Shikonin Derivatives from Onsoma visianii Decrease Expression of Phosphorylated STAT3 in Leukemia Cells and Exert Antitumor Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Line
2.3. MTT Assay
2.4. Assessment of Cell Death by Flow Cytometry
2.5. Cell Cycle Analysis
2.6. Flow Cytometric Analysis
2.7. Immunofluorescence Staining
2.8. RNA Extraction and Real-Time qRT-PCR
2.9. In Vivo Testing of Antitumor Activity
2.10. Statistical Analysis
3. Results
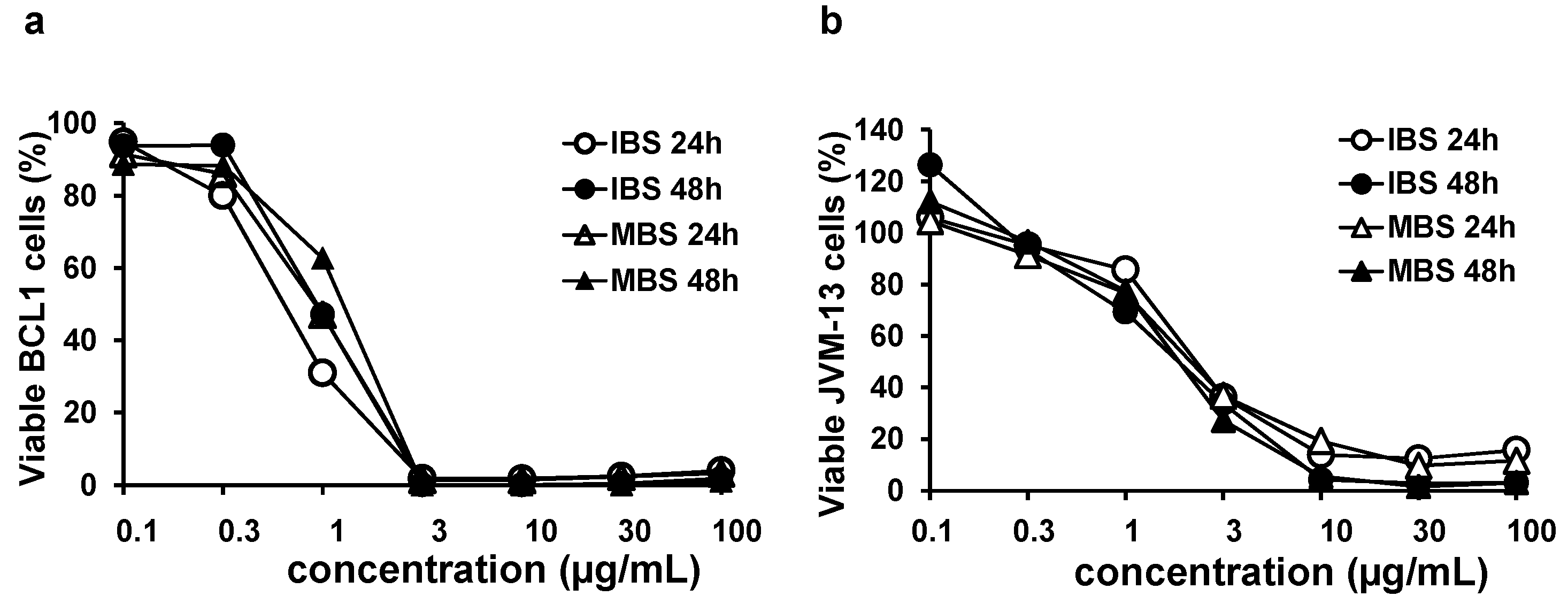
3.1. Shikonin Derivates Significantly Reduce the Viability of BCL1 and JVM-13 Cells
3.2. IBS and MBS Induce Apoptosis of BCL1 and JVM-13 Cells
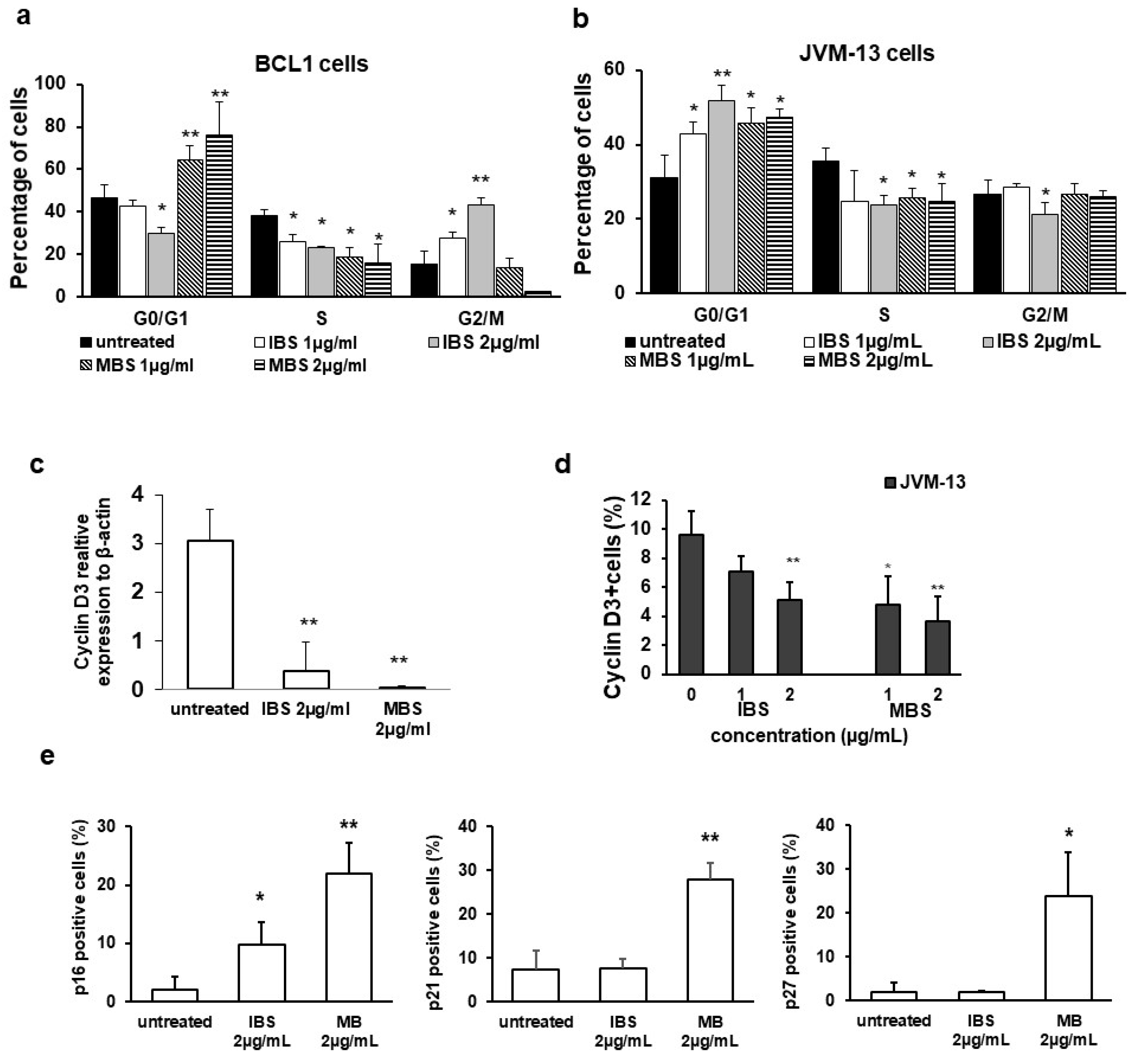
3.3. Derivatives of Shikonin Show Antiproliferative Effect against BCL1 Cells
3.4. Shikonin Derivatives Inhibit Expression of pSTAT3 and STAT3 Regulated Genes
3.5. Shikonin Derivatives Reduced BCL1 Cell Growth In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Andújar, I.; Ríos, J.L.; Giner, R.M.; Recio, M.C. Pharmacological properties of shikonin—A review of literature since 2002. Planta Med. 2013, 79, 1685–1697. [Google Scholar] [CrossRef] [Green Version]
- Vukic, M.D.; Vukovic, N.L.; Djelic, G.T.; Popovic, S.L.J.; Zaric, M.M.; Baskic, D.D.; Krstic, G.B.; Tesevic, V.V.; Kacaniova, M.M. Antibacterial and cytotoxic activities of naphthoquinone pigments from Onosma visianii Clem. EXCLI J. 2017, 16, 73–88. [Google Scholar]
- Wiench, B.; Chen, Y.R.; Paulsen, M.; Hamm, R.; Schröder, S.; Yang, N.S.; Efferth, T. Integration of Different “-omics” Technologies Identifies Inhibition of the IGF1R-Akt-mTOR Signaling Cascade Involved in the Cytotoxic Effect of Shikonin against Leukemia Cells. Evid. Based Complementary Altern. Med. 2013, 2013, 818709. [Google Scholar] [CrossRef]
- Johnston, P.A.; Grandis, J.R. STAT3 signaling: Anticancer strategies and challenges. Mol. Interv. 2011, 11, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazan-Halevy, I.; Harris, D.; Liu, Z.; Liu, J.; Li, P.; Chen, X.; Shanker, S.; Ferrajoli, A.; Keating, M.J.; Estrov, Z. STAT3 is constitutively phosphorylated on serine 727 residues, binds DNA, and activates transcription in CLL cells. Blood 2010, 115, 2852–2863. [Google Scholar] [CrossRef] [Green Version]
- Maryam, A.; Mehmood, T.; Yan, Q.; Li, Y.; Khan, M.; Ma, T. Proscillaridin A Promotes Oxidative Stress and ER Stress, Inhibits STAT3 Activation, and Induces Apoptosis in A549 Lung Adenocarcinoma Cells. Oxid. Med. Cell Longev. 2018, 2018, 3853409. [Google Scholar] [CrossRef]
- Amini, S.; Fathi, F.; Mobalegi, J.; Sofimajidpour, H.; Ghadimi, T. The expressions of stem cell markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3, Dppa4, and Esrrb in bladder, colon, and prostate cancer, and certain cancer cell lines. Anat. Cell Biol. 2014, 47, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Noone, A.M.; Howlader, N.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2015; Based on November 2017 SEER Data Submission, Posted to the SEER Web Site; National Cancer Institute: Bethesda, MD, USA, April 2018. [Google Scholar]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with venetoclax inrelapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Rozovski, U.; Harris, D.M.; Li, P.; Liu, Z.; Jain, P.; Veletic, I.; Ferrajoli, A.; Burger, J.; Thompson, P.; Jain, N.; et al. Activation of the B-cell receptor successively activates NF-κB and STAT3 in chronic lymphocytic leukemia cells. Int. J. Cancer 2017, 141, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef] [Green Version]
- Galton, D.A.; Goldman, J.M.; Wiltshaw, E.; Catovsky, D.; Henry, K.; Goldenberg, G.J. Prolymphocytic leukaemia. Br. J. Haematol. 1974, 27, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Dearden, C. How I treat prolymphocytic leukemia. Blood 2012, 120, 538–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molyneux, G.; Andrews, M.; Sones, W.; York, M.; Barnett, A.; Quirk, E.; Yeung, W.; Turton, J. Haemotoxicity of busulphan, doxorubicin, cisplatin and cyclophosphamide in the female BALB/c mouse using a brief regimen of drug administration. Cell Biol. Toxicol. 2011, 27, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Severin, F.; Frezzato, F.; Visentin, A.; Martini, V.; Trimarco, V.; Carraro, S.; Tibaldi, E.; Brunati, A.M.; Piazza, F.; Semenzato, G.; et al. In Chronic Lymphocytic Leukemia the JAK2/STAT3 Pathway Is Constitutively Activated and Its Inhibition Leads to CLL Cell Death Unaffected by the Protective Bone Marrow Microenvironment. Cancers 2019, 11, 1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.C.; Shi, L.H.; Wang, X.J.; Wang, S.X.; Wan, X.Q.; Liu, S.R.; Wang, Y.F.; Lu, Z.; Wang, L.H.; Ding, Y. Stat3/Oct-4/c-Myc signal circuit for regulating stemness-mediated doxorubicin resistance of triple-negative breast cancer cells and inhibitory effects of WP1066. Int. J. Oncol. 2018, 53, 339–348. [Google Scholar] [CrossRef]
- Thakur, R.; Trivedi, R.; Rastogi, N.; Singh, M.; Mishra, D.P. Inhibition of STAT3, FAK and Src mediated signaling reduces cancer stem cell load, tumorigenic potential and metastasis in breast cancer. Sci. Rep. 2015, 5, 10194. [Google Scholar] [CrossRef]
- Messmer, B.T.; Messmer, D.; Allen, S.L.; Kolitz, J.E.; Kudalkar, P.; Cesar, D.; Murphy, E.J.; Koduru, P.; Ferrarini, M.; Zupo, S.; et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J. Clin. Investig. 2005, 115, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Yu, C.R.; Li, W.H.; Li, W. XInduction of apoptosis by shikonin through a ROS/JNK-mediated process in Bcr/Ablpositive chronic myelogenous leukemia (CML) cells. Cell Res. 2008, 18, 879–888. [Google Scholar] [CrossRef] [Green Version]
- Vukic, M.D.; Vukovic, N.L.; Obradovic, A.D.; Popovic, S.L.; Zaric, M.M.; Djurdjevic, P.M.; Markovic, S.D.; Baskic, D.D. Naphthoquinone rich Onosma visianii Clem (Boraginaceae) root extracts induce apoptosis and cell cycle arrest in HCT-116 and MDA-MB-231 cancer cell lines. Nat. Prod. Res. 2018, 32, 2712–2716. [Google Scholar] [CrossRef]
- Vukic, M.D.; Vukovic, N.L.; Obradovic, A.; Matic, M.; Djukic, M.; Avdovic, E. Redox status, DNA and HSA binding study of naturally occurring naphthoquinone derivatives. EXCLI J. 2020, 19, 48–70. [Google Scholar]
- Chen, Y.; Wang, T.; Du, J.; Li, Y.; Wang, X.; Zhou, Y.; Yu, X.; Fan, W.; Zhu, Q.; Tong, X.; et al. The Critical Role of PTEN/PI3K/AKT Signaling Pathway in Shikonin-Induced Apoptosis and Proliferation Inhibition of Chronic Myeloid Leukemia. Cell Physiol. Biochem. 2018, 47, 981–993. [Google Scholar] [CrossRef]
- Shan, Z.L.; Zhong, L.; Xiao, C.L.; Gan, L.G.; Xu, T.; Song, H.; Yang, R.; Li, L.; Liu, B.Z. Shikonin suppresses proliferation and induces apoptosis in human leukemia NB4 cells through modulation of MAPKs and c-Myc. Mol. Med. Rep. 2017, 16, 3055–3060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henkels, K.M.; Turchi, J.J. Cisplatin-induced Apoptosis Proceeds by Caspase-3-dependent and -independent Pathways in Cisplatin-resistant and -sensitive Human Ovarian Cancer Cell Lines. Cancer Res. 1999, 59, 3077–3083. [Google Scholar] [PubMed]
- Alves, N.L.; Derks, I.A.; Berk, E.; Spijker, R.; van Lier, R.A.; Eldering, E. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity 2006, 24, 703–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gara, R.K.; Srivastava, V.K.; Duggal, S.; Bagga, J.K.; Bhatt, M.; Sanyal, S.; Mishra, D.P. Shikonin selectively induces apoptosis in human prostate cancer cells through the endoplasmic reticulum stress and mitochondrial apoptotic pathway. J. Biomed. Sci. 2015, 22, 26. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Zhu, Z.; Li, H.; Li, Y. Shikonin inhibits proliferation, migration, invasion and promotes apoptosis in NCI-N87 cells via inhibition of PI3K/AKT signal pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2662–2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshpande, A.; Sicinski, P.; Hinds, P.W. Cyclins and cdks in development and cancer: A perspective. Oncogene 2005, 24, 2909–2915. [Google Scholar] [CrossRef]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Hannon, G.J.; Zhang, H.; Casso, D.; Kobayashi, R.; Beach, D. p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366, 701–704. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhong, Y.; Long, X.; Zhu, Z.; Zhou, Y.; Ye, H.; Zeng, X.; Zheng, X. Deoxyshikonin isolated from Arnebia euchroma inhibits colorectal cancer by down-regulating the PI3K/Akt/mTOR pathway. Pharm. Biol. 2019, 57, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrielli, B.G.; Sarcevic, B.; Sinnamon, J.; Castellano, W.M.; Wang, X.Q.; Ellem, K.A. A cyclin D-Cdk4 activity required for G2 phase cell cycle progression is inhibited in ultraviolet radiation-induced G2 phase delay. J. Biol. Chem. 1999, 274, 13961–13969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Ma, J.; Su, M.; Shao, D.; Zhao, J.; Zhao, T.; Song, Z.; Meng, Y.; Jiao, P. Down-regulation of STAT3 induces the apoptosis and G1 cell cycle arrest in esophageal carcinoma ECA109 cells. Cancer Cell Int. 2018, 18, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Deng, J.; Ren, H.Y.; Jia, P.; Zhang, W.; Li, M.Q.; Li, S.W.; Zhou, Q. HSTAT3 influences the characteristics of stem cells in cervical carcinoma. Oncol. Lett. 2017, 14, 2131–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Target | Sense and Antisense |
|---|---|
| Mouse Bax | 5’-ACACCTGAGCTGACCTTG-3´ 5´-AGCCCATGATGGTTCTGATC-3´ |
| Mouse caspase-3 | 5´-AAATTCAAGGGACGGGTCAT-3´ 5´-ATTGACACAATACACGGGATCTGT-3´ |
| Mouse cyclin D3 | 5´- CCGTGATTGCGCACGACTTC-3´ 5´-TCTGTGGGAGTGCTGGTCTG-3´ |
| Mouse Nanog | 5´-AAGCAGAAGATGCGGACTGT-3´ 5´-GTGCTGAGCCCTTCTGAATC-3´ |
| Mouse Oct4 | 5´-CAAGGCAAGGGAGGTAGACA-3´ 5´-ATGAGTGACAGACAGGCCAG-3´ |
| Mouse c-Myc | 5´-CGGACACACAACGTCTTGGAA-3´ 5´-AGGATGTAGGCGGTGGCTTTT-3´ |
| Mouse β-actin | 5´-AGCTGCGTTTTACACCCTTT-3´ 5´-AAGCCATGCCAATGTTGTCT -3´ |
| Human Bax | 5´-ATGGACGGGTCCGGGGAGCA-3´ 5´-CCCAGTTGAAGTTGCCGTCA3-3´ |
| Human Bcl-2 | 5´-CTTTGAGTTCGGTGGGGTCA-3´ 5´-GGGCCGTACAGTTCCACAAA-3´ |
| Human cMyc | 5´-AAAGGCCCCCAAGGTAGTTA-3´ 5´-GCACAAGAGTTCCGTAGCTG-3´ |
| Human Nanog | 5´-ACATGCAACCTGAAGACGTGTG-3´ 5´-CATGGAAACCAGAACACGTGG-3´ |
| Human Sox2 | 5´-GAGCTTTGCAGGAAGTTTGC-3´ 5´-GCAAGAAGCCTCTCCTTGAA-3´ |
| Human Oct4 | 5´-ACATCAAAGCTCTGCAGAAAGAACT-3´ 5´-CTGAATACCTTCCCAAATAGAACCC-3´ |
| Human β-actin | 5´-CACCATTGGCAATGAGCGGTTC-3´ 5´-AGGTCTTTGCGGATGTCCACGT-3´ |
| Compound | IC50 ± SD (μg/mL) | |
|---|---|---|
| 24 h | 48 h | |
| Isobutyrylshikonin (IBS) | 0.86 ± 0.16 | 0.95 ± 0.15 |
| α-methylbutyrylshikonin (MBS) | 1.07 ± 0.19 | 1.02 ± 0.20 |
| Acetylshikonin | 3.82 ± 0.20 | 3.88 ± 0.20 |
| β-hydroxyisovalerylshikonin | ≥100 | ≥100 |
| Deoxyshikonin | ≥100 | ≥100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorovic, Z.; Milovanovic, J.; Arsenijevic, D.; Vukovic, N.; Vukic, M.; Arsenijevic, A.; Djurdjevic, P.; Milovanovic, M.; Arsenijevic, N. Shikonin Derivatives from Onsoma visianii Decrease Expression of Phosphorylated STAT3 in Leukemia Cells and Exert Antitumor Activity. Nutrients 2021, 13, 1147. https://doi.org/10.3390/nu13041147
Todorovic Z, Milovanovic J, Arsenijevic D, Vukovic N, Vukic M, Arsenijevic A, Djurdjevic P, Milovanovic M, Arsenijevic N. Shikonin Derivatives from Onsoma visianii Decrease Expression of Phosphorylated STAT3 in Leukemia Cells and Exert Antitumor Activity. Nutrients. 2021; 13(4):1147. https://doi.org/10.3390/nu13041147
Chicago/Turabian StyleTodorovic, Zeljko, Jelena Milovanovic, Dragana Arsenijevic, Nenad Vukovic, Milena Vukic, Aleksandar Arsenijevic, Predrag Djurdjevic, Marija Milovanovic, and Nebojsa Arsenijevic. 2021. "Shikonin Derivatives from Onsoma visianii Decrease Expression of Phosphorylated STAT3 in Leukemia Cells and Exert Antitumor Activity" Nutrients 13, no. 4: 1147. https://doi.org/10.3390/nu13041147






