, L.; He, K. Can Magnesium Enhance Exercise Performance? Nutrients 2017, 9, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, F.H.; Lukaski, H.C. Update on the relationship between magnesium and exercise. Magnes. Res. 2006, 19, 180. [Google Scholar]
- Setaro, L.; Santos-Silva, P.R.; Nakano, E.Y.; Sales, C.H.; Nunes, N.; Greve, J.M.; Colli, C. Magnesium status and the physical performance of volleyball players: Effects of magnesium supplementation. J. Sports Sci. 2013, 32, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Casoni, I.; Guglielmini, C.; Graziano, L.; Reali, M.; Mazzotta, D.; Abbasciano, V. Changes of Magnesium Concentrations in Endurance Athletes. Int. J. Sports Med. 1990, 11, 234–237. [Google Scholar] [CrossRef]
- Reno, A.M.; Green, M.; Killen, L.G.; O’Neal, E.K.; Pritchett, K.; Hanson, Z. Effects of Magnesium Supplementation on Muscle Soreness and Performance. J. Strength Cond. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.A.; Ismail, Y.; Ismail, A.A. Clinical assessment of magnesium status in the adult: An overview. In Magnesium in Human Health and Disease; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9781627030441. [Google Scholar]
- Filippi, J.; Al-Jaouni, R.; Wiroth, J.B.; Hébuterne, X.; Schneider, S.M. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm. Bowel Dis. 2006, 12, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Van Langenberg, D.; Della Gatta, P.; Warmington, S.A.; Kidgell, D.J.; Gibson, P.R.; Russell, A.P. Objectively measured muscle fatigue in Crohn’s disease: Correlation with self-reported fatigue and associated factors for clinical application. J. Crohn’s Coliti 2014, 8, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Naser, S.A. Domino effect of hypomagnesemia on the innate immunity of Crohn’s disease patients. World J. Diabetes 2014, 5, 527–535. [Google Scholar] [CrossRef]
- Habtezion, A.; Silverberg, M.S.; Parkes, R.; Mikolainis, S.; Steinhart, A.H. Risk Factors for Low Bone Density in Crohn’s Disease. Inflamm. Bowel Dis. 2002, 8, 87–92. [Google Scholar] [CrossRef]
- Mukai, A.; Yamamoto, S.; Matsumura, K. Hypocalcemia secondary to hypomagnesemia in a patient with Crohn’s disease. Clin. J. Gastroenterol. 2015, 8, 22–25. [Google Scholar] [CrossRef]
- Taylor, L.; Almutairdi, A.; Shommu, N.; Fedorak, R.; Ghosh, S.; Reimer, R.A.; Panaccione, R.; Raman, M. Cross-Sectional Analysis of Overall Dietary Intake and Mediterranean Dietary Pattern in Patients with Crohn’s Disease. Nutrients 2018, 10, 1761. [Google Scholar] [CrossRef] [Green Version]
- Pierote, N.R.; Braz, A.F.; Barros, S.L.; Neto, J.M.M.; Parente, J.M.L.; Silva, M.D.C.M.; Beserra, M.S.; Soares, N.R.M.; Marreiro, D.N.; Nogueira, N.D.N. Effect of mineral status and glucocorticoid use on bone mineral density in patients with Crohn’s disease. Nutrients 2018, 48, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Phuong Nguyen, G. Iron Deficiency, Zinc, Magnesium, Vitamin Deficiencies in Crohn’s Disease: Substitute or Not? Dig. Dis. 2016, 34, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Weisshof, R.; Chermesh, I. Micronutrient deficiencies in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Balamtekin, N.; Aksoy, Ç.; Baysoy, G.; Uslu, N.; Demir, H.; Köksal, G.; Saltık-Temizel, İ.N.; Özen, H.; Gürakan, F.; Yüce, A. Is compliance with gluten-free diet sufficient? Diet composition of celiac patients. Turk. J. Pediatr. 2015, 57, 374. [Google Scholar] [PubMed]
- Caruso, R.; Pallone, F.; Stasi, E.; Romeo, S.; Monteleone, G. Appropriate nutrient supplementation in celiac disease. Ann. Med. 2013, 45, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Kupper, C. Dietary guidelines and implementation for celiac disease. Gastroenterology 2005, 128, S121–S127. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, C.; Di Leo, G.; Ronfani, L.; Martelossi, S.; Not, T.; Ventura, A. Bone Metabolism in Celiac Disease. J. Pediatr. 2008, 153, 262–265. [Google Scholar] [CrossRef]
- Martin, J.; Geisel, T.; Maresch, C.; Krieger, K.; Stein, J. Inadequate Nutrient Intake in Patients with Celiac Disease: Results from a German Dietary Survey. Digestion 2013, 87, 240–246. [Google Scholar] [CrossRef]
- Fernández, C.B.; Varela-Moreiras, G.; Úbeda, N.; Alonso-Aperte, E. Nutritional Status in Spanish Children and Adolescents with Celiac Disease on a Gluten Free Diet Compared to Non-Celiac Disease Controls. Nutrients 2019, 11, 2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Nardo, G.; Villa, M.P.; Conti, L.; Ranucci, G.; Pacchiarotti, C.; Principessa, L.; Raucci, U.; Parisi, P. Nutritional Deficiencies in Children with Celiac Disease Resulting from a Gluten-Free Diet: A Systematic Review. Nutrients 2019, 11, 1588. [Google Scholar] [CrossRef] [Green Version]
- González, T.; Larretxi, I.; Vitoria, J.C.; Castaño, L.; Simón, E.; Churruca, I.; Navarro, V.; Lasa, A. Celiac Male’s Gluten-Free Diet Profile: Comparison to that of the Control Population and Celiac Women. Nutrients 2018, 10, 1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babio, N.; Alcázar, M.; Castillejo, G.; Recasens, M.; Martínez-Cerezo, F.; Gutiérrez-Pensado, V.; Masip, G.; Vaqué, C.; Vila-Martí, A.; Torres-Moreno, M.; et al. Patients With Celiac Disease Reported Higher Consumption of Added Sugar and Total Fat Than Healthy Individuals. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 63–69. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac disease: Understanding the gluten-free diet. Eur. J. Nutr. 2017, 56, 449–459. [Google Scholar] [CrossRef]
- Barbagallo, M. Magnesium and type 2 diabetes. World J. Diabetes 2015, 6, 1152–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, D.P.; Sharma, R.; Bansal, D.D. Implications of Magnesium Deficiency in Type 2 Diabetes: A Review. Biol. Trace Element Res. 2009, 134, 119–129. [Google Scholar] [CrossRef]
- Lopez-Ridaura, R.; Willett, W.C.; Rimm, E.B.; Liu, S.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care 2003, 27, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadass, S.; Basu, S.; Srinivasan, A. SERUM magnesium levels as an indicator of status of Diabetes Mellitus type 2. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 42–45. [Google Scholar] [CrossRef]
- Serefko, A.; Szopa, A.; Poleszak, E. Magnesium and depression. Magnes. Res. 2016, 29, 112–119. [Google Scholar] [CrossRef]
- Long, S.; Romani, A.M. Role of Cellular Magnesium in Human Diseases. Austin J. Nutr. Food Sci. 2014, 2, 1051. [Google Scholar]
- Prior, P.L.; Vaz, M.J.; Ramos, A.C.; Galduróz, J.C.F. Influence of Microelement Concentration on the Intensity of Alcohol Withdrawal Syndrome. Alcohol Alcohol. 2015, 50, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Grochowski, C.; Blicharska, E.; Baj, J.; Mierzwińska, A.; Brzozowska, K.; Forma, A.; Maciejewski, R. Serum iron, Magnesium, Copper, and Manganese Levels in Alcoholism: A Systematic Review. Molecules 2019, 24, 1361. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.A.; Ismail, N.A. Magnesium: A Mineral Essential for Health Yet Generally Underestimated or Even Ignored. J. Nutr. Food Sci. 2016, 6, 4. [Google Scholar] [CrossRef]
- Marles, R.J. Mineral nutrient composition of vegetables, fruits and grains: The context of reports of apparent historical declines. J. Food Compos. Anal. 2017, 56, 93–103. [Google Scholar] [CrossRef]
- Mayer, A. Historical changes in the mineral content of fruits and vegetables. Br. Food J. 1997, 99, 207–211. [Google Scholar] [CrossRef]
- Cazzola, R.; Della Porta, M.; Manoni, M.; Iotti, S.; Pinotti, L.; Maier, J.A. Going to the roots of reduced magnesium dietary intake: A tradeoff between climate changes and sources. Heliyon 2020, 6, e05390. [Google Scholar] [CrossRef] [PubMed]
- Olza, J.; Aranceta-Bartrina, J.; Gonzalez-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, A. Reported Dietary Intake, Disparity between the Reported Consumption and the Level Needed for Adequacy and Food Sources of Calcium, Phosphorus, Magnesium and Vitamin D in the Spanish Population: Findings from the ANIBES Study. Nutrients 2017, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium Fertilization Improves Crop Yield in Most Production Systems: A Meta-Analysis. Front. Plant Sci. 2020, 10, 1727. [Google Scholar] [CrossRef] [Green Version]
- Bohn, T.; Walczyk, T.; Leisibach, S.; Hurrell, R. Chlorophyll-bound Magnesium in Commonly Consumed Vegetables and Fruits: Relevance to Magnesium Nutrition. J. Food Sci. 2006, 69, S347–S350. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop. J. 2016, 4, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Melse-Boonstra, A. Bioavailability of Micronutrients From Nutrient-Dense Whole Foods: Zooming in on Dairy, Vegetables, and Fruits. Front. Nutr. 2020, 7, 101. [Google Scholar] [CrossRef]
- Roe, M.; Bell, S.; Oseredczuk, M.; Christensen, T.; Westenbrink, S.; Pakkala, H.; Presser, K.; Finglas, P. Updated food composition database for nutrient intake. EFSA Support. Publ. 2013, 10, 355E. [Google Scholar] [CrossRef] [Green Version]
- Departamento de Agricultura de Estados Unidos (USDA). FoodData Central. 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 10 February 2021).
- Ellam, S.; Williamson, G. Cocoa and Human Health. Annu. Rev. Nutr. 2013, 33, 105–128. [Google Scholar] [CrossRef]
- Brink, E.J.; Beynen, A.C. Nutrition and magnesium absorption: A review. Prog. Food Nutr. Sci. 1992, 16, 125–162. [Google Scholar]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Lopez, H.W.; Krespine, V.; Guy, C.; Messager, A.; Demigne, C.; Remesy, C. Prolonged Fermentation of Whole Wheat Sourdough Reduces Phytate Level and Increases Soluble Magnesium. J. Agric. Food Chem. 2001, 49, 2657–2662. [Google Scholar] [CrossRef]
- Lopez, H.W.; Leenhardt, F.; Coudray, C.; Remesy, C. Minerals and phytic acid interactions: Is it a real problem for human nutrition? Int. J. Food Sci. Technol. 2002, 37, 727–739. [Google Scholar] [CrossRef]
- Gibson, R.; Dahdouh, S.; Grande, F.; Najera, S.; Fialon, M.; Vincent, A.; King, J.; Bailey, K.; Raboy, V.; Charrondiere, U.R. New phytate data collection: Implications for nutrient reference intakes for minerals, programmes and policies. Ann. Nutr. Metab. 2017, 71, 209–210. [Google Scholar]
- Severo, J.S.; Morais, J.B.S.; De Freitas, T.E.C.; Cruz, K.J.C.; De Oliveira, A.R.S.; Poltronieri, F.; Marreiro, D.D.N. Metabolic and nutritional aspects of magnesium. Nutr. Clin. Diet. Hosp. 2015, 35, 67–74. [Google Scholar]
- Vegarud, G.E.; Langsrud, T.; Svenning, C. Mineral-binding milk proteins and peptides; occurrence, biochemical and technological characteristics. Br. J. Nutr. 2000, 84, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Kitano, T.; Esashi, T.; Azami, S. Effect of protein intake on mineral (calcium, magnesium, and phosphorus) balance in Japanese males. J. Nutr. Sci. Vitaminol. 1988, 34, 387–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, S.J.; Kohrt, W.M.; Warren, M.P.; Kraenzlin, M.I.; Bonjour, J.-P. Food fortification for bone health in adulthood: A scoping review. Eur. J. Clin. Nutr. 2016, 70, 1099–1105. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Song, Y.; Manson, J.E.; Signorello, L.B.; Zhang, S.M.; Shrubsole, M.J.; Ness, R.M.; Seidner, D.L.; Dai, Q. Magnesium, vitamin D status and mortality: Results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. 2013, 11, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieńkowski, P. Commentary on: Pouteau et al. Superiority of magnesium and vitamin B6 over magnesium alone on severe stress in healthy adults with low magnessemia: A randomized, single-blind clinical trial. PLoS ONE 2018, 13, e0208454. [Google Scholar] [CrossRef]
- Pouteau, E.; Kabir-Ahmadi, M.; Noah, L.; Mazur, A.; Dye, L.; Hellhammer, J.; Pickering, G.; DuBray, C. Superiority of combined magnesium (MG) and vitamin B6 (VITB6) supplementation over magnesium alone on severe stress in adults with low magnesemia: A randomised, single blind trial. Clin. Nutr. 2018, 37, S289–S290. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Milne, D.B. A moderately high intake compared to a low intake of zinc depresses magnesium balance and alters indices of bone turnover in postmenopausal women. Eur. J. Clin. Nutr. 2004, 58, 703–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, S. The multifaceted and widespread pathology of magnesium deficiency. Med. Hypotheses 2001, 56, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Wallace, T.C. Combating COVID-19 and Building Immune Resilience: A Potential Role for Magnesium Nutrition? J. Am. Coll. Nutr. 2020, 39, 685–693. [Google Scholar] [CrossRef]
- Takahashi, Y.; Imaizumi, Y. Hardness in Drinking Water. Eisei Kagaku 1988, 34, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Van Der Aa, M. Classification of mineral water types and comparison with drinking water standards. Environ. Earth Sci. 2003, 44, 554–563. [Google Scholar] [CrossRef] [Green Version]
- Maraver, F.; Vitoria, I.; Ferreira-Pêgo, C.; Armijo, F.; Salas-Salvadó, J. Magnesium in tap and bottled mineral water in Spain and its contribution to nutritional recommendations. Nutr. Hosp. 2015, 31, 2297–2312. [Google Scholar] [PubMed]
- Verhas, M.; De La Guéronnière, V.; Grognet, J.-M.; Paternot, J.; Hermanne, A.; Winkel, P.V.D.; Gheldof, R.; Martin, P.; Fantino, M.; Rayssiguier, Y. Magnesium bioavailability from mineral water. A study in adult men. Eur. J. Clin. Nutr. 2002, 56, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Sabatier, M.; Arnaud, M.J.; Kastenmayer, P.; Rytz, A.; Barclay, D.V. Meal effect on magnesium bioavailability from mineral water in healthy women. Am. J. Clin. Nutr. 2002, 75, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dórea, J.G. Magnesium in Human Milk. J. Am. Coll. Nutr. 2000, 19, 210–219. [Google Scholar] [CrossRef]
- Yang, Z.; Huffman, S.L. Review of fortified food and beverage products for pregnant and lactating women and their impact on nutritional status. Matern. Child Nutr. 2011, 7, 19–43. [Google Scholar] [CrossRef]
- Reidy, K.C.; Bailey, R.L.; Deming, D.M.; O’Neill, L.; Carr, B.T.; Lesniauskas, R.; Johnson, W. Food Consumption Patterns and Micronutrient Density of Complementary Foods Consumed by Infants Fed Commercially Prepared Baby Foods. Nutr. Today 2018, 53, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Gillis, L.; Gillis, A. Nutrient Inadequacy in Obese and Non-Obese Youth. Can. J. Diet. Pract. Res. 2005, 66, 237–242. [Google Scholar] [CrossRef]
- Poitevin, E. Determination of calcium, copper, iron, magnesium, manganese, potassium, phosphorus, sodium, and zinc in fortified food products by microwave digestion and inductively coupled plasma-optical emission spectrometry: Single-laboratory validation and ring tri. J. AOAC Int. 2012, 95, 177–185. [Google Scholar] [CrossRef]
- Food and Drug Administration. Food Labeling: Revision of the Nutrition and Supplement Facts Labels. Final rule. Fed. Regist. 2016, 81, 33741–37999. [Google Scholar]
- Food and Drug Administration. Food Labeling: Serving Sizes of Foods That Can Reasonably Be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendmen. Fed. Regist. 2016, 81, 34000–34047. [Google Scholar]
- Ates, M.; Kizildag, S.; Yuksel, O.; Hosgorler, F.; Yuce, Z.; Guvendi, G.; Kandis, S.; Karakilic, A.; Koc, B.; Uysal, N. Dose-Dependent Absorption Profile of Different Magnesium Compounds. Biol. Trace Elem. Res. 2019, 192, 244–251. [Google Scholar] [CrossRef]
- Schweigel, M.; Martens, H. Magnesium transport in the gastrointestinal tract. Front. Biosci. 2000, 3, D666–D677. [Google Scholar] [CrossRef] [Green Version]
- Vormann, J. Magnesium: Nutrition and metabolism. Mol. Aspects Med. 2003, 24, 27–37. [Google Scholar] [CrossRef]
- Vormann, J. Magnesium: Nutrition and Homoeostasis. AIMS Public Health 2016, 3, 329–340. [Google Scholar] [CrossRef]
- Ranade, V.V.; Somberg, J.C. Bioavailability and Pharmacokinetics of Magnesium After Administration of Magnesium Salts to Humans. Am. J. Ther. 2001, 8, 345–357. [Google Scholar] [CrossRef]
- Cosaro, E.; Bonafini, S.; Montagnana, M.; Danese, E.; Trettene, M.; Minuz, P.; Delva, P.; Fava, C. Effects of magnesium supplements on blood pressure, endothelial function and metabolic parameters in healthy young men with a family history of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.F.; Marakis, G.; Christie, S.; Byng, M. Mg citrate found more bioavailable than other Mg preparations in a randomised, double-blind study. Magnes. Res. 2003, 16, 183–191. [Google Scholar] [PubMed]
- Uysal, N.; Kizildag, S.; Yuce, Z.; Guvendi, G.; Kandis, S.; Koc, B.; Karakilic, A.; Camsari, U.M.; Ates, M. Timeline (Bioavailability) of Magnesium Compounds in Hours: Which Magnesium Compound Works Best? Biol. Trace Elem. Res. 2019, 187, 128–136. [Google Scholar] [CrossRef]
- Coudray, C.; Rambeau, M.; Feillet-Coudray, C.; Gueux, E.; Tressol, J.C.; Mazur, A.; Rayssiguier, Y. Study of magnesium bioavailability from ten organic and inorganic Mg salts in Mg-depleted rats using a stable isotope approach. Magnes. Res. 2005, 18, 215–223. [Google Scholar]
- Hillier, K. Magnesium Oxide. In xPharm: The Comprehensive Pharmacology Reference; Elsevier BV: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Hunter, L.A.; Gibbins, K.J. Magnesium Sulfate: Past, Present, and Future. J. Midwifery Women’s Heal. 2011, 56, 566–574. [Google Scholar] [CrossRef]
- Durlach, J.; Guiet-Bara, A.; Pagès, N.; Bac, P.; Bara, M. Magnesium chloride or magnesium sulfate: A genuine question. Magnes. Res. 2005, 18, 187–192. [Google Scholar] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Magnesium citrate malate as a source of magnesium added for nutritional purposes to food supplements. EFSA J. 2018, 16, e05484. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I.; Senmaru, M.; Terasaki, T.; Tsuji, A. Na+- and Cl−-Dependent transport of taurine at the blood-brain barrier. Biochem. Pharmacol. 1995, 50, 1783–1793. [Google Scholar] [CrossRef]
- Tsuji, A.; Tamai, I. Sodium- and chloride-dependent transport of taurine at the blood-brain barrier. Single Mol. Single Cell Seq. 1996, 403, 385–391. [Google Scholar]
- Covington, A.K.; Danish, E.Y. Measurement of Magnesium Stability Constants of Biologically Relevant Ligands by Simultaneous Use of pH and Ion-Selective Electrodes. J. Solut. Chem. 2009, 38, 1449–1462. [Google Scholar] [CrossRef]
- Farruggia, G.; Castiglioni, S.; Sargenti, A.; Marraccini, C.; Cazzaniga, A.; Merolle, L.; Iotti, S.; Cappadone, C.; Maier, J.A.M. Effects of supplementation with different Mg salts in cells: Is there a clue? Magnes. Res. 2014, 27, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Chen, C.; Liu, W.; Zhou, T.; Xun, P.; He, K.; Chen, P. The effect of magnesium supplementation on muscle fitness: A meta-analysis and systematic review. Magnes. Res. 2017, 30, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.O.; DeLucia, M.C.; Zhang, J.H.; Bejnerowicz, G.; Tartamella, L.; Dziura, J.; Petersen, K.F.; Befroy, D.; Cohen, D. A Randomized Controlled Study of Effects of Dietary Magnesium Oxide Supplementation on Bone Mineral Content in Healthy Girls. J. Clin. Endocrinol. Metab. 2006, 91, 4866–4872. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Laukkanen, J.A. Low serum magnesium levels are associated with increased risk of fractures: A long-term prospective cohort study. Eur. J. Epidemiol. 2017, 32, 593–603. [Google Scholar] [CrossRef] [Green Version]
- Musso, C.G. Magnesium metabolism in health and disease. Int. Urol. Nephrol. 2009, 41, 357–362. [Google Scholar] [CrossRef]
- Nanduri, A.; Saleem, S.; Khalaf, M. Severe hypermagnesemia. Chest 2020, 158, A1016. [Google Scholar] [CrossRef]
- National Institutes of Health NIH Magnesium—Health Professional Fact Sheet. Fact Sheet Healyh Prof. 2018. Available online: https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/ (accessed on 10 February 2021).
- Moodie, E. Modern Trends in Animal Health and HUSBANDRY Hypocalcaemia and Hypomagnesaemia. Br. Vet. J. 1965, 121, 338–349. [Google Scholar] [CrossRef]
- Murphy, E. Mysteries of Magnesium Homeostasis. Circ. Res. 2000, 86, 245–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romani, A.M.P. Magnesium in health and disease. Met. Ions Life Sci. 2013, 2013, 49–79. [Google Scholar]
- Glasdam, S.M.; Glasdam, S.; Peters, G.H. The Importance of Magnesium in the Human Body: A Systematic Literature Review. Adv. Clin. Chem. 2016, 73, 169–193. [Google Scholar] [PubMed] [Green Version]
- Workinger, J.L.; Doyle, R.P.; Bortz, J. Challenges in the Diagnosis of Magnesium Status. Nutrients 2018, 10, 1202. [Google Scholar] [CrossRef]
- Ismail, Y.; Ismail, A.A.; Ismail, A.A.A. The underestimated problem of using serum magnesium measurements to exclude magnesium deficiency in adults; A health warning is needed for “normal” results. Clin. Chem. Lab. Med. 2010, 48, 323–327. [Google Scholar] [CrossRef]
- Rylander, R.; Remer, T.; Berkemeyer, S.; Vormann, J. Acid-Base Status Affects Renal Magnesium Losses in Healthy, Elderly Persons. J. Nutr. 2006, 136, 2374–2377. [Google Scholar] [CrossRef]
- Löwik, M.R.; Van Dokkum, W.; Kistemaker, C.; Schaafsma, G.; Ockhuizen, T. Body composition, health status and urinary magnesium excretion among elderly people (Dutch Nutrition Surveillance System). Magnes. Res. 1993, 6, 223–232. [Google Scholar] [PubMed]
- Ware, E.B.; Smith, J.A.; Zhao, W.; Ganesvoort, R.T.; Curhan, G.C.; Pollak, M.; Mount, D.B.; Turner, S.T.; Chen, G.; Shah, R.J.; et al. Genome-wide Association Study of 24-Hour Urinary Excretion of Calcium, Magnesium, and Uric Acid. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 448–460. [Google Scholar] [CrossRef] [Green Version]
- Gant, C.M.; Soedamah-Muthu, S.S.; Binnenmars, S.H.; Bakker, S.J.L.; Navis, G.; Laverman, G.D. Higher Dietary Magnesium Intake and Higher Magnesium Status Are Associated with Lower Prevalence of Coronary Heart Disease in Patients with Type 2 Diabetes. Nutrients 2018, 10, 307. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Sun, J.; Deng, X.; Huang, X.; Sun, W.; Xu, Y.; Xu, M.; Lu, J.; Bi, Y. Low Serum Magnesium Level Is Associated with Microalbuminuria in Chinese Diabetic Patients. Int. J. Endocrinol. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Suliburska, J.; Bogdański, P.; Szulińska, M.; Pupek-Musialik, D. Short-Term Effects of Sibutramine on Mineral Status and Selected Biochemical Parameters in Obese Women. Biol. Trace Element Res. 2012, 149, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haigney, M.C.; Silver, B.; Tanglao, E.; Silverman, H.S.; Hill, J.D.; Shapiro, E.; Gerstenblith, G.; Schulman, S.P. Noninvasive Measurement of Tissue Magnesium and Correlation With Cardiac Levels. Circulation 1995, 92, 2190–2197. [Google Scholar] [CrossRef]
- Shechter, M.; Sharir, M.; Paul, M.J.; James, L.; Burton, F.; Noel, S.C.; Merz, B. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation 2000, 102, 2353–2358. [Google Scholar] [CrossRef] [Green Version]
- Silver, B.B. Development of Cellular Magnesium Nano-Analysis in Treatment of Clinical Magnesium Deficiency. J. Am. Coll. Nutr. 2004, 23, 732S–737S. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Welch, A.A.; Adelnia, F.; Bergeron, C.M.; Reiter, D.A.; Dominguez, L.J.; Brennan, N.A.; Fishbein, K.W.; Spencer, R.G.; Ferrucci, L. Age and Muscle Function Are More Closely Associated With Intracellular Magnesium, as Assessed by 31P Magnetic Resonance Spectroscopy, Than With Serum Magnesium. Front. Physiol. 2019, 10, 1454. [Google Scholar] [CrossRef]
- Iotti, S.; Malucelli, E. In vivo assessment of Mg2+ in human brain and skeletal muscle by 31P-MRS. Magnes. Res. 2008, 21, 157–162. [Google Scholar]
- McCully, K.K.; Turner, T.N.; Langley, J.; Zhao, Q. The reproducibility of measurements of intramuscular magnesium concentrations and muscle oxidative capacity using 31P MRS. Dyn. Med. 2009, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Malucelli, E.; Lodi, R.; Martinuzzi, A.; Tonon, C.; Barbiroli, B.; Iotti, S. Free Mg2+ concentration in the calf muscle of glycogen phosphorylase and phosphofructokinase deficiency patients assessed in different metabolic conditions by 31P MRS. Dyn. Med. 2005, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Pironi, L.; Malucelli, E.; Guidetti, M.; Lanzoni, E.; Farruggia, G.; Pinna, A.D.; Barbiroli, B.; Iotti, S. The complex relationship between magnesium and serum parathyroid hormone: A study in patients with chronic intestinal failure. Magnes. Res. 2009, 22, 37–43. [Google Scholar] [CrossRef]
- Nelander, M.; Weis, J.; Bergman, L.; Larsson, A.; Wikstrom, A.K.; Wikstrom, J. Cerebral magnesium levels in preeclampsia; A phosphorus magnetic resonance spectroscopy study. Am. J. Hypertens. 2017, 30, 667–672. [Google Scholar] [CrossRef]
- Mairiang, E.; Hanpanich, P.; Sriboonlue, P. In vivo 31P-MRS assessment of muscle-pH, cytolsolic-[Mg2+] and phosphorylation potential after supplementing hypokaliuric renal stone patients with potassium and magnesium salts. Magn. Reson. Imaging 2004, 22, 715–719. [Google Scholar] [CrossRef]
- Reyngoudt, H.; Kolkovsky, A.L.L.; Carlier, P.G. Free intramuscular Mg2+ concentration calculated using both31P and1H NMRS-based pH in the skeletal muscle of Duchenne muscular dystrophy patients. NMR Biomed. 2019, 32, e4115. [Google Scholar] [CrossRef] [PubMed]
- Schutten, J.C.; Gomes-Neto, A.W.; Navis, G.; Gansevoort, R.T.; Dullaart, R.P.F.; Kootstra-Ros, J.E.; Danel, R.M.; Goorman, F.; Gans, R.O.B.; De Borst, M.H.; et al. Lower Plasma Magnesium, Measured by Nuclear Magnetic Resonance Spectroscopy, is Associated with Increased Risk of Developing Type 2 Diabetes Mellitus in Women: Results from a Dutch Prospective Cohort Study. J. Clin. Med. 2019, 8, 169. [Google Scholar] [CrossRef] [Green Version]
- Lutz, N.W.; Bernard, M. Multiparametric quantification of heterogeneity of metal ion concentrations, as demonstrated for [Mg2+] by way of 31P MRS. J. Magn. Reson. 2018, 294, 71–82. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Zapata, C.; Franco, D. Proteins and amino acids. In Innovative Thermal and Non-Thermal Processing; Barba, F.J., Saraiba, J.M.A., Cravotto, G., Lorenzo, J.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 139–168. ISBN 9781469816593. [Google Scholar]
- Christian, G.D. Medicine, trace elements, and atomic absorption spectroscopy. Anal. Chem. 1969, 41, 24A–40A. [Google Scholar] [CrossRef]
- DiPietro, E.; Bashor, M.; Stroud, P.; Smarr, B.; Burgess, B.; Turner, W.; Neese, J. Comparison of an inductively coupled plasma-atomic emission spectrometry method for the determination of calcium, magnesium, sodium, potassium, copper and zinc with atomic absorption spectroscopy and flame photometry methods. Sci. Total Environ. 1988, 74, 249–262. [Google Scholar] [CrossRef]
- Uğurlu, V.; Binay, Ç.; Şimşek, E.; Bal, C. Cellular Trace Element Changes in Type 1 Diabetes Patients. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 180–186. [Google Scholar] [CrossRef]
- Millart, H.; Durlach, V.; Durlach, J. Red blood cell magnesium concentrations: Analytical problems and significance. Magnes. Res. 1995, 8, 65–76. [Google Scholar]
- Tashiro, M.; Inoue, H.; Konishi, M. Magnesium Homeostasis in Cardiac Myocytes of Mg-Deficient Rats. PLoS ONE 2013, 8, e73171. [Google Scholar] [CrossRef] [PubMed]
- Schilling, K.; Larner, F.; Saad, A.; Roberts, R.; Kocher, H.M.; Blyuss, O.; Halliday, A.N.; Crnogorac-Jurcevic, T. Urine metallomics signature as an indicator of pancreatic cancer. Metallomics 2020, 12, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yan, L.; Guo, T.; Yang, S.; Liu, Y.; Xie, Q.; Ni, D.; Wang, J. Association between Serum Essential Metal Elements and the Risk of Schizophrenia in China. Sci. Rep. 2020, 10, 10875. [Google Scholar] [CrossRef] [PubMed]
- Günzel, D.; Schlue, W.-R. Determination of [Mg2+]i—An update on the use of Mg2+-selective electrodes. BioMetals 2002, 15, 237–249. [Google Scholar] [CrossRef]
- Kamochi, M.; Aibara, K.; Nakata, K.; Murakami, M.; Nandate, K.; Sakamoto, H.; Sata, T.; Shigematsu, A. Profound ionized hypomagnesemia induced by therapeutic plasma exchange in liver failure patients. Transfusion 2002, 42, 1598–1602. [Google Scholar] [CrossRef]
- Fu, C.-Y.; Chen, S.-J.; Cai, N.-H.; Liu, Z.-H.; Zhang, M.; Wang, P.-C.; Zhao, J.-N. Increased risk of post-stroke epilepsy in Chinese patients with a TRPM6 polymorphism. Neurol. Res. 2019, 41, 378–383. [Google Scholar] [CrossRef]
- Ordak, M.; Maj-Zurawska, M.; Matsumoto, H.; Bujalska-Zadrozny, M.; Kieres-Salomonski, I.; Nasierowski, T.; Muszynska, E.; Wojnar, M. Ionized magnesium in plasma and erythrocytes for the assessment of low magnesium status in alcohol dependent patients. Drug Alcohol Depend. 2017, 178, 271–276. [Google Scholar] [CrossRef]
- International Federation of Clinica Ben Rayana; Burnett, R.W.; Covington, A.K.; D’Orazio, P.; Fogh-Andersen, N.; Jacobs, E.; Külpmann, W.R.; Kuwa, K.; Larsson, L.; Lewenstam, A.; et al. IFCC Guideline for sampling, measuring and reporting ionized magnesium in plasma. Clin. Chem. Lab. Med. 2008, 46, 21–26. [Google Scholar] [CrossRef]
- Maj-Żurawska, M.; Lewenstam, A. Selectivity coefficients of ion-selective magnesium electrodes used for simultaneous determination of magnesium and calcium ions. Talanta 2011, 87, 295–301. [Google Scholar] [CrossRef]
- Lvova, L.; Gonçalves, C.G.; Di Natale, C.; Legin, A.; Kirsanov, D.; Paolesse, R. Recent advances in magnesium assessment: From single selective sensors to multisensory approach. Talanta 2018, 179, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, F.; Diehl, H. Indicator for the Titration of Calcium Plus Magnesium with (Ethylenedinitrilo)tetraacetate. Anal. Chem. 1960, 32, 1123–1127. [Google Scholar] [CrossRef]
- Abernethy, M.H.; Fowler, R.T. Micellar improvement of the calmagite compleximetric measurement of magnesium in plasma. Clin. Chem. 1982, 28, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Malucelli, E.; Procopio, A.; Fratini, M.; Gianoncelli, A.; Notargiacomo, A.; Merolle, L.; Sargenti, A.; Castiglioni, S.; Cappadone, C.; Farruggia, G.; et al. Single cell versus large population analysis: Cell variability in elemental intracellular concentration and distribution. Anal. Bioanal. Chem. 2018, 410, 337–348. [Google Scholar] [CrossRef]
- Chromý, V.; Svoboda, V.; Štěpánová, I. Spectrophotometric determination of magnesium in biological fluids with xylidyl blue II. Biochem. Med. 1973, 7, 208–217. [Google Scholar] [CrossRef]
- Wimmer, M.C.; Artiss, J.D.; Zak, B. A kinetic colorimetric procedure for quantifying magnesium in serum. Clin. Chem. 1986, 32, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Trapani, V.; Schweigel-Röntgen, M.; Cittadini, A.; Wolf, F.I. Intracellular Magnesium Detection by Fluorescent Indicators. Methods Enzymol. 2012, 505, 421–444. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, X.; Li, M.; Liao, N.; Bi, A.; Jiang, Y.; Liu, S.; Gong, Z.; Zeng, W. Fluorescent probes for the detection of magnesium ions (Mg2+): From design to application. RSC Adv. 2018, 8, 12573–12587. [Google Scholar] [CrossRef] [Green Version]
- Picone, G.; Cappadone, C.; Farruggia, G.; Malucelli, E.; Iotti, S. The assessment of intracellular magnesium: Different strategies to answer different questions. Magnes. Res. 2020, 33, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Komatsu, H.; Ikeda, T.; Saito, N.; Araki, S.; Citterio, D.; Hisamoto, H.; Kitamura, Y.; Kubota, T.; Nakagawa, J.; et al. Design and Synthesis of Mg2+-Selective Fluoroionophores Based on a Coumarin Derivative and Application for Mg2+ Measurement in a Living Cell. Anal. Chem. 2002, 74, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Iwasawa, N.; Citterio, D.; Suzuki, Y.; Kubota, T.; Tokuno, K.; Kitamura, Y.; Oka, K.; Suzuki, K. Design and Synthesis of Highly Sensitive and Selective Fluorescein-Derived Magnesium Fluorescent Probes and Application to Intracellular 3D Mg2+ Imaging. J. Am. Chem. Soc. 2004, 126, 16353–16360. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yokoyama, K. Development of Functional Fluorescent Molecular Probes for the Detection of Biological Substances. Biosensors 2015, 5, 337–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Q.; Buccella, D. Highly selective, red emitting BODIPY-based fluorescent indicators for intracellular Mg2+ imaging. J. Mater. Chem. B 2018, 6, 7247–7256. [Google Scholar] [CrossRef]
- Gruskos, J.J.; Zhang, G.; Buccella, D. Visualizing Compartmentalized Cellular Mg2+ on Demand with Small-Molecule Fluorescent Sensors. J. Am. Chem. Soc. 2016, 138, 14639–14649. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Shindo, Y.; Hotta, K.; Citterio, D.; Nishiyama, S.; Suzuki, K.; Oka, K. Design and synthesis of a FlAsH-type Mg2+ fluorescent probe for specific protein labeling. J. Am. Chem. Soc. 2014, 136, 2374–2381. [Google Scholar] [CrossRef]
- Farruggia, G.; Iotti, S.; Prodi, L.; Montalti, M.; Zaccheroni, N.; Savage, P.B.; Trapani, V.; Sale, P.; Wolf, F.I. 8-Hydroxyquinoline derivatives as fluorescent sensors for magnesium in living cells. J. Am. Chem. Soc. 2006, 128, 344–350. [Google Scholar] [CrossRef]
- Farruggia, G.; Iotti, S.; Prodi, L.; Zaccheroni, N.; Montalti, M.; Savage, P.B.; Andreani, G.; Trapani, V.; Wolf, F.I. A Simple Spectrofluorometric Assay to Measure Total Intracellular Magnesium by a Hydroxyquinoline Derivative. J. Fluoresc. 2009, 19, 11–19. [Google Scholar] [CrossRef]
- Farruggia, G.; Iotti, S.; Lombardo, M.; Marraccini, C.; Petruzziello, D.; Prodi, L.; Sgarzi, M.; Trombini, C.; Zaccheroni, N. Microwave Assisted Synthesis of a Small Library of SubstitutedN,N′-Bis((8-hydroxy-7-quinolinyl)methyl)-1,10-diaza-18-crown-6 Ethers. J. Org. Chem. 2010, 75, 6275–6278. [Google Scholar] [CrossRef]
- Sargenti, A.; Farruggia, G.; Zaccheroni, N.; Marraccini, C.; Sgarzi, M.; Cappadone, C.; Malucelli, E.; Procopio, A.; Prodi, L.; Lombardo, M.; et al. Synthesis of a highly Mg2+-selective fluorescent probe and its application to quantifying and imaging total intracellular magnesium. Nat. Protoc. 2017, 12, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Sargenti, A.; Farruggia, G.; Malucelli, E.; Cappadone, C.; Merolle, L.; Marraccini, C.; Andreani, G.; Prodi, L.; Zaccheroni, N.; Sgarzi, M.; et al. A novel fluorescent chemosensor allows the assessment of intracellular total magnesium in small samples. Analyst 2014, 139, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Merolle, L.; Sponder, G.; Sargenti, A.; Mastrototaro, L.; Cappadone, C.; Farruggia, G.; Procopio, A.; Malucelli, E.; Parisse, P.; Gianoncelli, A.; et al. Overexpression of the mitochondrial Mg channel MRS2 increases total cellular Mg concentration and influences sensitivity to apoptosis. Metallomics 2018, 10, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Kumar, R.; Singh, A.K.; Mohiyuddin, S.; Gopinath, P. Systematic approach of chromone skeleton for detecting Mg2+, ion: Applications for sustainable cytotoxicity and cell imaging possibilities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 235, 118290. [Google Scholar] [CrossRef] [PubMed]
- Decelle, J.; Veronesi, G.; Gallet, B.; Stryhanyuk, H.; Benettoni, P.; Schmidt, M.; Tucoulou, R.; Passarelli, M.; Bohic, S.; Clode, P.; et al. Subcellular Chemical Imaging: New Avenues in Cell Biology. Trends Cell Biol. 2020, 30, 173–188. [Google Scholar] [CrossRef]
- De Santis, S.; Sotgiu, G.; Crescenzi, A.; Taffon, C.; Felici, A.C.; Orsini, M. On the chemical composition of psammoma bodies microcalcifications in thyroid cancer tissues. J. Pharm. Biomed. Anal. 2020, 190, 113534. [Google Scholar] [CrossRef] [PubMed]
- Picone, G.; Cappadone, C.; Pasini, A.; Lovecchio, J.; Cortesi, M.; Farruggia, G.; Lombardo, M.; Gianoncelli, A.; Mancini, L.; Ralf, H.M.; et al. Analysis of Intracellular Magnesium and Mineral Depositions during Osteogenic Commitment of 3D Cultured Saos2 Cells. Int. J. Mol. Sci. 2020, 21, 2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zghoul, N.; Alam-Eldin, N.; Mak, I.T.; Silver, B.; Weglicki, W.B. Hypomagnesemia in diabetes patients: Comparison of serum and intracellular measurement of responses to magnesium supplementation and its role in inflammation. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Becker, R.A.; Cluff, K.; Duraisamy, N.; Casale, G.P.; Pipinos, I.I. Analysis of ischemic muscle in patients with peripheral artery disease using X-ray spectroscopy. J. Surg. Res. 2017, 220, 79–87. [Google Scholar] [CrossRef]
- Malucelli, E.; Iotti, S.; Gianoncelli, A.; Fratini, M.; Merolle, L.; Notargiacomo, A.; Marraccini, C.; Sargenti, A.; Cappadone, C.; Farruggia, G.; et al. Quantitative Chemical Imaging of the Intracellular Spatial Distribution of Fundamental Elements and Light Metals in Single Cells. Anal. Chem. 2014, 86, 5108–5115. [Google Scholar] [CrossRef]
- Hughes, D. Chapter 49 Cultural Influences on Medical Knowledge. In Handbook of the Philosophy of Medicine; Schramme, T., Edwards, S., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 1–18. [Google Scholar]
- Pradelli, L.; Ghetti, G. A general model for the estimation of societal costs of lost production and informal care in Italy. Farmeconomia. Health Econ. Ther. Pathw. 2017, 18, A365. [Google Scholar]
- Yang, W.; Dall, T.M.; Beronjia, K.; Lin, J.; Semilla, A.P.; Chakrabarti, R.; Hogan, P.F.; Petersen, M.P. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar]
- Deuschl, G.; Beghi, E.; Fazekas, F.; Varga, T.; Christoforidi, K.A.; Sipido, E.; Bassetti, C.L.; Vos, T.; Feigin, V.L. The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health 2020, 5, e551–e567. [Google Scholar] [CrossRef]
- Cruz, K.J.C.; De Oliveira, A.R.S.; Pinto, D.P.; Morais, J.B.S.; Lima, F.D.S.; Colli, C.; Torres-Leal, F.L.; Marreiro, D.D.N. Influence of Magnesium on Insulin Resistance in Obese Women. Biol. Trace Element Res. 2014, 160, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Günther, T. The biochemical function of Mg2+ in insulin secretion, insulin signal transduction and insulin resistance. Magnes. Res. 2010, 23, 5–18. [Google Scholar] [CrossRef]
- Castellanos-Gutiérrez, A.; Sánchez-Pimienta, T.G.; Carriquiry, A.; Da Costa, T.H.M.; Ariza, A.C. Higher dietary magnesium intake is associated with lower body mass index, waist circumference and serum glucose in Mexican adults. Nutr. J. 2018, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morán, M.; Mendía, L.E.S.; Galván, G.Z.; Guerrero-Romero, F. The role of magnesium in type 2 diabetes: A brief based-clinical review. Magnes. Res. 2011, 24, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, B.F.; Clegg, D.J. Electrolyte and Acid–Base Disturbances in Patients with Diabetes Mellitus. N. Engl. J. Med. 2015, 373, 548–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, S.C.; Wolk, A. Magnesium intake and risk of type 2 diabetes: A meta-analysis. J. Intern. Med. 2007, 262, 208–214. [Google Scholar] [CrossRef]
- Schulze, M.B.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Fiber and magnesium intake and incidence of type 2 diabetes: A prospective study and meta-analysis. Arch. Intern. Med. 2007, 167, 956–965. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.Y.; Xun, P.; He, K.; Qin, L.Q. Magnesium intake and risk of type 2 diabetes meta-analysis of prospective cohort studies. Diabetes Care 2011, 34, 2116–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hruby, A.; Meigs, J.B.; O’Donnell, C.J.; Jacques, P.F.; McKeown, N.M. Higher Magnesium Intake Reduces Risk of Impaired Glucose and Insulin Metabolism and Progression From Prediabetes to Diabetes in Middle-Aged Americans. Diabetes Care 2013, 37, 419–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerreroromero, F.; Simentalmendia, L.E.; Hernández-Ronquillo, G.; Rodriguezmoran, M. Oral magnesium supplementation improves glycaemic status in subjects with prediabetes and hypomagnesaemia: A double-blind placebo-controlled randomized trial. Diabetes Metab. 2015, 41, 202–207. [Google Scholar] [CrossRef]
- Zhao, B.; Deng, H.; Li, B.; Chen, L.; Zou, F.; Hu, L.; Wei, Y.; Zhang, W. Association of magnesium consumption with type 2 diabetes and glucose metabolism: A systematic review and pooled study with trial sequential analysis. Diabetes Metab. Res. Rev. 2020, 36, e3243. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Huang, Y.-L. Chromium, zinc and magnesium status in type 1 diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 588–592. [Google Scholar] [CrossRef]
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Abraham, G.E.; Grewal, H. A total dietary program emphasizing magnesium instead of calcium. Effect on the mineral density of calcaneous bone in postmenopausal women on hormonal therapy. J. Reprod. Med. 1990, 35, 503–507. [Google Scholar]
- Stendig-Lindberg, G.; Tepper, R.; Leichter, I. Trabecular bone density in a two year controlled trial of peroral magnesium in osteoporosis. Magnes. Res. 1993, 6, 155–163. [Google Scholar]
- Aydın, H.; Deyneli, O.; Yavuz, D.; Gozu, H.; Mutlu, N.; Kaygusuz, I.; Akalın, S.; Kaygusuz, I. Short-Term Oral Magnesium Supplementation Suppresses Bone Turnover in Postmenopausal Osteoporotic Women. Biol. Trace Element Res. 2009, 133, 136–143. [Google Scholar] [CrossRef]
- Tucker, K.L.; Hannan, M.T.; Chen, H.; Cupples, L.A.; Wilson, P.W.; Kiel, D.P. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am. J. Clin. Nutr. 1999, 69, 727–736. [Google Scholar] [CrossRef]
- Mederle, O.A.; Balas, M.; Ioanoviciu, S.D.; Gurban, C.-V.; Tudor, A.; Borza, C. Correlations between bone turnover markers, serum magnesium and bone mass density in postmenopausal osteoporosis. Clin. Interv. Aging 2018, 13, 1383–1389. [Google Scholar] [CrossRef] [Green Version]
- Farsinejad-Marj, M.; Saneei, P.; Esmaillzadeh, A. Dietary magnesium intake, bone mineral density and risk of fracture: A systematic review and meta-analysis. Osteoporos. Int. 2016, 27, 1389–1399. [Google Scholar] [CrossRef]
- Orchard, T.S.; Larson, J.C.; Alghothani, N.; Bout-Tabaku, S.; Cauley, J.A.; Chen, Z.; Lacroix, A.Z.; Wactawski-Wende, J.; Jackson, R.D. Magnesium intake, bone mineral density, and fractures: Results from the Women’s Health Initiative Observational Study. Am. J. Clin. Nutr. 2014, 99, 926–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronese, N.; Stubbs, B.; Solmi, M.; Noale, M.; Vaona, A.; Demurtas, J.; Maggi, S. Dietary magnesium intake and fracture risk: Data from a large prospective study. Br. J. Nutr. 2017, 117, 1570–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, A.A.; Skinner, J.; Hickson, M. Dietary Magnesium May Be Protective for Aging of Bone and Skeletal Muscle in Middle and Younger Older Age Men and Women: Cross-Sectional Findings from the UK Biobank Cohort. Nutrients 2017, 9, 1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Cunha, M.M.L.; Trepout, S.; Messaoudi, C.; Wu, T.-D.; Ortega, R.; Guerquin-Kern, J.-L.; Marco, S. Overview of chemical imaging methods to address biological questions. Micron 2016, 84, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Rosique-Esteban, N.; Guasch-Ferré, M.; Hernández-Alonso, P.; Salas-Salvadó, J. Dietary Magnesium and Cardiovascular Disease: A Review with Emphasis in Epidemiological Studies. Nutrients 2018, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Rosanoff, A. Magnesium and hypertension. Clin. Calcium 2005, 15, 255–260. [Google Scholar]
- Touyz, R.M.; Milne, F.J.; Reinach, S.G. Intracellular Mg2+, Ca2+, Na2+ and K+ in platelets and erythrocytes of essential hypertension patients: Relation to blood pressure. Clin. Exp. Hypertens. Part A Theory Pract. 1992, 14, 1189–1209. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, H.O.; Nicolson, D.; Campbell, F.; Cook, J.V.; Beyer, F.R.; Ford, G.A.; Mason, J. Magnesium supplementation for the management of primary hypertension in adults. Cochrane Database Syst. Rev. 2006, 2006, CD004640. [Google Scholar] [CrossRef]
- Kass, L.S.; Weekes, J.; Carpenter, L.W. Effect of magnesium supplementation on blood pressure: A meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 411–418. [Google Scholar] [CrossRef]
- Fang, X.; Han, H.; Li, M.; Liang, C.; Fan, Z.; Aaseth, J.; He, J.; Montgomery, S.; Cao, Y. Dose-Response Relationship between Dietary Magnesium Intake and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Regression Analysis of Prospective Cohort Studies. Nutrients 2016, 8, 739. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.T.; Xun, P.; Song, Y.; Rosanoff, A.; Shechter, M.; He, K. The effect of magnesium supplementation on blood pressure in individuals with insulin resistance, prediabetes, or noncommunicable chronic diseases: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 106, 921–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Gobbo, L.C.; Imamura, F.; Wu, J.H.Y.; Otto, M.C.D.O.; Chiuve, S.E.; Mozaffarian, D. Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2013, 98, 160–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepura, O.B.; Martynow, A.I. Magnesium orotate in severe congestive heart failure (MACH). Int. J. Cardiol. 2009, 131, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Vierling, W.; Liebscher, D.H.; Micke, O.; Von Ehrlich, B.; Kisters, K. Magnesium deficiency and therapy in cardiac arrhythmias: Recommendations of the German Society for Magnesium Research. DMW—Dtsch. Med. Wochenschr. 2013, 138, 1165–1171. [Google Scholar]
- Drew, B.J.; Ackerman, M.J.; Funk, M.; Gibler, W.B.; Kligfield, P.; Menon, V.; Philippides, G.J.; Roden, D.M.; Zareba, W. Prevention of Torsade de Pointes in Hospital Settings. Circulationa 2010, 121, 1047–1060. [Google Scholar] [CrossRef]
- Ferrè, S.; Baldoli, E.; Leidi, M.; Maier, J.A. Magnesium deficiency promotes a pro-atherogenic phenotype in cultured human endothelial cells via activation of NFkB. Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 952–958. [Google Scholar] [CrossRef]
- Peacock, J.M.; Ohira, T.; Post, W.; Sotoodehnia, N.; Rosamond, W.; Folsom, A.R. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2010, 160, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Joosten, M.M.; Gansevoort, R.T.; Mukamal, K.J.; Van Der Harst, P.; Geleijnse, J.M.; Feskens, E.J.M.; Navis, G.; Bakker, S.J.L.; The PREVEND Study Group. Urinary and plasma magnesium and risk of ischemic heart disease. Am. J. Clin. Nutr. 2013, 97, 1299–1306. [Google Scholar] [CrossRef] [Green Version]
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.M.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018, 124, 2785–2800. [Google Scholar] [CrossRef] [Green Version]
- Workeneh, B.T.; Uppal, N.N.; Jhaveri, K.D.; Rondon-Berrios, H. Hypomagnesemia in the Cancer Patient. Kidney360 2021, 2, 154–166. [Google Scholar] [CrossRef]
- Gray, A.; Dang, B.N.; Moore, T.B.; Clemens, R.; Pressman, P. A review of nutrition and dietary interventions in oncology. SAGE Open Med. 2020, 8, 2050312120926877. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-C.; Wu, C.-F.; Chen, C.-W.; Shi, C.-S.; Huang, W.-S.; Kuan, F.-C. Hypomagnesemia and clinical benefits of anti-EGFR monoclonal antibodies in wild-type KRAS metastatic colorectal cancer: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaszczyk, U.; Duda-Chodak, A. Magnesium: Its role in nutrition and carcinogenesis. Rocz. Państwowego Zakładu Hig. 2013, 64, 3. [Google Scholar]
- Tao, M.; Dai, Q.; Millen, A.E.; Nie, J.; Edge, S.B.; Trevisan, M.; Shields, P.G.; Freudenheim, J. Abstract 884: Associations of intakes of magnesium and calcium and survival among women with breast cancer: Results from Western New York Exposures and Breast Cancer (WEB) Study. Epidemiology 2015, 75, 884. [Google Scholar] [CrossRef]
- Huang, W.-Q.; Long, W.-Q.; Mo, X.-F.; Zhang, N.-Q.; Luo, H.; Lin, F.-Y.; Huang, J.; Zhang, C.-X. Direct and indirect associations between dietary magnesium intake and breast cancer risk. Sci. Rep. 2019, 9, 5764. [Google Scholar] [CrossRef]
- Liu, M.; Yang, H.; Mao, Y. Magnesium and liver disease. Ann. Transl. Med. 2019, 7, 578. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zou, Q.; Liu, L.; Zhu, X.; Jia, Q.; Wang, L.; Yan, R. Inhibitory effect of magnesium cantharidate on human hepatoma SMMC-7721 cell proliferation by blocking MAPK signaling pathway. Chin. J. Cell. Mol. Immunol. 2017, 33, 347–351. [Google Scholar]
- Liu, Y.; Xu, Y.; Ma, H.; Wang, B.; Xu, L.; Zhang, H.; Song, X.; Gao, L.; Liang, X.; Ma, C. Hepatitis B virus X protein amplifies TGF-ß promotion on HCC motility through down-regulating PPM1a. Oncotarget 2016, 7, 33125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesselink, E.; Kok, D.E.; Bours, M.J.L.; De Wilt, J.H.W.; Van Baar, H.; Van Zutphen, M.; Geijsen, A.M.J.R.; Keulen, E.T.P.; Hansson, B.M.E.; Ouweland, J.V.D.; et al. Vitamin D, magnesium, calcium, and their interaction in relation to colorectal cancer recurrence and all-cause mortality. Am. J. Clin. Nutr. 2020, 111, 1007–1017. [Google Scholar] [CrossRef] [Green Version]
- Dai, Q.; Shu, X.-O.; Deng, X.; Xiang, Y.-B.; Li, H.; Yang, G.; Shrubsole, M.J.; Ji, B.; Cai, H.; Chow, W.-H.; et al. Modifying effect of calcium/magnesium intake ratio and mortality: A population-based cohort study. BMJ Open 2013, 3, e002111. [Google Scholar] [CrossRef] [Green Version]
- Wark, P.A.; Lau, R.; Norat, T.; Kampman, E. Magnesium intake and colorectal tumor risk: A case-control study and meta-analysis. Am. J. Clin. Nutr. 2012, 96, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Selvaraj, S.; Varma, A.; Derry, S.; Sahmoun, A.E.; Singh, B.B. Increase in Serum Ca2+/Mg2+ Ratio Promotes Proliferation of Prostate Cancer Cells by Activating TRPM7 Channels. J. Biol. Chem. 2013, 288, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steck, S.E.; Omofuma, O.O.; Su, L.J.; Maise, A.A.; Woloszynska-Read, A.; Johnson, C.S.; Zhang, H.; Bensen, J.T.; Fontham, E.T.H.; Mohler, J.L.; et al. Calcium, magnesium, and whole-milk intakes and high-aggressive prostate cancer in the North Carolina–Louisiana Prostate Cancer Project (PCaP). Am. J. Clin. Nutr. 2018, 107, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Sahmoun, A.E.; Singh, B.B. Does a higher ratio of serum calcium to magnesium increase the risk for postmenopausal breast cancer? Med. Hypotheses 2010, 75, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Chatterjee, P.K.; Madankumar, S.; Mehdi, S.F.; Xue, X.; Metz, C.N. Magnesium deficiency with high calcium-to-magnesium ratio promotes a metastatic phenotype in the CT26 colon cancer cell line. Magnes. Res. 2020, 33, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Sasazuki, S.; Inoue, M.; Iwasaki, M.; Sawada, N.; Takachi, R.; Tsugane, S.; Members of the JPHC Study Group. High Dietary Intake of Magnesium May Decrease Risk of Colorectal Cancer in Japanese Men. J. Nutr. 2010, 140, 779–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folsom, A.R.; Hong, C.-P. Magnesium Intake and Reduced Risk of Colon Cancer in a Prospective Study of Women. Am. J. Epidemiol. 2005, 163, 232–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, P.A.V.D.; Smits, K.M.; Goldbohm, R.A.; Weijenberg, M.P. Magnesium intake and colorectal cancer risk in the Netherlands Cohort Study. Br. J. Cancer 2007, 96, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Mahabir, S.; Wei, Q.; Barrera, S.L.; Dong, Y.Q.; Etzel, C.J.; Spitz, M.R.; Forman, M.R. Dietary magnesium and DNA repair capacity as risk factors for lung cancer. Carcinogenesis 2008, 29, 949–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, R.J.; Robinson, D.P.; Stolzenberg-Solomon, R.Z.; Bamlet, W.R.; De Andrade, M.; Oberg, A.L.; Rabe, K.G.; Anderson, K.E.; Olson, J.E.; Sinha, R.; et al. Nutrients from Fruit and Vegetable Consumption Reduce the Risk of Pancreatic Cancer. J. Gastrointest. Cancer 2012, 44, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.; Xun, P.; Yokota, K.; White, E.; He, K. Magnesium intake and incidence of pancreatic cancer: The VITamins and Lifestyle study. Br. J. Cancer 2015, 113, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Ravell, J.C.; Chauvin, S.D.; He, T.; Lenardo, M. An Update on XMEN Disease. J. Clin. Immunol. 2020, 40, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Afridi, H.I.; Kazi, T.G.; Talpur, F.N. Correlation of Calcium and Magnesium Levels in the Biological Samples of Different Types of Acute Leukemia Children. Biol. Trace Element Res. 2018, 186, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Slahin, G.; Ertem, U.; Duru, F.; Birgen, D.; Yuuksek, N. High Prevelance of Chronic Magnesium Deficiency in T Cell Lymphoblastic Leukemia and Chronic Zinc Deficiency in Children with Acute Lymphoblastic Leukemia and Malignant Lymphoma. Leuk. Lymphoma 2000, 39, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Sikora, P.; Borzȩcka, H.; Kołła̧taj, B.; Majewski, M.; Wieczorkiewicz-Płaza, A.; Zaja̧czkowska, M. The diagnosis of familial hypomagnesemia with hypercalciuria and nephrocalcinosis in a girl with acute lymphoblastic leukemia—Case report. Pol. Merkur. Lek. 2006, 118, 430–432. [Google Scholar]
- Canbolat, O.; Kavutcu, M.; Durak, I. Magnesium contents of leukemic lymphocytes. BioMetals 1994, 7, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.A.; Halton, J.M.; Bradley, C.; Wu, B.; Barr, R.D. Bone and mineral abnormalities in childhood acute lymphoblastic leukemia: Influence of disease, drugs and nutrition. Int. J. Cancer 1998, 8, 35–39. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium in Disease Prevention and Overall Health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef] [PubMed]
- Pickering, G.; Mazur, A.; Trousselard, M.; Bienkowski, P.; Yaltsewa, N.; Amessou, M.; Noah, L.; Pouteau, E. Magnesium Status and Stress: The Vicious Circle Concept Revisited. Nutrients 2020, 12, 3672. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Charles, A. Glutamate and Its Receptors as Therapeutic Targets for Migraine. Neurotherapeutics 2018, 15, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linde, M.; Gustavsson, A.; Stovner, L.J.; Steiner, T.J.; Barré, J.; Katsarava, Z.; Lainez, J.M.; Lampl, C.; Lantéri-Minet, M.; Rastenyte, D.; et al. The cost of headache disorders in Europe: The Eurolight project. Eur. J. Neurol. 2011, 19, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Nattagh-Eshtivani, E.; Sani, M.A.; Dahri, M.; Ghalichi, F.; Ghavami, A.; Arjang, P.; Tarighat-Esfanjani, A. The role of nutrients in the pathogenesis and treatment of migraine headaches: Review. Biomed. Pharmacother. 2018, 102, 317–325. [Google Scholar] [CrossRef]
- Lodi, R.; Iotti, S.; Cortelli, P.; Pierangeli, G.; Cevoli, S.; Clementi, V.; Soriani, S.; Montagna, P.; Barbiroli, B. Deficient energy metabolism is associated with low free magnesium in the brains of patients with migraine and cluster headache. Brain Res. Bull. 2001, 54, 437–441. [Google Scholar] [CrossRef]
- Choi, H.; Parmar, N. The use of intravenous magnesium sulphate for acute migraine: Meta-analysis of randomized controlled trials. Eur. J. Emerg. Med. 2014, 21, 2–9. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Yeh, T.-H.; Huang, Y.-C.; Chen, P.-Y. Effects of Intravenous and Oral Magnesium on Reducing Migraine: A Meta-analysis of Randomized Controlled Trials. Pain Physician 2016, 19, E97–E112. [Google Scholar]
- Serefko, A.; Szopa, A.; Wlaź, P.; Nowak, G.; Radziwoń-Zaleska, M.; Skalski, M.; Poleszak, E. Magnesium in depression. Pharmacol. Rep. 2013, 65, 547–554. [Google Scholar] [CrossRef]
- Barragán-Rodríguez, L.; Rodríguez-Morán, M.; Guerrero-Romero, F. Efficacy and safety of oral magnesium supplementation in the treatment of depression in the elderly with type 2 diabetes: A randomized, equivalent trial. Magnes. Res. 2008, 21, 218–223. [Google Scholar]
- Jacka, F.N.; Maes, M.; Pasco, J.A.; Williams, L.J.; Berk, M. Nutrient intakes and the common mental disorders in women. J. Affect. Disord. 2012, 141, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, E.K.; Littenberg, B. Magnesium intake and depression in adults. J. Am. Board Fam. Med. 2015, 28, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eby, G.A.; Eby, K.L. Rapid recovery from major depression using magnesium treatment. Med. Hypotheses 2006, 67, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Di Martino, M.; Pellegrino, P. Magnesium in the gynaecological practice: A literature review. Magnes. Res. 2017, 30, 1–7. [Google Scholar] [PubMed]
- Tarleton, E.K.; Littenberg, B.; MacLean, C.D.; Kennedy, A.G.; Daley, C. Role of magnesium supplementation in the treatment of depression: A randomized clinical trial. PLoS ONE 2017, 12, e0180067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Lv, J.; Wang, W.; Zhang, D. Dietary magnesium and calcium intake and risk of depression in the general population: A meta-analysis. Aust. N. Z. J. Psychiatry 2017, 51, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Barkerhaliski, M.L.; White, H.S. Glutamatergic Mechanisms Associated with Seizures and Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022863. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.B.; Prasad, C.; Kobrzynski, M.; Campbell, C.; Filler, G. Seizures Related to Hypomagnesemia: A Case Series and Review of the Literature. Child Neurol. Open 2016, 3, 2329048X16674834. [Google Scholar] [CrossRef] [Green Version]
- Maresova, P.; Klimova, B.; Novotny, M.; Kuca, K. Alzheimer’s and Parkinson’s Diseases: Expected Economic Impact on Europe-A Call for a Uniform European Strategy. J. Alzheimer’s Dis. 2016, 54, 1123–1133. [Google Scholar] [CrossRef]
- Zádori, D.; Veres, G.; Szalárdy, L.; Klivényi, P.; Vécsei, L. Alzheimer’s Disease: Recent Concepts on the Relation of Mitochondrial Disturbances, Excitotoxicity, Neuroinflammation, and Kynurenines. J. Alzheimer’s Dis. 2018, 62, 523–547. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Wheatley, E.G.; Villeda, S.A. Mechanisms of Hippocampal Aging and the Potential for Rejuvenation. Annu. Rev. Neurosci. 2017, 40, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-P.; Li, L.; Bao, J.; Wang, Z.-H.; Zeng, J.; Liu, E.-J.; Li, X.-G.; Huang, R.-X.; Gao, D.; Li, M.-Z.; et al. Magnesium Protects Cognitive Functions and Synaptic Plasticity in Streptozotocin-Induced Sporadic Alzheimer’s Model. PLoS ONE 2014, 9, e108645. [Google Scholar] [CrossRef] [Green Version]
- Slutsky, I.; Abumaria, N.; Wu, L.-J.; Huang, C.; Zhang, L.; Li, B.; Zhao, X.; Govindarajan, A.; Zhao, M.-G.; Zhuo, M.; et al. Enhancement of Learning and Memory by Elevating Brain Magnesium. Neuron 2010, 65, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Veronese, N.; Zurlo, A.; Solmi, M.; Luchini, C.; Trevisan, C.; Bano, G.; Manzato, E.; Sergi, G.; Rylander, R. Magnesium Status in Alzheimer’s Disease: A Systematic Review. Am. J. Alzheimers. Dis. Other Demen. 2016, 31, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; You, J.; Su, Y.; Wang, Q. The Effect of Magnesium Deficiency on Neurological Disorders: A Narrative Review Article. Iran. J. Public Health 2019, 48, 379–387. [Google Scholar] [CrossRef]
- Barbiroli, B.; Martinelli, P.; Patuelli, A.; Lodi, R.; Iotti, S.; Cortelli, P.; Montagna, P. Phosphorus magnetic resonance spectroscopy in multiple system atrophy and Parkinson’s disease. Mov. Disord. 1999, 14, 430–435. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Fukushima, W.; Sasaki, S.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Dietary intake of metals and risk of Parkinson’s disease: A case-control study in Japan. J. Neurol. Sci. 2011, 306, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Hemamy, M.; Heidari-Beni, M.; Askari, G.; Karahmadi, M.; Maracy, M. Effect of Vitamin D and Magnesium Supplementation on Behavior Problems in Children with Attention-Deficit Hyperactivity Disorder. Int. J. Prev. Med. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Archana, E.; Pai, P.; Prabhu, B.K.; Shenoy, R.P.; Prabhu, K.; Rao, A. Altered Biochemical Parameters in Saliva of Pediatric Attention Deficit Hyperactivity Disorder. Neurochem. Res. 2012, 37, 330–334. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; El-Mazary, A.-A.M.; Maher, R.M.; Saber, M.M. Zinc, ferritin, magnesium and copper in a group of Egyptian children with attention deficit hyperactivity disorder. Ital. J. Pediatr. 2011, 37, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousain-Bosc, M.; Roche, M.; Polge, A.; Pradal-Prat, D.; Rapin, J.; Bali, J.P. Improvement of neurobehavioral disorders in children supplemented with magnesium-vitamin B6: I. Attention deficit hyperactivity disorders. Magnes. Res. 2006, 19, 46–52. [Google Scholar] [PubMed]
- El Baza, F.; AlShahawi, H.A.; Zahra, S.; AbdelHakim, R.A. Magnesium supplementation in children with attention deficit hyperactivity disorder. Egypt. J. Med. Hum. Genet. 2016, 17, 63–70. [Google Scholar] [CrossRef] [Green Version]
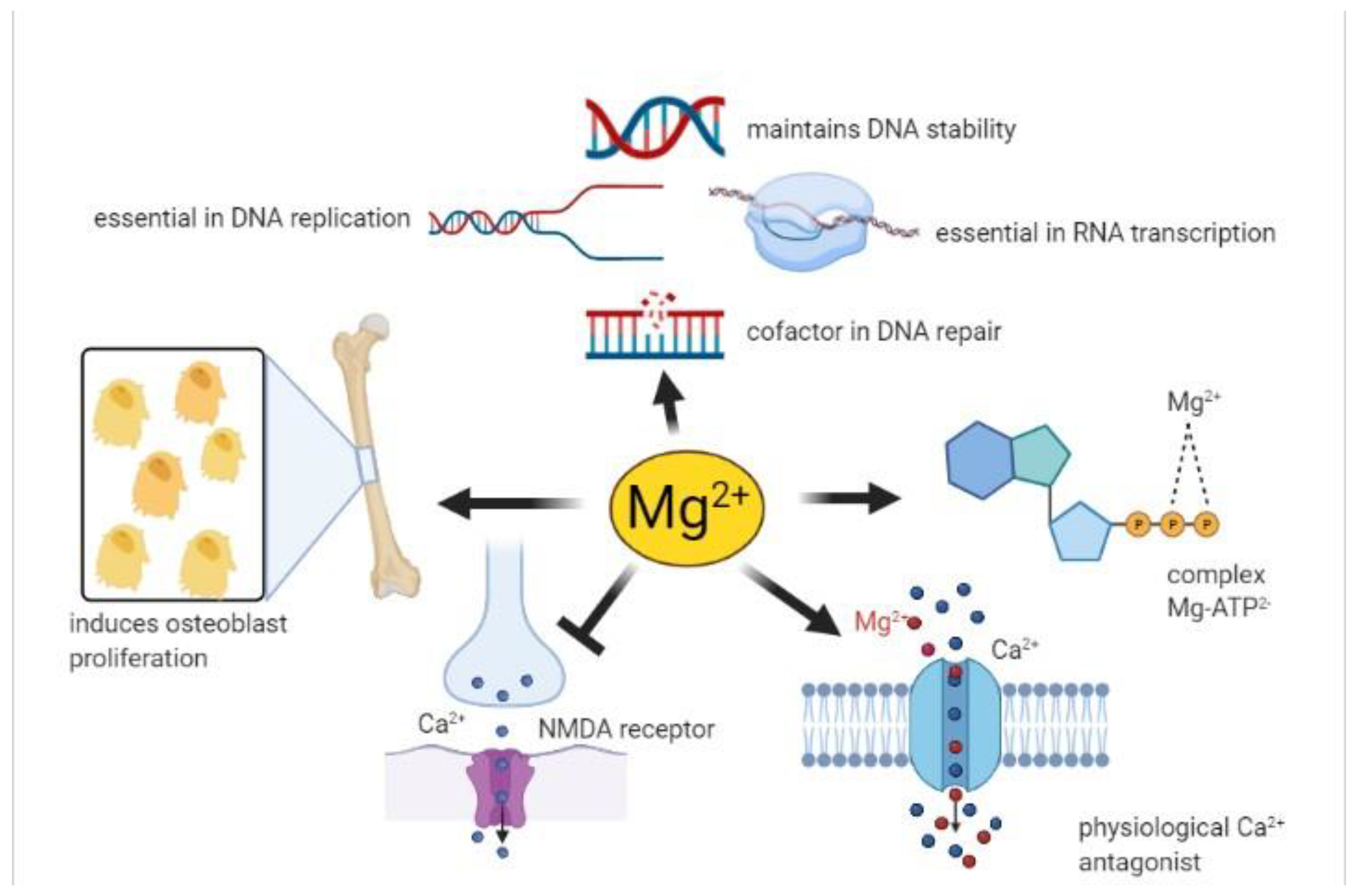
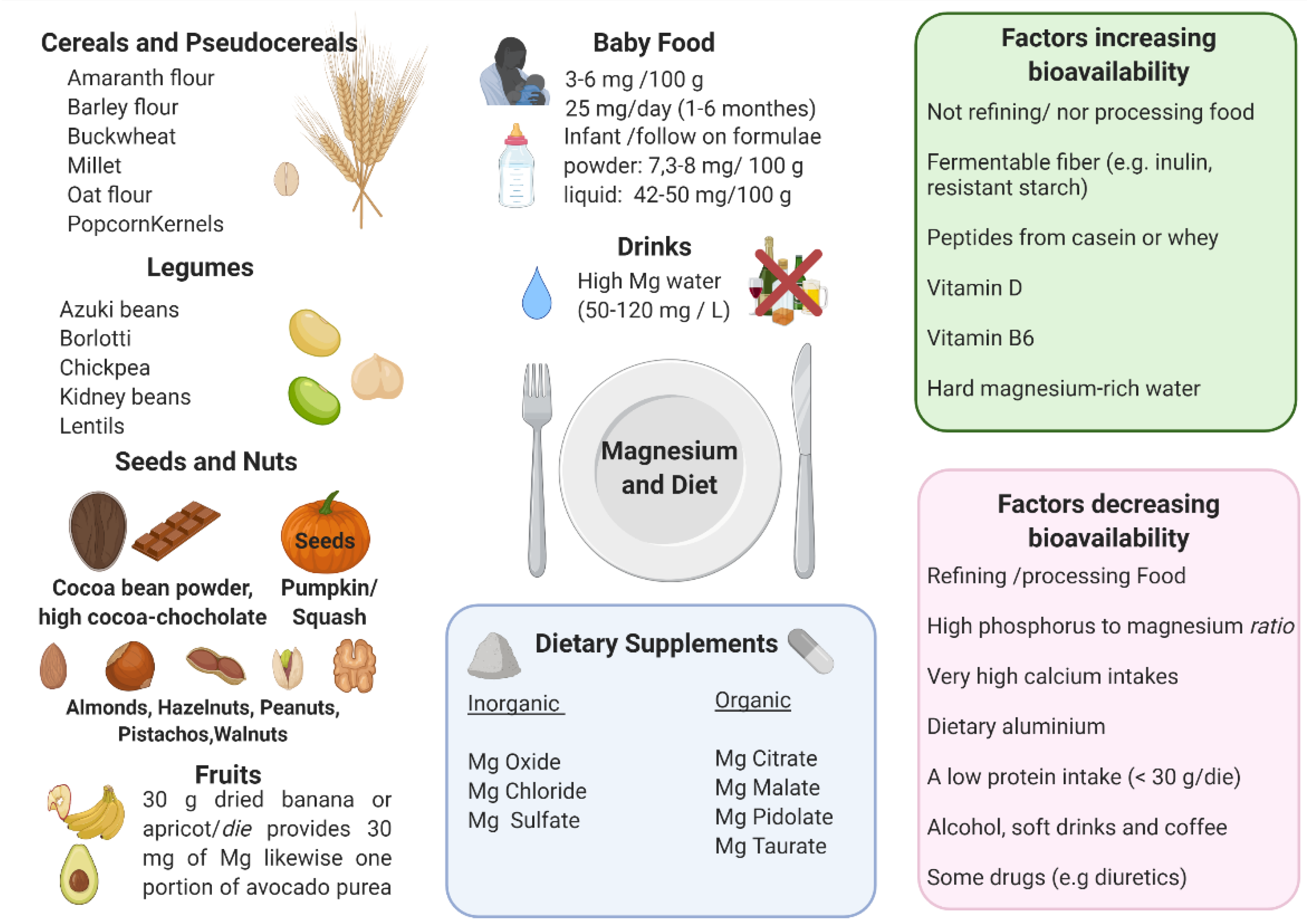
| LOCALIZATION | ENZYME | Mg-ATP2− | FREE Mg2+ | REF |
|---|---|---|---|---|
| Cytosol: glycolytic pathway | Hexokinase | - | [53] | |
| Phosphofructokinase | - | |||
| Phosphoglycerate kinase | - | |||
| Pyruvate kinase | - | |||
| Aldolase | - | |||
| Enolase | - | |||
| Mitochondrion | Pyruvate dehydrogenase phosphatase | - | [65] | |
| Isocitrate dehydrogenase | - | [55] | ||
| α-Ketoglutarate dehydrogenase | - | [56] | ||
| Fo/F1-ATPase | - | [58] | ||
| Muscle cytosol/Heart mitochondrion | Creatine kinase | - | [53] | |
| Liver, cytosol | Phosphoenolpyruvate carboxykinase | - | [40] | |
| Glucose-6-phosphatase | - | |||
| β-subunit of the insulin receptor | Receptor tyrosine kinase activity | - | [62,63] |
| Life Stage | PRI (mg) | AR (mg) | UL * (mg) | RDA-DRI (mg) | DRV-AI (mg) | LARN (mg) |
|---|---|---|---|---|---|---|
| Birth to 6 months | - | Nd | 30 | |||
| Infants 7–12 months | 80 | Nd | Nd | 75 | 80 | 80 |
| Children 1–3 years | 80 | 65 | 250 | 80 | 170 | 80 |
| Children 4–6 years | 100 | 85 | 250 | 130 | 230 | 100 |
| Children 7–10 years | 150 | 130 | 250 | 240 | 230 | 150 |
| Teen boys 11–18 years | 240 | 170–200 | 250 | 410 | 300 | 240 |
| Teen girls 11–18 years | 240 | 170–200 | 250 | 360 | 250 | 240 |
| Men | 240 | 170 | 250 | 400–420 | 350 | 240 |
| Women | 240 | 170 | 250 | 310–320 | 300 | 240 |
| Pregnant | 240 | 170 | 250 | 350–400 | 300 | 240 |
| Breastfeeding | 240 | 170 | 250 | 310–360 | 300 | 240 |
| Food | EFSA (mg/100 g) | CREA (mg/100 g) | USDA (mg/Measure) | Measure and Weight |
|---|---|---|---|---|
| Wheat/Cereal bran | 451 | 550 | 354 | 1 cup, 50 g |
| Pumpkin and squash seed, dried | 429 | 592 | 764 | 1 cup, 46 g |
| Cocoa powder | 545 | 499 | 29 | 1 ts 1, 6 g |
| Sunflower seeds dried | 346 | n.a 2 | 173 | 1 cup, 130 g |
| Wheat germ | 276 | 255 | 275 | 1 cup, 115 g |
| Amaranth flour | 266 | 266 | 476 | 1 cup, 193 g |
| Cashews dried | 258 | 260 | 352 | 1 cup, 137 g |
| Sweet, dried almonds | 251 | 264 | 386 | 1 cup, 143 g |
| Peanuts, roasted | 229 | 175 | 260 | 1 cup, 146 g |
| Quinoa | n.a | 189 | 335 | 1 cup, 170 g |
| Pecans | 168 | 121 | 132 | 1 cup, 109 g |
| Hazelnuts, dried | 163 | 163 | 187 | 1 cup, 187 g |
| Beans, dried | 158 | 170 | 258 | 1 cup, 184 g |
| Walnuts, dried | 150 | 158 | 185 | 1 cup, 169 g |
| Chickpeas, dried | 150 | 131 | 158 | 1 cup, 100 g |
| Pistachios, dried | 147 | 160 | 149 | 1 cup, 123 g |
| Millet, shelled | 136 | 160 | 228 | 1 cup, 200 g |
| Wheat flour, hard | 136 | 120 | 164 | 1 cup, 120 g |
| Oat flour | 131 | n.a 2 | 150 | 1 cup, 169 g |
| Buckwheat flour, whole-groats | 121 | 231 | 301 | 1 cup, 120 g |
| Macadamia | 115 | 120 | 156 | 1 cup, 132 g |
| Wholemeal pasta | 111 | 101 | 95 | 1 cup, 90 g |
| Lentils, dried | 101 | 83.1 | 113 | 1 cup, 100 g |
| Magnesium Deficiency | Magnesium Toxicity |
|---|---|
| Hypocalcemia, hypokalemia | Diarrhea, nausea and vomiting |
| Osteoporosis | Muscle weakness |
| Cardiovascular disorders | Low blood pressure |
| Neurological disorders | Loss of deep tendon reflexes |
| Diabetes | Sinoatrial or atrioventricular node blocks |
| Tumors | Respiratory paralysis |
| Covid-19 | Cardiac arrest |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. https://doi.org/10.3390/nu13041136
Fiorentini D, Cappadone C, Farruggia G, Prata C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients. 2021; 13(4):1136. https://doi.org/10.3390/nu13041136
Chicago/Turabian StyleFiorentini, Diana, Concettina Cappadone, Giovanna Farruggia, and Cecilia Prata. 2021. "Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency" Nutrients 13, no. 4: 1136. https://doi.org/10.3390/nu13041136
APA StyleFiorentini, D., Cappadone, C., Farruggia, G., & Prata, C. (2021). Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients, 13(4), 1136. https://doi.org/10.3390/nu13041136