Relationship between Mediterranean Diet Adherence and Saliva Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Anthropometric Parameters
2.2. Mediterranean Diet Adhesion Patterns Assessment
2.3. Saliva Collection and Cleaning
2.4. Saliva Laboratory Analysis
2.4.1. Saliva Flow Rate, pH, and Total Protein Concentration
2.4.2. SDS-PAGE Separation and Protein Profile Analysis
2.4.3. Salivary Amylase Enzymatic Activity
2.5. Statistical Analysis
3. Results
3.1. MD Adherence Participants’ Characteristics
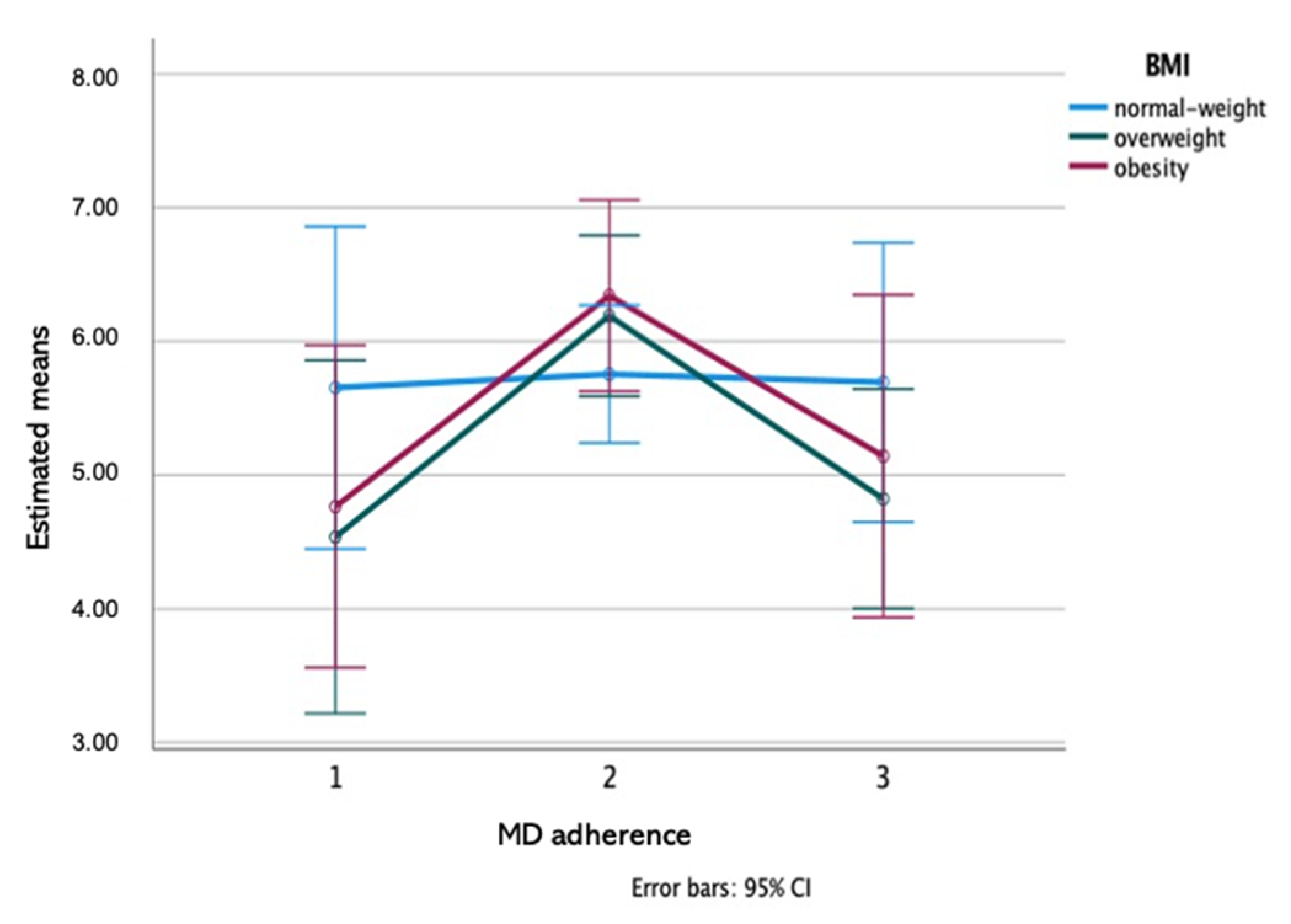
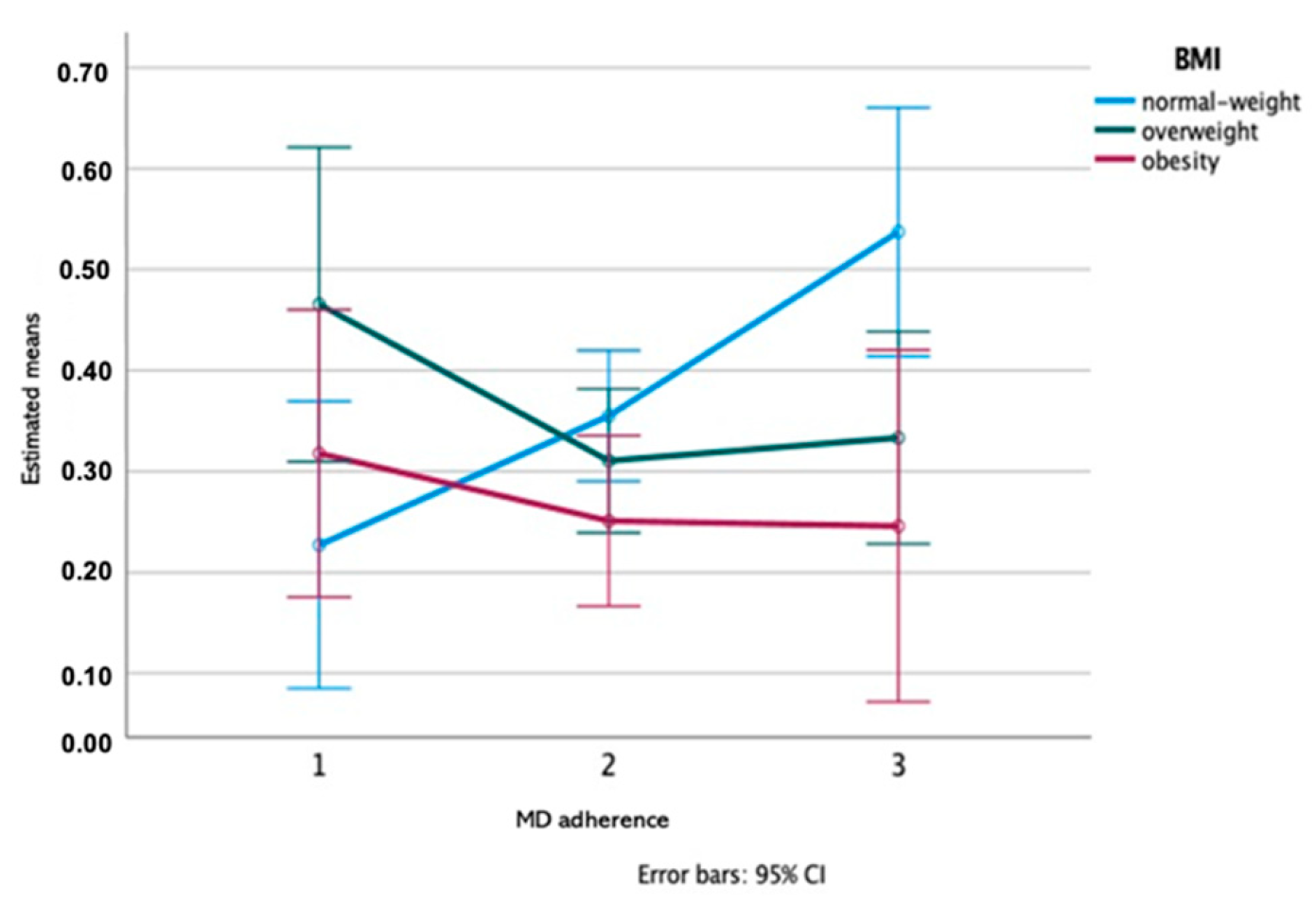
3.2. Variations in Saliva Composition among MD Adherence Groups
3.3. Association of Saliva Composition and Polyphenol/Flavanol Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouzas, C.; Bibiloni, M.D.M.; Julibert, A.; Ruiz-Canela, M.; Salas-Salvadó, J.; Corella, D.; Zomeño, M.D.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. Adherence to the Mediterranean Lifestyle and Desired Body Weight Loss in a Mediterranean Adult Population with Overweight: A PREDIMED-Plus Study. Nutrients 2020, 12, 2114. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Kyriacou, A.; Evans, J.M.; Economides, N.; Kyriacou, A. Adherence to the Mediterranean diet by the Greek and Cypriot population: A systematic review. Eur. J. Public Heal. 2015, 25, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- León-Muñoz, L.M.; Guallar-Castillón, P.; Graciani, A.; López-García, E.; Mesas, A.E.; Aguilera, M.T.; Banegas, J.R.; Rodríguez-Artalejo, F. Adherence to the Mediterranean Diet Pattern Has Declined in Spanish Adults. J. Nutr. 2012, 142, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Notarnicola, M.; Cisternino, A.M.; Inguaggiato, R.; Guerra, V.; Reddavide, R.; Donghia, R.; Rotolo, O.; Zinzi, I.; Leandro, G.; et al. Trends in adherence to the Mediterranean diet in South Italy: A cross sectional study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Gregório, M.J.; de Sousa, S.M.; Chkoniya, V.; Graça, P. Estudo de adesão ao padrão alimentar Mediterrânico; Lisboa. 2020. Available online: https://repositorio-aberto.up.pt/bitstream/10216/131751/2/438182.pdf (accessed on 8 April 2021).
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R.; et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Tvarijonaviciute, A.; Martinez-Subiela, S.; Lopez-Jornet, P.; Lamy, E. Saliva in Health and Disease The Present and Future of a Unique Sample for Diagnosis, 1st ed.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Dinnella, C.; Recchia, A.; Vincenzi, S.; Tuorila, H.; Monteleone, E. Temporary Modification of Salivary Protein Profile and Individual Responses to Repeated Phenolic Astringent Stimuli. Chem. Senses 2009, 35, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Dinnella, C.; Recchia, A.; Tuorila, H.; Monteleone, E. Individual astringency responsiveness affects the acceptance of phenol-rich foods. Appetite 2011, 56, 633–642. [Google Scholar] [CrossRef]
- Rodrigues, L.; Da Costa, G.; Cordeiro, C.; Pinheiro, C.C.; Amado, F.; Lamy, E. Relationship between saliva protein composition and 6-n -Propylthiouracil bitter taste responsiveness in young adults. J. Sens. Stud. 2017, 32, e12275. [Google Scholar] [CrossRef]
- Martin, L.E.; Nikonova, L.V.; Kay, K.E.; Torregrossa, A.-M. Altering salivary protein profile can increase acceptance of a novel bitter diet. Appetite 2019, 136, 8–17. [Google Scholar] [CrossRef]
- Lamy, E.; Da Costa, G.; Santos, R.; Capela e Silva, F.; Potes, J.; Pereira, A.; Coelho, A.V.; Baptista, E.S. Effect of condensed tannin ingestion in sheep and goat parotid saliva proteome. J. Anim. Physiol. Anim. Nutr. 2010, 95, 304–312. [Google Scholar] [CrossRef]
- Lamy, E.; Graça, G.; Da Costa, G.; Franco, C.; Capela e Silva, F.; Baptista, E.S.; Coelho, A.V. Changes in mouse whole saliva soluble proteome induced by tannin-enriched diet. Proteome Sci. 2010, 8, 1–12. [Google Scholar] [CrossRef]
- Crawford, C.R.; Running, C.A. Addition of chocolate milk to diet corresponds to protein concentration changes in human saliva. Physiol. Behav. 2020, 225, 113080. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and managing the global epidemic. WHO Technical Report Series 894; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Lopes, C.; Aro, A.; Azevedo, A.; Ramos, E.; Barros, H. Intake and Adipose Tissue Composition of Fatty Acids and Risk of Myocardial Infarction in a Male Portuguese Community Sample. J. Am. Diet. Assoc. 2007, 107, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes among High-Risk Subjects: The PREDIMED Trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- Gregório, M.J.; Rodrigues, A.M.; Salvador, C.; Dias, S.S.; De Sousa, R.D.; Mendes, J.M.; Coelho, P.S.; Branco, J.C.; Lopes, C.; Martínez-González, M.A.; et al. Validation of the Telephone-Administered Version of the Mediterranean Diet Adherence Screener (MEDAS) Questionnaire. Nutr. 2020, 12, 1511. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; Du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Lamy, E.; Simões, C.; Rodrigues, L.; Costa, A.R.; Vitorino, R.; Amado, F.; Antunes, C.; Carmo, I.D. Changes in the salivary protein profile of morbidly obese women either previously subjected to bariatric surgery or not. J. Physiol. Biochem. 2015, 71, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Santos, V.; Barrambana, S.; Simões, C.; Carreira, L.; Infante, P.; Capela e Silva, F. Saliva Protein Composition Relates with Interindividual Variations in Bread Sensory Ratings. Starch Stärke 2021, 73. [Google Scholar] [CrossRef]
- Carreira, L.; Castelo, P.M.; Simões, C.; Capela e Silva, F.; Viegas, C.; Lamy, E. Changes in Salivary Proteome in Response to Bread Odour. Nutrients 2020, 12, 1002. [Google Scholar] [CrossRef]
- Mandel, A.L.; Gachons, C.P.D.; Plank, K.L.; Alarcon, S.; Breslin, P.A.S. Individual Differences in AMY1 Gene Copy Number, Salivary α-Amylase Levels, and the Perception of Oral Starch. PLoS ONE 2010, 5, e13352. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Vitorino, R.; Osório, H.; Fernandes, A.; Venâncio, A.; Mateus, N.; Amado, F.; De Freitas, V. Reactivity of Human Salivary Proteins Families Toward Food Polyphenols. J. Agric. Food Chem. 2011, 59, 5535–5547. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.J.; Lilley, T.H.; Haslam, E.; Williamson, M.P. Multiple Interactions between Polyphenols and a Salivary Proline-Rich Protein Repeat Result in Complexation and Precipitation†. Biochem. 1997, 36, 5566–5577. [Google Scholar] [CrossRef] [PubMed]
- Notario-Barandiaran, L.; Project, O.B.O.T.I.; Valera-Gran, D.; Gonzalez-Palacios, S.; Garcia-De-La-Hera, M.; Fernández-Barrés, S.; Pereda-Pereda, E.; Fernández-Somoano, A.; Guxens, M.; Iñiguez, C.; et al. High adherence to a mediterranean diet at age 4 reduces overweight, obesity and abdominal obesity incidence in children at the age of 8. Int. J. Obes. 2020, 44, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Marrugat, J.; Vila, J.; Covas, M.I.; Elosua, R. Adherence to the Traditional Mediterranean Diet Is Inversely Associated with Body Mass Index and Obesity in a Spanish Population. J. Nutr. 2004, 134, 3355–3361. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.; Medina-Remón, A.; Martínez-González, M.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Rodrigues, L.; Costa, G.; Cordeiro, C.; Pinheiro, C.; Amado, F.; Lamy, E. Salivary proteome and glucose levels are related with sweet taste sensitivity in young adults. Food Nutr. Res. 2017, 61, 1389208. [Google Scholar] [CrossRef] [PubMed]
- Dsamou, M.; Morzel, M.; Le Corre, L.; Séverin, I.; Chagnon, M.-C. Caffeine increases the expression of cystatin SN in human submandibular acinar-like HSG cells. Arch. Oral Biol. 2013, 58, 1511–1516. [Google Scholar] [CrossRef]
- Da Costa, G.; Lamy, E.; Capela e Silva, F.; Andersen, J.; Baptista, E.S.; Coelho, A.V. Salivary Amylase Induction by Tannin-Enriched Diets as a Possible Countermeasure Against Tannins. J. Chem. Ecol. 2008, 34, 376–387. [Google Scholar] [CrossRef]
- Martin, L.E.; Kay, K.E.; Torregrossa, A.-M. Bitter-Induced Salivary Proteins Increase Detection Threshold of Quinine, But Not Sucrose. Chem. Senses 2019, 44, 379–388. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, A.-M.; Nikonova, L.; Bales, M.B.; Leal, M.V.; Smith, J.C.; Contreras, R.J.; Eckel, L.A. Induction of Salivary Proteins Modifies Measures of Both Orosensory and Postingestive Feedback during Exposure to a Tannic Acid Diet. PLoS ONE 2014, 9, e105232. [Google Scholar] [CrossRef]
- Santos, J.; Saus, E.; Smalley, S.; Cataldo, L.; Alberti, G.; Parada, J.; Gratacòs, M.; Estivill, X. Copy Number Polymorphism of the Salivary Amylase Gene: Implications in Human Nutrition Research. J. Nutr. Nutr. 2012, 5, 117–131. [Google Scholar] [CrossRef]
- Mennella, I.; Fogliano, V.; Vitaglione, P. Salivary lipase and α-amylase activities are higher in overweight than in normal weight subjects: Influences on dietary behavior. Food Res. Int. 2014, 66, 463–468. [Google Scholar] [CrossRef]
- Aldossari, N.M.; El Gabry, E.E.; Gawish, G.E. Association between salivary amylase enzyme activity and obesity in Saudi Arabia. Med. 2019, 98, e15878. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Padilla, M.; Maldonado-Montero, E.F.; Enguix-Armada, A.; Del Paso, G.A.R. Salivary Alpha-Amylase Mediates the Increase in Hunger Levels in Adolescents with Excess Weight after Viewing Food Images. Child. Obes. 2020, 16, 53–58. [Google Scholar] [CrossRef]
- Mosca, A.C.; Stieger, M.; Neyraud, E.; Brignot, H.; Van De Wiel, A.; Chen, J. How are macronutrient intake, BMI, ethnicity, age, and gender related to the composition of unstimulated saliva? A case study. J. Texture Stud. 2018, 50, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Falchi, M.; Moustafa, J.S.E.-S.; Takousis, P.; Pesce, F.; Bonnefond, A.; Andersson-Assarsson, J.C.; Sudmant, P.H.; Dorajoo, R.; Al-Shafai, M.N.; Bottolo, L.; et al. Low copy number of the salivary amylase gene predisposes to obesity. Nat. Genet. 2014, 46, 492–497. [Google Scholar] [CrossRef]

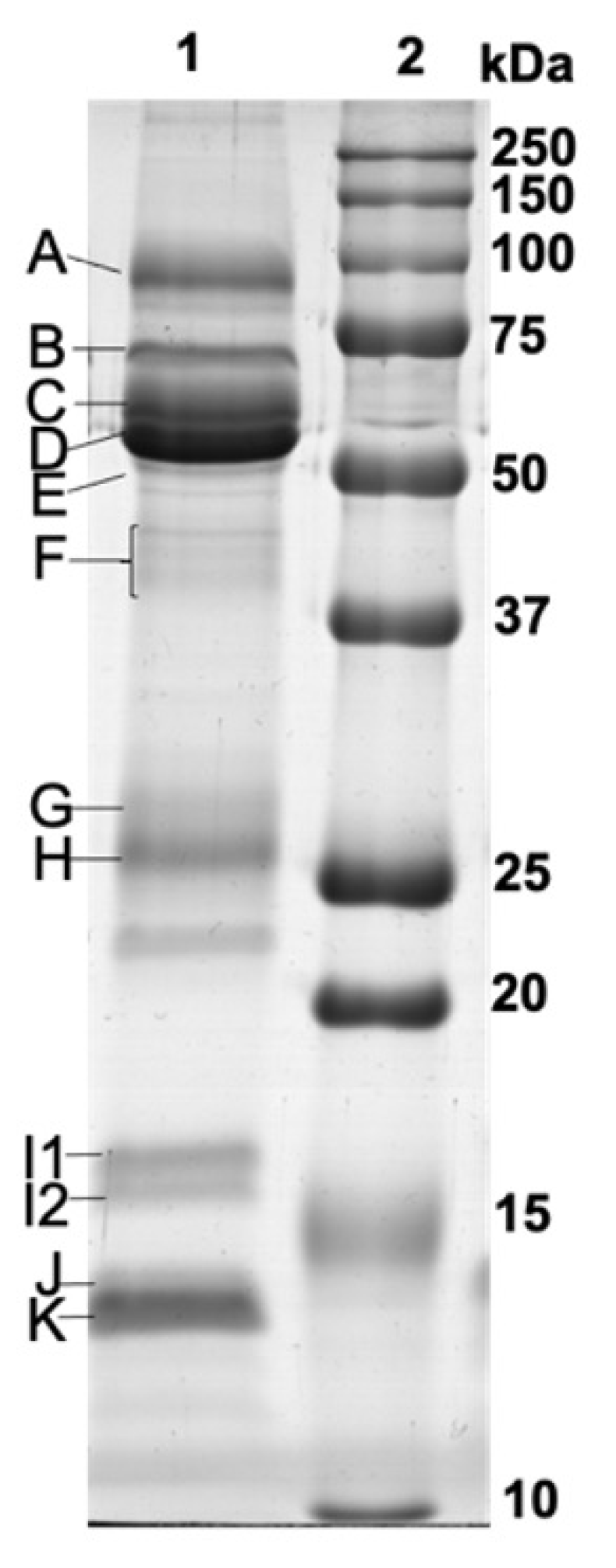
| MD Adherence | n | Band A | Band B | Band C | Band D | Band E | Band F | Band G | Band H | Band I1 | Band I2 | Band J | Band K | Amy_ Protein (U/mg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 (low) | 18 | 5.7 ± 1.2 | 7.7 ± 2.3 | 9.8 ± 2.2 | 8.1 ± 1.7 | 2.1 ± 0.8 | 7.5 ± 1.3 | 4.4 ± 0.8 | 7.2 ± 1.5 | 3.4 ± 1.0 | 3.2 ± 1.3 | 7.5 ± 2.7 | 5.0 ± 1.1 A | 0.3 ± 0.2 |
| Group 2 (medium) | 76 | 5.7 ± 1.4 | 7.1 ± 2.2 | 9.0 ± 1.8 | 8.4 ± 2.7 | 1.8 ± 0.4 | 8.1 ± 1.3 | 4.3 ± 0.7 | 7.4 ± 1.5 | 3.5 ± 0.7 | 3.1 ± 0.8 | 8.1 ± 2.7 | 6.0 ± 1.5 B | 0.3 ± 0.2 |
| Group 3 (high) | 28 | 5.7 ± 1.6 | 7.0 ± 2.5 | 9.4 ± 2.4 | 8.6 ± 2.4 | 1.8 ± 0.4 | 7.8 ± 1.3 | 3.9 ± 0.9 | 7.4 ± 1.5 | 3.4 ± 0.7 | 3.2 ± 0.8 | 7.9 ± 1.9 | 5.1 ± 1.7 A | 0.4 ± 0.2 |
| p-value | ||||||||||||||
| MD adherence | 0.880 | 0.597 | 0.506 | 0.947 | 0.206 | 0.389 | 0.093 | 0.883 | 0.931 | 0.600 | 0.615 | 0.005 * | 0.324 | |
| BMI | 0.649 | 0.816 | 0.924 | 0.146 | 0.284 | 0.921 | 0.203 | 0.213 | 0.145 | 0.471 | 0.675 | 0.431 | 0.108 | |
| MD adher × BMI | 0.292 | 0.230 | 0.966 | 0.139 | 0.077 | 0.930 | 0.428 | 0.178 | 0.330 | 0.142 | 0.292 | 0.264 | 0.019 * | |
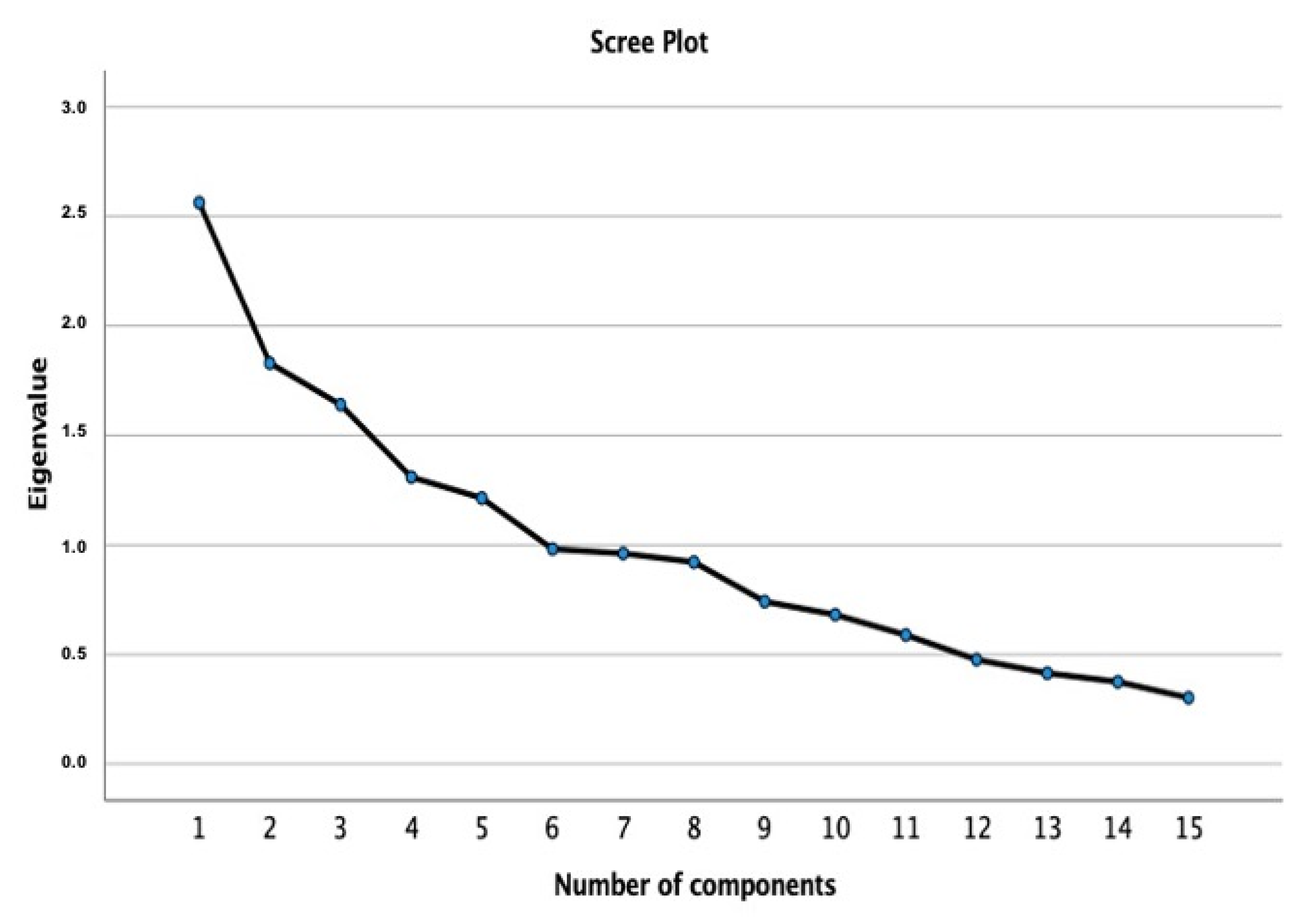
| Components | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| band A | 0.768 | ||||
| band B | −0.442 | ||||
| band C | 0.581 | 0.538 | 0.320 | ||
| band D | 0.761 | ||||
| band E | 0.639 | ||||
| band F | 0.449 | −0.435 | 0.438 | ||
| band G | −0.307 | 0.478 | |||
| band H | 0.661 | −0.367 | |||
| band I1 | 0.628 | ||||
| band I2 | 0.635 | ||||
| band J | 0.799 | ||||
| band K | −0.344 | −0.435 | 0.373 | −0.338 | |
| flavanoids intake | 0.754 | 0.403 | |||
| polyphenol intake | 0.761 | ||||
| Amy_protein (U/µg) | 0.778 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louro, T.; Simões, C.; Penetra, M.J.; Carreira, L.; Castelo, P.M.; Luis, H.; Moreira, P.; Lamy, E. Relationship between Mediterranean Diet Adherence and Saliva Composition. Nutrients 2021, 13, 1246. https://doi.org/10.3390/nu13041246
Louro T, Simões C, Penetra MJ, Carreira L, Castelo PM, Luis H, Moreira P, Lamy E. Relationship between Mediterranean Diet Adherence and Saliva Composition. Nutrients. 2021; 13(4):1246. https://doi.org/10.3390/nu13041246
Chicago/Turabian StyleLouro, Teresa, Carla Simões, Maria João Penetra, Laura Carreira, Paula Midori Castelo, Henrique Luis, Pedro Moreira, and Elsa Lamy. 2021. "Relationship between Mediterranean Diet Adherence and Saliva Composition" Nutrients 13, no. 4: 1246. https://doi.org/10.3390/nu13041246
APA StyleLouro, T., Simões, C., Penetra, M. J., Carreira, L., Castelo, P. M., Luis, H., Moreira, P., & Lamy, E. (2021). Relationship between Mediterranean Diet Adherence and Saliva Composition. Nutrients, 13(4), 1246. https://doi.org/10.3390/nu13041246









