Combined Effects of Exercise and Phytoanabolic Extracts in Castrated Male and Female Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics and Experimental Animals
2.2. Surgical Castration
2.3. Climbing Exercise Protocol
2.4. Treatment with Ergogenic Phytocompounds
2.5. Determination of Active Principles in Lyophilized Extracts
2.6. Voluntary Locomotor Activity
2.7. Rota-Rod Performance
2.8. Grasping Test
2.9. Euthanasia and Sample Collection
2.10. Biochemical Analysis
2.11. Cytokine Determination
2.12. Histological Analysis of Skeletal Muscle and Adipose Tissue
2.13. Statistical Analysis
3. Results
3.1. Analysis of Bioactive Compounds in Lyophilized Extracts
3.2. Castration Effects Prior to Exercise and Treatments
3.3. Changes in Body Weight after Exercise and Treatments
3.4. Combined Effects of Ergogenic Extracts and Exercise on Skeletal Muscle Weight
3.5. Adipose Depots and the Effects of Exercise and Ergogenic Extracts
3.6. Exercise and Treatment with Phytoanabolic Extracts and Their Effects on Functional Parameters
3.7. Skeletal Muscle Alterations after Exercise and Treatment with Anabolic Extracts
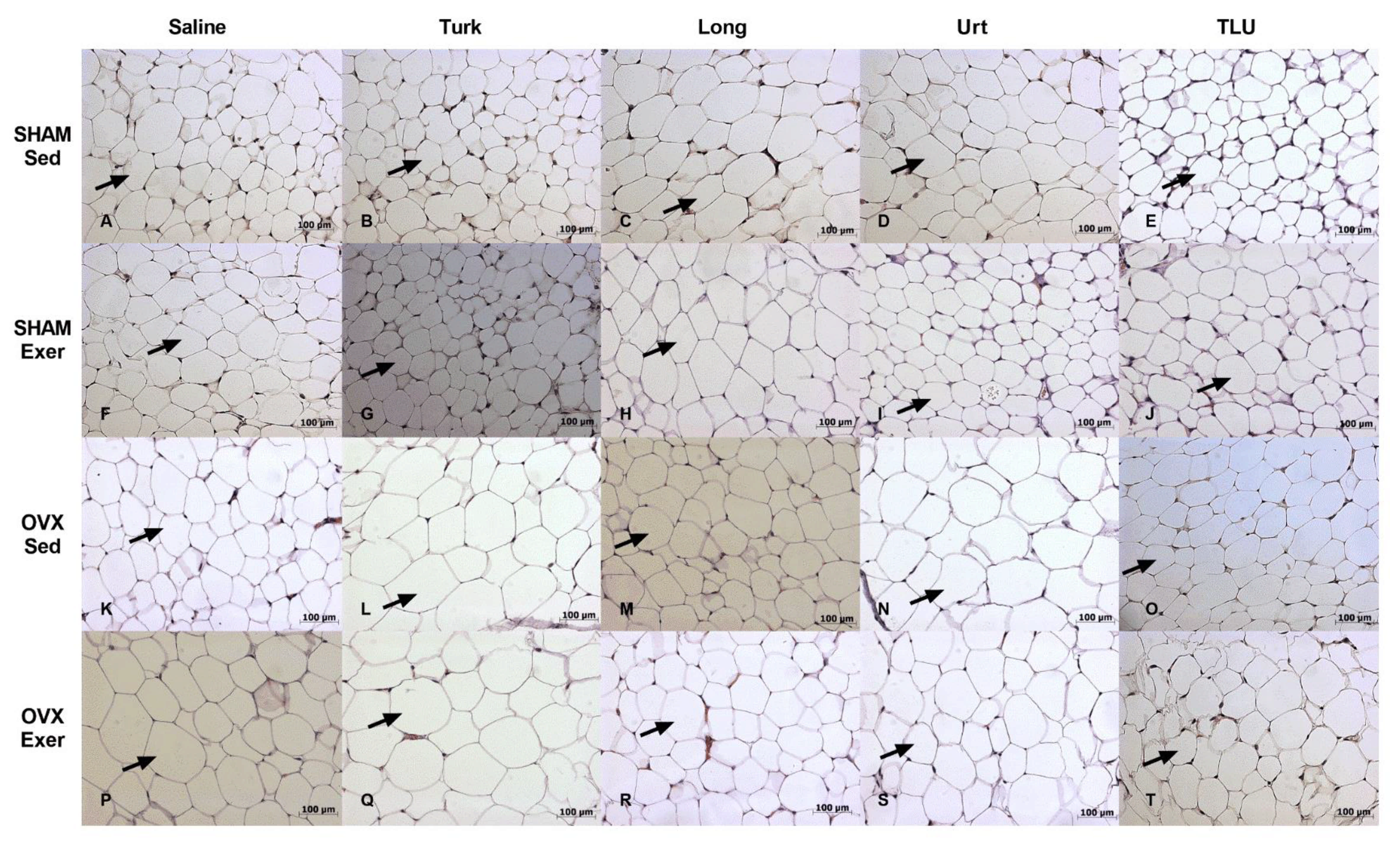
3.8. Histological Evaluation of Adipose Tissue
3.9. Evaluation of Changes in Weights of Kidney, Liver, Brain and Bone
3.10. Biochemical and Inflammatory Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Morley, J.E. Hormones and Sarcopenia. Curr. Pharm. Des. 2017, 23, 4484–4492. [Google Scholar] [CrossRef] [PubMed]
- Messier, V.; Rabasa-Lhoret, R.; Barbat-Artigas, S.; Elisha, B.; Karelis, A.D.; Aubertin-Leheudre, M. Menopause and sarcopenia: A potential role for sex hormones. Maturitas 2011, 68, 331–336. [Google Scholar] [CrossRef]
- Urban, R.J.; Al, E.T. Translational studies in older men using testosterone to treat sarcopenia. Trans. Am. Clin. Climatol. Assoc. 2014, 125, 27–44. [Google Scholar] [PubMed]
- Basualto-alarcón, C.; Varela, D.; Duran, J.; Maass, R.; Estrada, M. Sarcopenia and androgens: A link between pathology and treatment. Front. Endocrinol. 2014, 5, 217. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulou, S.K. Sarcopenia: A contemporary health problem among older adult populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachex Sarcopenia Muscle 2015, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Beckwée, D.; Delaere, A.; Aelbrecht, S.; Baert, B.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Butmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef]
- Chisamore, M.J.; Gentile, M.A.; Dillon, G.M.; Baran, M.; Gambone, C.; Riley, S.; Schmidt, A.; Flores, O.; Wilkinson, H.; Alves, S.E. A novel selective androgen receptor modulator (SARM) MK-4541 exerts anti-androgenic activity in the prostate cancer xenograft R-3327G and anabolic activity on skeletal muscle mass & function in castrated mice. J. Steroid Biochem. Mol. Biol. 2016, 163, 88–97. [Google Scholar] [CrossRef]
- Coss, C.C.; Jones, A.; Hancock, M.L.; Steiner, M.S.; Dalton, J.T. Selective androgen receptor modulators for the treatment of late onset male hypogonadism. Asian J. Androl. 2014, 256–261. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. Recent advances in pharmacological, hormonal, and nutritional intervention for sarcopenia. Pflugers Arch. Eur. J. Physiol. 2018, 470, 449–460. [Google Scholar] [CrossRef]
- Teixeira, C.J.; Ribeiro, L.M.; Veras, K.; Araujo, L.C.; da Cunha Araujo, L.C.; Curi, R.; de Oliveira Carvalho, C.R. Dehydroepiandrosterone supplementation is not bene fi cial in the late postmenopausal period in diet-induced obese rats. Life Sci. 2018, 202, 110–116. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Favero, G.; Rodella, L.F. Impact of Melatonin on Skeletal Muscle and Exercise. Cells 2020, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Tamaki, M.; Miyashita, K.; Hagiwara, A.; Wakino, S.; Inoue, H.; Fujii, K.; Fujii, C.; Endo, S.; Uto, A.; Mitsuishi, M.; et al. Ghrelin treatment improves physical decline in sarcopenia model mice through muscular enhancement and mitochondrial activation. Endocr. J. 2017, 64, S47–S51. [Google Scholar] [CrossRef] [Green Version]
- Gagliano-Juca, T.; Basaria, S. Testosterone replacement therapy and cardiovascular risk. Nat. Rev. Cardiol. 2019. [Google Scholar] [CrossRef]
- Kathryn Korkidakis, A.; Reid, R.L. Testosterone in Women: Measurement and Therapeutic Use. J. Obstet. Gynaecol. Can. 2017, 39, 124–130. [Google Scholar] [CrossRef]
- Rondanelli, M.; Miccono, A.; Peroni, G.; Guerriero, F.; Morazzoni, P.; Riva, A.; Guido, D.; Perna, S. A systematic review on the effects of botanicals on skeletal muscle health in order to prevent sarcopenia. Evid. Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Weng, W.; Gao, R.; Liu, Y.; Monacelli, F. New Insights for Cellular and Molecular Mechanisms of Aging and Aging-Related Diseases: Herbal Medicine as Potential Therapeutic Approach. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Luan, F.; Han, K.; Li, M.; Zhang, T.; Liu, D.; Yu, L.; Lv, H. Ethnomedicinal uses, phytochemistry, pharmacology, and toxicology of species from the genus ajuga L.: A systematic review. Am. J. Chin. Med. 2019, 47, 959–1003. [Google Scholar] [CrossRef]
- Isenmann, E.; Ambrosio, G.; Felix, J.; Mazzarino, M.; De, X.; Zimmer, P.; Kazlauskas, R.; Goebel, C.; Botrè, F.; Diel, P.; et al. Ecdysteroids as non-conventional anabolic agent: Performance enhancement by ecdysterone supplementation in humans. Arch. Toxicol. 2019, 93, 1807–1816. [Google Scholar] [CrossRef]
- Parr, M.K.; Ambrosio, G.; Wuest, B.; Mazzarino, M.; de la Torre, X.; Sibilia, F.; Joseph, J.F.; Diel, P.; Botrè, F. Targeting the administration of ecdysterone in doping control samples. Forensic Toxicol. 2020, 38, 172–184. [Google Scholar] [CrossRef]
- Bhat, R.; Karim, A.A. Fitoterapia Tongkat Ali (Eurycoma longifolia Jack): A review on its ethnobotany and pharmacological importance. Fitoterapia 2010, 81, 669–679. [Google Scholar] [CrossRef]
- Al-Salahi, O.; Kit-Lam, C.; Majid, A.; Al-Suede, F.; Mohammed Saghir, S.; Abdullah, W.; Ahamed, M.; Yusoff, N. Anti-angiogenic quassinoid-rich fraction from Eurycoma longifolia modulates endothelial cell function. Microvasc. Res. 2013, 90, 30–39. [Google Scholar] [CrossRef]
- Balan, D.; Chan, K.; Murugan, D.; AbuBakar, S.; Wong, P. Antiadipogenic effects of a standardized quassinoids—Enriched fraction and eurycomanone from Eurycoma longifolia. Phytother. Res. 2018, 1–14. [Google Scholar] [CrossRef]
- George, A.; Suzuki, N.; Abas, A.B.; Mohri, K.; Utsuyama, M.; Hirokawa, K.; Takara, T. Immunomodulation in Middle-Aged Humans Via the Ingestion of Physta® Standardized Root Water Extract of Eurycoma longifolia Jack—A Parallel Study. Phytother. Res. 2016, 635, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Choe, K.; Yoo, H.H. Review on a traditional herbal medicine, Eurycoma longifolia Jack (Tongkat Ali): Its traditional uses, chemistry, evidence-based pharmacology and toxicology. Molecules 2016, 21, 331. [Google Scholar] [CrossRef] [Green Version]
- Chinnappan, S.M.; George, A.; Ashok, G.; Choudhary, Y.K. Effect of herbal extract Eurycoma longifolia (Physta®) on female reproductive hormones and bone biochemical markers: An ovariectomised rat model study. BMC Complement. Med. Ther. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Tambi, M.I.B.M.; Imran, M.K.; Henkel, R.R. Standardised water-soluble extract of Eurycoma longifolia, Tongkat ali, as testosterone booster for managing men with late-onset hypogonadism? Andrologia 2012, 44, 226–230. [Google Scholar] [CrossRef]
- Chen, C.K.; Ooi, F.K.; Kasim, N.A.A.; Asari, M.A. Effects of Eurycoma Longifolia Jack Supplementation Combined with Resistance Training on Isokinetic Muscular Strength and Power, Anaerobic Power, and Urinary Testosterone: Epitestosterone Ratio in Young Males. Int. J. Prev. Med. 2019, 10, 118. [Google Scholar] [CrossRef]
- Allkanjari, O.; Vitalone, A. What do we know about phytotherapy of benign prostatic hyperplasia? Life Sci. 2015, 126, 42–56. [Google Scholar] [CrossRef]
- Dhouibi, R.; Affes, H.; Salem, M.B.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Screening of pharmacological uses of Urtica dioica and others bene fi ts. Prog. Biophys. Mol. Biol. 2019. [Google Scholar] [CrossRef]
- Ibrahim, M.; Rehman, K.; Razzaq, A.; Hussain, I.; Farooq, T.; Hussain, A.; Akash, M.S.H. Investigations of phytochemical constituents and their pharmacological properties isolated from the genus urtica: Critical review and analysis. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 25–66. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Raychaudhuri, S.; Kraus, O.; Shahinozzaman, M.; Lofti, L.; Obanda, D.N. Urtica dioica whole vegetable as a functional food targeting fat accumulation and insulin resistance—A preliminary study in a mouse pre-diabetic model. Nutrients 2020, 12, 1059. [Google Scholar] [CrossRef] [PubMed]
- Nahata, A.; Dixit, V.K. Ameliorative effects of stinging nettle (Urtica dioica) on testosterone-induced prostatic hyperplasia in rats. Andrologia 2012, 396–409. [Google Scholar] [CrossRef]
- MCTI-CONCEA. Diretriz Brasileira para o Cuidado e a Utilização de Animais em Atividades de Ensino ou de Pesquisa Científica—DBCA. Resolução Normativa CONCEA n. 30. 2 February 2016. Available online: http://www.mctic.gov.br/mctic/export/sites/institucional/institucional/concea/arquivos/legislacao/resolucoes_normativas/Resolucao-Normativa-CONCEA-n-30-de-02.02.2016-D.O.U.-de-03.02.2016-Secao-I-Pag.-03.pdf (accessed on 29 March 2017).
- Riedl, I.; Yoshioka, M.; St-amand, J. Concomitant modulation of transcripts related to fiber type determination and energy metabolism in skeletal muscle of female ovariectomized mice by estradiol injection. J. Steroid Biochem. Mol. Biol. 2010, 122, 91–99. [Google Scholar] [CrossRef]
- Corazza, A.V.; Paolillo, F.R.; Groppo, F.C.; Bagnato, V.S.; Caria, P.H.F. Phototherapy and resistance training prevent sarcopenia in ovariectomized rats. Lasers Med. Sci. 2013, 28, 1467–1474. [Google Scholar] [CrossRef]
- Zheng, W.; Hengevoß, J.; Soukup, S.; Kulling, S.; Xie, M.; Diel, P. An isoflavone enriched diet increases skeletal muscle adaptation in response to physical activity in ovariectomized rats. Mol. Nutr. Food Res. 2017, 61, 1–30. [Google Scholar] [CrossRef]
- Collins, B.C.; Laakkonen, E.K.; Lowe, D.A. Aging of the musculoskeletal system: How the loss of estrogen impacts muscle strength. Bone 2019, 123, 137–144. [Google Scholar] [CrossRef]
- Ikeda, K.; Horie-inoue, K.; Inoue, S. Functions of estrogen and estrogen receptor signaling on skeletal muscle. J. Steroid Biochem. Mol. Biol. 2019, 191, 105375. [Google Scholar] [CrossRef]
- Doulamis, I.; Tzani, A.; Konstantopoulos, P.; Daskalopoulou, A.; Spinos, T.; Bletsa, E.; Mitsopoulou, D.; Spinou, M.; Brinia, M.; Palaiopanos, K.; et al. Experimental hypogonadism: Insulin resistance, biochemical changes and effect of testosterone substitution. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 1–5. [Google Scholar] [CrossRef]
- Ropelle, E.R.; Pauli, J.R.; Zecchin, K.G.; Ueno, M.; de Souza, C.T.; Morari, J.; Faria, M.C.; Velloso, L.A.; Saad, M.J.; Carvalheira, J.B. A Central Role for Neuronal Amp Activated Protein Kinase (Ampk) in Cancer-Induced Anorexia. Endocrinology 2007, 148, 5220–5229. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Khamoui, A.V.; Jo, E.; Zourdos, M.C.; Panton, L.B.; Ormsbee, M.J.; Kim, J. Effect of conjugated linoleic acids and omega-3 fatty acids with or without resistance training on muscle mass in high-fat diet-fed middle-aged mice. Exp. Physiol. 2017, 11, 1500–1512. [Google Scholar] [CrossRef]
- Lee, S.; Barton, E.R.; Sweeney, H.L.; Farrar, R.P. Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J. Appl. Physiol. 2004, 96, 1097–1104. [Google Scholar] [CrossRef] [Green Version]
- Tashmukhamedova, M.; Almatov, K.; Syrov, V.; Sultanov, M.; Abidov, A. Comparative study of the effect of ecdysterone, turkesterone and nerobol on the function of rat liver mitochondria in experimental diabetes. Vopr. Med. Khim. 1986, 32, 24–28. [Google Scholar]
- Syrov, V. Mechanism of the anabolic action of phytoecdisteroids in mammals. Nauchnye Doki. Vyss. Shkoly Biol. Nauk. 1984, 11, 16–20. [Google Scholar]
- Syrov, V.; Kurmukov, A.; Sakhibov, A. Effect of turkesterone and nerobol on the activity of the protein synthesizing system of mouse liver. Vopr. Med. Khim. 1978, 24, 456–460. [Google Scholar]
- Syrov, V.; Tashmukhamedova, M.; Khushbaktova, Z.; Mirtalipov, D.; Mamatkhanov, A. Effect of phytoecdysteroids and nerobol on parameters of carbohydrate and lipid metabolism and phospholipid spectrum of liver mitochondrial membrane in experimental diabetes mellitus of rats. Ukr. Biokhim. Zhurnal 1978, 64, 61–67. [Google Scholar]
- Tashmukhamedova, M.; Almatov, K.; Syrov, V.; Sultanov, M.; Abidov, A. Effect of phytoecdisteroids and anabolic steroids on liver mitochondrial respiration and oxidative phosphorylation in alloxan diabetic rats. Nauchnye Doki. Vyss. Shkoly Biol. Nauk. 1985, 9, 37–39. [Google Scholar]
- Arthur, S.T.; Zwetsloot, K.A.; Lawrence, M.M.; Nieman, D.C.; Lila, M.A.; Grace, M.H.; Howden, R.; Cooley, I.D.; Keith, M.D.; Demick, J.L.; et al. Ajuga turkestanica increases Notch and Wnt signaling in aged skeletal muscle. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2584–2592. [Google Scholar]
- Low, B.; Das, P.K.; Chan, K. Acute, Reproductive Toxicity and Two-generation Teratology Studies of a Standardized Quassinoid- rich Extract of Eurycoma longifolia Jack in Sprague—Dawley Rats. Phytother. Res. 2014, 1029, 1022–1029. [Google Scholar] [CrossRef]
- Abdulghani, M.; Hussin, A.H.; Sulaiman, S.A.; Chan, K.L. The ameliorative effects of Eurycoma longifolia Jack on testosterone-induced reproductive disorders in female rats. Reprod. Biol. 2012, 12, 247–255. [Google Scholar] [CrossRef]
- Wahab, N.A.; Mokhtar, I.N.M.; Nurul, I.W.; Halim, H.A. Basic research the effect of Eurycoma longifolia Jack on spermatogenesis in estrogen-treated rats. Clinics 2010, 65, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Ang, H.; Lee, K. Effect of Eurycoma longifolia Jack on libido in middle-aged male rats. J. Basic Clin. Physiol. Pharmacol. 2002, 13, 249–254. [Google Scholar] [CrossRef]
- Ang, H.; Cheang, H. Effects of Eurycoma longifolia jack on laevator ani muscle in both uncastrated and testosterone-stimulated castrated intact male rats. Arch. Pharm. Res. 2001, 24, 437–440. [Google Scholar] [CrossRef]
- Patel, S.S.; Udayabanu, M. Urtica dioica extract attenuates depressive like behavior and associative memory dysfunction in dexamethasone induced diabetic mice. Metab. Brain Dis. 2014, 29, 121–130. [Google Scholar] [CrossRef]
- Dar, S.A.; Ganai, F.A.; Yousuf, A.R.; Balkhi, H.; Bhat, T.M.; Sharma, P.; Ahmad, S.; Ganai, F.A.; Yousuf, A.R.; Dar, S.A.; et al. Pharmacological and toxicological evaluation of Urtica dioica. Pharm. Biol. 2013, 51. [Google Scholar] [CrossRef]
- Jalili, C.; Salahshoor, M.R.; Naseri, A. Protective effect of Urtica dioica L. against nicotine-induced damage on sperm parameters, testosterone and testis tissue in mice. Iran. J. Reprod. Med. 2014, 12, 401–408. [Google Scholar]
- Obanda, D.N.; Ribnicky, D.; Yu, Y.; Stephens, J.; Cefalu, W.T. An extract of Urtica dioica L. mitigates obesity induced insulin resistance in mice skeletal muscle via protein phosphatase 2A (PP2A). Sci. Rep. 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.X.; Chen, R.S.; Shen, Y.; Yin, Z.Y. Phytosterols elevation in bamboo shoot residue through laboratorial scale solid-state fermentation using isolated Aspergillus niger CTBU. Appl. Biochem. Biotechnol. 2014, 172, 4078–4083. [Google Scholar] [CrossRef]
- Toledo, M.; Penna, F.; Busquets, S.; Lopez-Soberiano, F.J.; Argiles, J.M. Distinct Behaviour of Sorafenib in Experimental Cachexia-Inducing Tumours: The Role of STAT3. PLoS ONE 2014, 9, e113931. [Google Scholar] [CrossRef]
- Murphy, K.T.; Chee, A.; Trieu, J.; Naim, T.; Lynch, G.S. Importance of functional and metabolic impairments in the characterization of the C-26 murine model of cancer cachexia. Dis. Model. Mech. 2012, 5, 533–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.L.; Lee, M.C.; Hsu, Y.J.; Huang, W.C.; Huang, C.C.; Huang, S.W. Isolated soy protein supplementation and exercise improve fatigue-related biomarker levels and bone strength in ovariectomized mice. Nutrients 2018, 10, 1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Counts, B.R.; Fix, D.K.; Hetzler, K.L.; Carson, J.A. The Effect of Estradiol Administration on Muscle Mass Loss and Cachexia Progression in Female ApcMin/+ Mice. Front. Endocrinol. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, J.P.; Gao, S.; Puppa, M.J.; Sato, S.; Welle, S.L.; Carson, J.A. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol. Cell. Endocrinol. 2013, 365, 174–186. [Google Scholar] [CrossRef] [Green Version]
- Sciote, J.; Horton, M.; Zyman, Y.; Pascoe, G. Differential effects of diminished oestrogen and androgen levels on development of skeletal muscle fibres in hypogonadal mice. Acta Physiol. Scand. 2001, 172, 179–187. [Google Scholar] [CrossRef]
- Guo, S.; Huang, Y.; Zhang, Y.; Huang, H.; Hong, S.; Liu, T. Impacts of exercise interventions on different diseases and organ functions in mice. J. Sport Health Sci. 2020, 9, 53–73. [Google Scholar] [CrossRef]
- Krause Neto, W.; Silva, W.D.A.; Ciena, A.P.; Anaruma, C.A.; Gama, E.F. Vertical climbing for rodent resistance training: A discussion about training parameters. Int. J. Sport Sci. 2016, 6, 36–49. [Google Scholar] [CrossRef]
- Lee, S.; Farrar, R.P. Resistance training induces muscle-specific changes in muscle mass andfunction in rat. J. Exerc. Physiol. 2003, 6, 80–87. [Google Scholar]
- Ahtiainen, J.P.; Lensu, S.; Ruotsalainen, I.; Schumann, M.; Ihalainen, J.K.; Fachada, V.; Mendias, C.L.; Brook, M.S.; Smith, K.; Atherton, P.J.; et al. Physiological adaptations to resistance training in rats selectively bred for low and high response to aerobic exercise training. Exp. Physiol. 2018, 103, 1513–1523. [Google Scholar] [CrossRef] [Green Version]
- Bunratsami, S.; Udomuksorn, W.; Kumarnsit, E.; Vongvatcharanon, S.; Vongvatcharanon, U. Estrogen replacement improves skeletal muscle performance by increasing parvalbumin levels in ovariectomized rats. Acta Histochem. 2015. [Google Scholar] [CrossRef]
- Graber, T.G.; Fandrey, K.R. Novel individualized power training protocol preserves physical function in adult and older mice. GeroScience 2019, 165–183. [Google Scholar] [CrossRef]
- Elhag, H.E.E.A.; Sulaiman, A.Z.; Ajit, A.B. A Review on the Exraction Methods of Extracts and Phytochemicals from Eurycoma longifolia. In Proceedings of the National Conference for Postgraduate Research (NCON-PGR), Universiti Malaysia, Pahang, Malaysia, 24–25 September 2016; pp. 253–259. [Google Scholar]
- Mamatkhanov, A.U.; Yakubova, M.R.; Syrov, V.N. The technological isolation of turkesterone from the epigeal part of Ajuga turkestanica (Rgl.) Brig. is described, a method is proposed for the quantitative determination of turkesterone, and the results of an investigation of its biological activity. Chem. Nat. Compd. 1998, 34, 150–154. [Google Scholar] [CrossRef]
- Chrubasik, J.E.; Roufogalis, B.D.; Wagner, H.; Chrubasik, S. A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: Urticae radix. Phytomedicine 2007, 14, 568–579. [Google Scholar] [CrossRef]
- Chakraborty, D.; Pal, A. Quassinoids: Chemistry and Novel Detection Techniques; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9783642221446. [Google Scholar]
- Pandey, A.; Tripathi, S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J. Pharmacogn. Phytochem. 2014, 2, 115–119. [Google Scholar]
- Abidi, S.L. Chromatographic analysis of plant sterols in foods and vegetable oils. J. Chromatogr. A 2001, 935, 173–201. [Google Scholar] [CrossRef]
- Destrez, B.; Pinel, G.; Monteau, F.; Lafont, R.; Le, B. Detection and identification of 20-hydroxyecdysone metabolites in calf urine by liquid chromatography-high resolution or tandem mass spectrometry measurements and establishment of their kinetics of elimination after 20-hydroxyecdysone administration. Anal. Chim. Acta 2008, 7, 178–184. [Google Scholar] [CrossRef]
- Teh, C.; Murugaiyah, V.; Chan, K. Developing a validated liquid chromatography—Mass spectrometric method for the simultaneous analysis of five bioactive quassinoid markers for the standardization of manufactured batches of Eurycoma longifolia Jack extract as antimalarial medicaments. J. Chromatogr. A 2011, 1218, 1861–1877. [Google Scholar] [CrossRef]
- Bataglion, G.A.; Meurer, E.C.; De Albergaria, A.C.R.; Bícego, M.C.; Weber, R.R.; Eberlin, M.N. Determination of geochemically important sterols and triterpenols in sediments using UHPLC-MS/MS. Anal. Chem. 2015, 85, 7771–7778. [Google Scholar] [CrossRef]
- Seidlova-Wuttke, D.; Ehrhardt, C.; Wuttke, W. Metabolic effects of 20-OH-Ecdysone in ovariectomized rats. J. Steroid Biochem. Mol. Biol. 2010, 119, 121–126. [Google Scholar] [CrossRef]
- Kenny, A.M.; Kleppinger, A.; Wang, Y.; Prestwood, K.M. Effects of Ultra-Low-Dose Estrogen Therapy on Muscle and Physical Function in Older Women. J. Am. Geriatr. Soc. 2005, 53, 1973–1977. [Google Scholar] [CrossRef]
- Javed, A.A.; Mayhew, A.J.; Shea, A.K.; Raina, P. Association Between Hormone Therapy and Muscle Mass in Postmenopausal Women A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Parr, M.K.; Zhao, P.; Haupt, O.; Ngueu, S.T.; Hengevoss, J.; Fritzmeier, K.H.; Piechotta, M.; Schlorer, N.; Muhn, P.; Zheng, W.-Y.; et al. Estrogen receptor beta is involved in skeletal muscle hypertrophy induced by the phytoecdysteroid ecdysterone. Mol. Nutr. Food Res. 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Caldwell, M.E.; Greene, N.P. Muscle metabolism and atrophy: Let’s talk about sex. Biol. Sex. Differ. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Russel, N.; Grossmann, M. Estradiol as a male hormone. Eur. J. Endocrinol. 2019, 181, R23–R43. [Google Scholar] [CrossRef]
- Low, B.; Choi, S.; Abdul, H.; Kumar, P.; Chan, K. Eurycomanone, the major quassinoid in Eurycoma longifolia root extract increases spermatogenesis by inhibiting the activity of phosphodiesterase and aromatase in steroidogenesis. J. Ethnopharmacol. 2013, 1–7. [Google Scholar] [CrossRef]
- Ziaei, R.; Foshati, S.; Hadi, A.; Kermani, M.A.H.; Ghavami, A.; Clark, C.C.T.; Tarrahi, M.J. The effect of nettle (Urtica dioica) supplementation on the glycemic control of patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Phyther. Res. 2020, 34, 282–294. [Google Scholar] [CrossRef]
- Namjou, A.; Heidarian, E.; Rafieian-Kopaei, M. Effects of Urtica dioica hydro-alcoholic extract on blood serum glucose and lipid profiles of female Wistar rats with long-term estrogen deficiency. Vet Res. Forum. 2018, 9, 349–355. [Google Scholar] [CrossRef]
- Horstman, A.M.; Dillon, E.L.; Urban, R.J.; Sheffi, M. The Role of Androgens and Estrogens on Healthy Aging and Longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1140–1152. [Google Scholar] [CrossRef] [Green Version]
- Neergheen-bhujun, V.S. Underestimating the Toxicological Challenges Associated with the Use of Herbal Medicinal Products in Developing Countries. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Dinan, L.; Lafont, R. Effects and applications of arthropod steroid hormones (ecdysteroids) in mammals. J. Endocrinol. 2006, 191, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Quinn, M.A.; Xu, X.; Ronfani, M.; Cidlowski, J.A.; Quinn, M.A.; Xu, X.; Ronfani, M.; Cidlowski, J.A. Estrogen Deficiency Promotes Hepatic Steatosis via a Glucocorticoid Receptor-Dependent Mechanism in Mice. Cell Rep. 2018, 22, 2690–2701. [Google Scholar] [CrossRef] [Green Version]
- Harada, N.; Hanaoka, R.; Horiuchi, H.; Kitakaz, T.; Mitani, T. Castration influences intestinal microflora and induces abdominal obesity in high-fat diet-fed mice. Sci. Rep. 2016, 1–9. [Google Scholar] [CrossRef]
- Morrone, M.S.; Schnorr, C.E.; Behr, G.A.; Gasparotto, J.; Bortolin, R.C.; Martinello, B.; Henkin, B.S.; Rabello, T.K.; Zanotto-Filho, A.; Gelain, D.P.; et al. Adiposity Accumulation, Serum Cholesterol Alterations, and Oxidative Stress in Ovariectomized Rats. Oxid. Med. Cell. Longev. 2016, 2016, 5719291. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-satta, M.; Berna-erro, A.; Carrasco-garcia, E.; Alberro, A.; Saenz-antoñanzas, A.; Vergara, I.; Otaegui, D.; Matheu, A. Relevance of oxidative stress and inflammation in frailty based on human studies and mouse models. Aging 2020, 12, 9982–9999. [Google Scholar] [CrossRef]













| SHAM | OVX | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline/Sed | Saline/Exer | Turk/Sed | Turk/Exer | Long/Sed | Long/Exer | Urt/Sed | Urt/Exer | TLU/Sed | TLU/Exer | Saline/Sed | Saline/Exer | Turk/Sed | Turk/Exer | Long/Sed | Long/Exer | Urt/Sed | Urt/Exer | TLU/Sed | TLU/Exer | |
| Initial Weight (g) | 21.3 ± 0.6 | 19.8 ± 0.4 a | 20.1 ± 0.4 | 20.7 ± 0.5 | 20.4 ± 0.3 | 20.7 ± 0.5 | 20.5 ± 0.5 | 20.2 ± 0.6 | 20.6 ± 0.3 | 20.5 ± 0.3 | 20.4 ± 0.5 | 20.6 ± 0.5 | 20.1 ± 0.5 | 20.8 ± 0.5 | 20.4 ± 0.3 | 20.8 ± 0.4 | 20.7 ± 0.3 | 20.3 ± 0.4 | 20.2 ± 0.3 | 20.5 ± 0.4 |
| Final Weight (g) | 23.2 ± 0.6 | 21.0 ± 0.4 a | 22.4 ± 0.3 | 21.5 ± 0.4 a | 22.4 ± 0.4 | 21.7 ± 0.3 | 22.1 ± 0.2 | 21.6 ± 0.3 | 22.4 ± 0.4 | 21.1 ± 0.4 a | 23.5 ± 0.5 | 22.2 ± 0.5 | 23.0 ± 0.4 | 22.6 ± 0.5 | 23.6 ± 0.3 | 22.8 ± 0.3 | 23.1 ± 0.4 | 21.9 ± 0.5 b | 23.4 ± 0.5 | 22.0 ± 0.4 |
| Final-Initial (g) | 1.8 ± 0.3 | 1.1 ± 0.2 | 2.3 ± 0.2 | 1.4 ± 0.4 | 2.0 ± 0.3 | 1.0 ± 0.4 | 1.7 ± 0.4 | 1.4 ± 0.4 | 1.8 ± 0.3 | 0.6 ± 0.3 | 3.1 ± 0.5 a | 1.6 ± 0.5 b | 2.9 ± 0.3 | 1.7 ± 0.5 | 3.2 ± 0.3 | 2.0 ± 0.2 | 2.9 ± 0.3 | 1.7 ± 0.4 b | 3.1 ± 0.4 | 1.5 ± 0.3 b c |
| Gastrocnemius (mg) | 116.2 ± 4.1 | 110.7 ± 2.6 | 113.4 ± 2.8 | 108.9 ± 3.6 | 107.4 ± 1.4 | 113.5 ± 3.5 | 114.6 ± 2.7 | 110.1 ± 4.5 | 110.3 ± 3.6 | 111.0 ± 2.5 | 123.5 ± 4.4 | 116.5 ± 2.1 | 109.4 ± 2.7 b | 117.5 ± 3.3 | 122.6 ± 1.6 | 122.9 ± 1.2 | 116.7 ± 2.8 | 118.3 ± 2.6 | 112.4 ± 3.2 | 117.4 ± 3.3 |
| Tibial (mg) | 60.4 ± 2.0 | 56.6 ± 2.1 | 61.1 ± 1.6 | 60.3 ± 2.3 | 60.7 ± 2.3 | 62.0 ± 2.5 | 62.4 ± 2.3 | 62.3 ± 3.1 | 58.4 ± 2.0 | 56.4 ± 1.9 | 65.3 ± 1.5 | 61.1 ± 2.3 | 57.0 ± 4.1 | 59.7 ± 2.4 | 63.3 ± 1.9 | 65.9 ± 2.5 | 58.0 ± 3.0 | 61.4 ± 1.9 | 57.7 ± 3.2 | 61.8 ± 1.5 |
| Soleus (mg) | 5.7 ± 0.5 | 5.1 ± 0.5 | 5.6 ± 0.4 | 4.9 ± 0.4 | 4.5 ± 0.2 | 5.3 ± 0.4 | 5.7 ± 0.5 | 6.2 ± 0.8 | 5.0 ± 0.4 | 5.1 ± 0.3 | 5.3 ± 0.5 | 5.1 ± 0.7 | 5.9 ± 0.5 | 5.2 ± 0.3 | 6.2 ± 0.5 | 6.2 ± 0.5 | 4.9 ± 0.3 | 5.7 ± 0.5 | 6.1 ± 0.7 | 5.5 ± 0.4 |
| Sum of Muscle Weight (mg) | 182.3 ± 6.0 | 172.4 ± 4.6 | 180.6 ± 4.4 | 174.3 ± 4.9 | 168.2 ± 5.6 | 180.8 ± 6.1 | 182,6 ± 4.6 | 178.6 ± 7.7 | 174,9 ± 5.5 | 172.4 ± 4.0 | 194.1 ± 5.4 | 182.7 ± 4.0 | 172.3 ± 6.4 b | 182.4 ± 5.6 | 192.1 ± 3.2 | 190.9 ± 5.8 | 179.5 ± 5.3 | 185.4 ± 3.8 | 175.9 ± 5.7 | 183.4 ± 4.5 |
| Muscle weight over total weight (%) | 0.79 ± 0.02 | 0.82 ± 0.02 | 0.82 ± 0.02 | 0.80 ± 0.02 | 0.75 ± 0.02 | 0.83 ± 0.02 c | 0.82 ± 0.02 | 0.82 ± 0.03 | 0.78 ± 0.02 | 0.82 ± 0.01 | 0.81 ± 0.02 | 0.83 ± 0.02 | 0.74 ± 0.02 | 0.81 ± 0.02 | 0.81 ± 0.01 | 0.83 ± 0.02 | 0.77 ± 0.02 | 0.84 ± 0.01 | 0.75 ± 0.03 | 0.83 ± 0.01 c |
| iBAT (mg) | 168.0 ± 11.9 | 182.1 ± 13.6 | 145.3 ± 16.6 | 145.4 ± 15.1 | 181.3 ± 10.7 | 133.0 ± 16.3 | 167.1 ± 14.6 | 151.3 ± 18.3 | 197.4 ± 15.7 | 161.3 ± 10.8 | 172.0 ± 17.4 | 178.5 ± 22.1 | 213.2 ± 26.1 | 188.4 ± 12.7 | 197.5 ± 28.6 | 157.5 ± 22.9 | 154.2 ± 28.8 | 139.6 ± 21.3 | 214.6 ± 16.1 | 179.2 ± 23.3 |
| iWAT (mg) | 118.7 ± 8.2 | 111.2 ± 7.8 | 107.4 ± 8.9 | 99.7 ± 8.2 | 111.5 ± 6.8 | 112.6 ± 6.6 | 106.3 ± 5.1 | 111.4 ± 8.6 | 144.6 ± 5.6 | 120.9 ± 5.0 | 143.5 ± 12.5 | 118.9 ± 11.8 | 144.4 ± 13.1 | 121.0 ± 11.4 | 139.5 ± 12.3 | 114.1 ± 8.7 | 129.2 ± 12.5 | 115.7 ± 9.1 | 163.1 ± 15.3 | 142.2 ± 6.1 |
| iWAT over total weight (%) | 0.51 ± 0.03 | 0.53 ± 0.03 | 0.48 ± 0.03 | 0.46 ± 0.03 | 0.50 ± 0.03 | 0.52 ± 0.03 | 0.48 ± 0.03 | 0.51 ± 0.04 | 0.65 ± 0.03 | 0.57 ± 0.02 | 0.61 ± 0.05 | 0.53 ± 0.04 | 0.62 ± 0.05 | 0.53 ± 0.04 | 0.59 ± 0.05 | 0.50 ± 0.03 | 0.056 ± 0.05 | 0.53 ± 0.03 | 0.69 ± 0.06 | 0.65 ± 0.02 |
| SHAM | ORX | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline/Sed | Saline/Exer | Turk/Sed | Turk/Exer | Long/Sed | Long/Exer | Urt/Sed | Urt/Exer | TLU/Sed | TLU/Exer | Saline/Sed | Saline/Exer | Turk/Sed | Turk/Exer | Long/Sed | Long/Exer | Urt/Sed | Urt/Exer | TLU/Sed | TLU/Exer | |
| Initial Weight (g) | 27.9 ± 0.5 | 27.6 ± 0.6 | 26.8 ± 0.8 | 27.6 ± 0.5 | 27.5 ± 0.6 | 27.9 ± 0.9 | 27.3 ± 0.4 | 27.3 ± 0.6 | 26.8 ± 0.7 | 28.4 ± 0.5 | 28.3 ± 0.6 | 28.5 ± 0.6 | 27.5 ± 0.4 | 28.1 ± 0.4 | 27.5 ± 0.5 | 27.9 ± 0.4 | 27.7 ± 0.4 | 27.6 ± 0.6 | 27.7 ± 0.5 | 27.8 ± 0.7 |
| Final Weight (g) | 29.5 ± 0.8 | 28.6 ± 0.8 | 29.7 ± 0.9 | 28.8 ± 0.6 | 30.8 ± 0.5 | 29.4 ± 1.0 | 30.0 ± 0.6 | 28.3 ± 0.7 | 30.2 ± 0.8 | 29.6 ± 0.7 | 24.6 ± 0.6 | 23.9 ± 0.4 | 24.6 ± 0.6 | 23.4 ± 0.3 | 24.4 ± 0.6 | 23.3 ± 0.3 | 24.6 ± 0.3 | 23.2 ± 0.4 | 24.41 ± 0.5 | 23.1 ± 0.4 |
| Final-Initial (g) | 2.6 ± 0.3 | 1.0 ± 0.3 a | 2.9 ± 0.4 | 1.1 ± 0.4 a c | 3.3 ± 0.4 | 1.5 ± 0.3 c | 2.7 ± 0.5 | 1.0 ± 0.2 a c | 3.4 ± 0.4 | 1.1 ± 0.3 a c | −3.7 ± 0.3 a | −4.61 ± 0.5 | −3.28 ± 0.3 | −4.7 ± 0.2 b | −3.2 ± 0.5 | −4.6 ± 0.3 c | −3.0 ± 0.3 | −4.36 ± 0.4 | −3.3 ± 0.3 | −4.7 ± 0.3 c |
| Gastrocnemius (mg) | 159.0 ± 3.0 | 150.1 ± 4.2 | 154.2 ± 4.2 | 158.9 ± 3.7 | 154.9 ± 1.3 | 158.0 ± 4.2 | 155.7 ± 1.6 | 150.3 ± 3.5 | 162.2 ± 3.3 | 157.3 ± 3.4 | 129.7 ± 2.4 a | 132.1 ± 1.8 | 138.1 ± 2.7 | 130.9 ± 2.8 | 129.5 ± 2.0 | 130.6 ± 3.2 | 137.5 ± 1.4 | 134.0 ± 2.8 | 132.4 ± 2.9 | 126.1 ± 3.3 |
| Tibial (mg) | 88.2 ± 3.1 | 81.0 ± 1.3 | 83.5 ± 3.1 | 84.0 ± 2.9 | 86.0 ± 2.4 | 84.8 ± 3.9 | 90.1 ± 2.7 | 82.9 ± 2.6 | 85.5 ± 2.0 | 84.8 ± 3.2 | 69.4 ± 2.4 a | 68.5 ± 2.7 | 72.40 ± 3.2 | 72.7 ± 1.8 | 70.6 ± 1.9 | 69.3 ± 2.1 | 76.2 ± 3.1 | 72.0 ± 2.1 | 68,0 ± 2.7 | 67.1 ± 2.0 |
| Soleus (mg) | 8.4 ± 0.6 | 7.6 ± 0.5 | 7.3 ± 0.3 | 8.3 ± 0.5 | 7.3 ± 0.3 | 7.6 ± 0.6 | 8.5 ± 0.5 | 6.9 ± 0.6 | 7.6 ± 0.5 | 7.9 ± 0.5 | 6.0 ± 0.4 a | 7.1 ± 0.5 | 6.9 ± 0.5 | 6.2 ± 0.3 | 6.5 ± 0.5 | 6.6 ± 0.3 | 6.6 ± 0.4 | 6.2 ± 0.4 | 6.3 ± 0.4 | 6.3 ± 0.3 |
| Sum of Muscle Weight (mg) | 255.5 ± 5.3 | 238.7 ± 5.6 | 242.9 ± 7.7 | 256.9 ± 2.8 | 250.9 ± 3.9 | 250.3 ± 8.2 | 254.2 ± 4.3 | 240.1 ± 6.1 | 255.3 ± 4.8 | 250.1 ± 6.5 | 205.1 ± 4.5 a | 207.7 ± 3.3 | 217.3 ± 5.7 | 209.8 ± 3.5 | 206.5 ± 3.6 | 206.5 ± 4.8 | 220.3 ± 3.8 | 212.2 ± 4.7 | 206.7 ± 5.3 | 199.5 ± 4.2 |
| Muscle weight over total weight (%) | 0.83 ± 0.01 | 0.85 ± 0.02 | 0.80 ± 0.01 | 0.87 ± 0.01 c | 0.81 ± 0.01 | 0.84 ± 0.02 | 0.85 ± 0.02 | 0.85 ± 0.02 | 0.83 ± 0.01 | 0.84 ± 0.01 | 0.85 ± 0.01 | 0.87 ± 0.02 | 0.89 ± 0.01 | 0.90 ± 0.02 | 0.85 ± 0.02 | 0.89 ± 0.01 | 0.88 ± 0.01 | 0.91 ± 0.01 b | 0.85 ± 0.02 | 0.87 ± 0.01 |
| iBAT (mg) | 178.5 ± 5.8 | 184.1 ± 10.0 | 188.0 ± 10.0 | 185.6 ± 7.1 | 189.4 ± 9.8 | 171.5 ± 10.6 | 185.3 ± 6.9 | 184.1 ± 8.2 | 185.7 ± 8.1 | 180.2 ± 10.0 | 179.5 ± 10.9 | 174.6 ± 8.1 | 186.1 ± 12.3 | 190.6 ± 10.2 | 183.4 ± 13.5 | 170.9 ± 7.7 | 193.7 ± 12.1 | 164.9 ± 10.4 | 177.5 ± 9.9 | 179.5 ± 5.6 |
| iWAT (mg) | 111.1 ± 3.86 | 103.5 ± 11.6 | 118.7 ± 8.8 | 106.2 ± 8.9 | 125.8 ± 6.9 | 101.5 ± 6.3 | 106.2 ± 9.1 | 107.9 ± 6.1 | 127.5 ± 8.4 | 113.9 ± 9.4 | 159.9 ± 9.3 a | 127.9 ± 7.1 | 161.0 ± 13.1 | 125.7 ± 8.2 | 169.2 ± 16.6 | 128.8 ± 8.2 c | 149.9 ± 8.3 | 122.3 ± 9.4 b | 148.2 ± 8.3 | 130.8 ± 6.5 |
| iWAT over total weight (%) | 0.34 ± 0.02 | 0.37 ± 0.04 | 0.40 ± 0.03 | 0.37 ± 0.03 | 0.41 ± 0.02 | 0.35 ± 0.02 | 0.36 ± 0.03 | 0.39 ± 0.03 | 0.42 ± 0.03 | 0.39 ± 0.03 | 0.65 ± 0.03 a | 0.54 ± 0.03 b | 0.65 ± 0.04 | 0.54 ± 0.03 | 0.67 ± 0.06 | 0.58 ± 0.04 | 0.63 ± 0.03 | 0.51 ± 0.03 b | 0.61 ± 0.03 | 0.57 ± 0.03 |
| SHAM | OVX | |||||||
|---|---|---|---|---|---|---|---|---|
| Saline/Sed | Saline/Exer | TLU/Sed | TLU/Exer | Saline/Sed | Saline/Exer | TLU/Sed | TLU/Exer | |
| Cholesterol (mg/dL) | 76.7 ± 8.2 | 87.7 ± 5.2 | 79.2 ± 4.4 | 83.3 ± 9.3 | 100.0 ± 6.7 | 85.12 ± 11.16 | 89.1 ± 3.2 | 87.1 ± 4.9 |
| HDL (mg/dL) | 51.5 ± 5.2 | 56.3 ± 4.3 | 48.7 ± 2.8 | 58.99 ± 2.1 | 59.0 ± 3.8 | 58.2 ± 5.7 | 57.5 ± 3.0 | 55.3 ± 3.8 |
| Non-HDL (mg/dL) | 30.4 ± 4.5 | 31.5 ± 2.3 | 30.5 ± 2.1 | 36.9 ± 3.6 | 41.0 ± 3.2 a | 35.1 ± 2.2 | 31.6 ± 2.2 | 31.8 ± 1.8 |
| Triglycerides (mg/DL) | 67.4 ± 7.3 | 66.1 ± 2.2 | 62.2 ± 6.1 | 74.9 ± 5.2 | 74.2 ± 5.7 | 70.8 ± 7.5 | 66.0 ± 8.2 | 70.4 ± 5.8 |
| TGO (U/L) | 56.5 ± 14.0 | 61.9 ± 13.8 | 48.9 ± 7.9 | 53.6 ± 9.5 | 60.9 ± 12.3 | 63.8 ± 7.8 | 44.4 ± 10.4 | 54.8 ±13.8 |
| TGP (U/L) | 32.6 ± 3.0 | 58.1 ± 17.6 | 38.3 ± 8.2 | 45.3 ± 10.2 | 57.1 ± 5.2 | 39.8 ± 11.8 | 31.2 ± 6.7 | 21.6 ± 18.7 |
| IL-1β | ND | ND | ND | ND | ND | ND | ND | ND |
| TNF | ND | ND | ND | ND | ND | ND | ND | ND |
| IL-10 | ND | ND | ND | ND | ND | ND | ND | ND |
| SHAM | ORX | |||||||
|---|---|---|---|---|---|---|---|---|
| Saline/Sed | Saline/Exer | TLU/Sed | TLU/Exer | Saline/Sed | Saline/Exer | TLU/Sed | TLU/Exer | |
| Cholesterol (mg/dL) | 103.0 ± 7.0 | 109.3 ± 11.2 | 91.9 ± 4.4 | 89.4 ± 5.2 | 106.1 ± 8.8 | 96.2 ± 10.1 | 99.2 ± 1.9 | 99.4 ± 6.4 |
| HDL (mg/dL) | 69.9 ± 7.5 | 71.2 ± 5.8 | 55.0 ± 3.4 | 61.0 ± 6.3 | 62.7 ± 5.0 | 64.0 ± 5.8 | 64.6 ± 5.0 | 67.0 ± 4.5 |
| Non-HDL (mg/dL) | 33.1 ± 2.2 | 24.6 ± 2.1 | 36.9 ± 4.1 | 28.4 ± 5.6 | 43.4 ± 6.5 | 32.3 ± 5.8 | 34.5 ± 5.5 | 32.4 ± 4.4 |
| Triglycerides (mg/DL) | 103.9 ± 1.5 | 104.5 ± 5.2 | 107.4 ± 19.1 | 102.3 ± 10.6 | 98.1 ± 12.7 | 93.74 ± 12.12 | 112.1 ± 15.3 | 106.9 ± 13.8 |
| TGO (U/L) | 101.2 ± 11.5 | 78.8 ± 9.1 | 118.0 ± 10.4 | 86.8 ± 12.3 | 90.3 ± 19.8 | 84.0 ± 14.3 | 95.2 ± 17.3 | 64.0 ± 9.5 |
| TGP (U/L) | 53.0 ± 10.9 | 48.5 ± 6.9 | 35.2 ± 4.3 | 38.8 ± 4.5 | 35.33 ± 4.9 | 37.5 ± 7.1 | 38.4 ± 9.1 | 37.6 ± 4.7 |
| IL-1β | ND | ND | ND | ND | ND | ND | ND | ND |
| TNF | ND | ND | ND | ND | ND | ND | ND | ND |
| IL-10 | ND | ND | ND | ND | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, J.P.; Silva, L.C.; Nunes, M.S.; Rübensam, G.; Oliveira, J.R.; Silva, R.B.M.; Campos, M.M. Combined Effects of Exercise and Phytoanabolic Extracts in Castrated Male and Female Mice. Nutrients 2021, 13, 1177. https://doi.org/10.3390/nu13041177
Martins JP, Silva LC, Nunes MS, Rübensam G, Oliveira JR, Silva RBM, Campos MM. Combined Effects of Exercise and Phytoanabolic Extracts in Castrated Male and Female Mice. Nutrients. 2021; 13(4):1177. https://doi.org/10.3390/nu13041177
Chicago/Turabian StyleMartins, Jerônimo P., Lucia C. Silva, Matheus S. Nunes, Gabriel Rübensam, Jarbas R. Oliveira, Rodrigo B. M. Silva, and Maria M. Campos. 2021. "Combined Effects of Exercise and Phytoanabolic Extracts in Castrated Male and Female Mice" Nutrients 13, no. 4: 1177. https://doi.org/10.3390/nu13041177
APA StyleMartins, J. P., Silva, L. C., Nunes, M. S., Rübensam, G., Oliveira, J. R., Silva, R. B. M., & Campos, M. M. (2021). Combined Effects of Exercise and Phytoanabolic Extracts in Castrated Male and Female Mice. Nutrients, 13(4), 1177. https://doi.org/10.3390/nu13041177







