Whole and Isolated Protein Fractions Differentially Affect Gastrointestinal Integrity Markers in C57Bl/6 Mice Fed Diets with a Moderate-Fat Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Purified Diet Rationale and Composition
2.2. Purified Diets—Animal Growth
2.3. Plasma Endotoxin Assays
2.4. In Vivo Gastrointestinal Permeability and Motility
2.5. Plasma Cytokines and IgA
2.6. Intestinal Enzyme Assays
2.7. Histology
2.8. Gene Expression
2.9. Cecal Metabolite Content
2.10. Chow-Fed Reference Mice
2.11. Statistical Analyses
3. Results
3.1. Animal Growth and Development
3.2. In Vivo Gastrointestinal Permeability and Motility
3.3. Systemic Inflammatory Markers
3.4. Intestinal Enzyme Assays
3.5. Histology
3.6. Gene Expression
3.7. Cecal Metabolite Content
3.8. Chow-Fed Reference Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, N.; Lewis, P.; Samuelson, D.; Liboni, K.; Neu, J. Glutamine regulates Caco-2 cell tight junction proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G726–G733. [Google Scholar] [CrossRef]
- Hamilton, M.K.; Boudry, G.; Lemay, D.G.; Raybould, H.E. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G840–G851. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.C.; Wisniewski, P.J.; Noji, M.; McGuinness, L.R.; Haggblom, M.M.; Lightfoot, S.A.; Joseph, L.B.; Kerkhof, L.J. The Effect of Diet and Exercise on Intestinal Integrity and Microbial Diversity in Mice. PLoS ONE 2016, 11, e0150502. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.Y.; Ha, C.W.; Campbell, C.R.; Mitchell, A.J.; Dinudom, A.; Oscarsson, J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE 2012, 7, e34233. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Alaniz, R.C.; Wood, T.K.; Jayaraman, A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Brahe, L.K.; Astrup, A.; Larsen, L.H. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes. Rev. 2013, 14, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Brun, P.; Castagliuolo, I.; Leo, V.D.; Buda, A.; Pinzani, M.; Palù, G.; Martines, D. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G518–G525. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.F.; Collado, M.C.; Ferreira, C.L.; Bressan, J.; Peluzio Mdo, C. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr. Res. 2012, 32, 637–647. [Google Scholar] [CrossRef]
- Candido, F.G.; Valente, F.X.; Grzeskowiak, L.M.; Moreira, A.P.B.; Rocha, D.; Alfenas, R.C.G. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: Mechanisms and clinical implications on obesity. Int. J. Food Sci. Nutr. 2018, 69, 125–143. [Google Scholar] [CrossRef]
- United States Department of Agriculture. United States Department of Agriculture. ChooseMyPlate.gov. Available online: https://www.choosemyplate.gov/dairy (accessed on 22 May 2020).
- Zemel, M.B. The role of dairy foods in weight management. J. Am. Coll. Nutr. 2005, 24, 537S–546S. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Shi, H.; Greer, B.; Dirienzo, D.; Zemel, P.C. Regulation of adiposity by dietary calcium. FASEB J. 2000, 14, 1132–1138. [Google Scholar] [CrossRef]
- Larrson, S.C.; Bergkvist, L.; Rutegard, J.; Giovannucci, E.; Wolk, A. Calcium and dairy food intakes are inversely associated with colorectal cancer risk in the Cohort of Swedish Men. Am. J. Clin. Nutr. 2006, 83, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B. Mechanisms of dairy modulation of adiposity. J. Nutr. 2003, 133, 252S–256S. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.P.; Dunn, T.N.; Drayton, J.B.; Oort, P.J.; Adams, S.H. A high calcium diet containing nonfat dry milk reduces weight gain and associated adipose tissue inflammation in diet-induced obese mice when compared to high calcium alone. Nutr. Metab. 2012, 9, 3. [Google Scholar] [CrossRef]
- Snow, D.R.; Jimenez-Flores, R.; Ward, R.E.; Cambell, J.; Young, M.J.; Nemere, I.; Hintze, K.J. Dietary milk fat globule membrane reduces the incidence of aberrant crypt foci in Fischer-344 rats. J. Agric. Food Chem. 2010, 58, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.R.; Ward, R.E.; Olsen, A.; Jimenez-Flores, R.; Hintze, K.J. Membrane-rich milk fat diet provides protection against gastrointestinal leakiness in mice treated with lipopolysaccharide. J. Dairy Sci. 2011, 94, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Spitsberg, V.L. Invited Review: Bovine milk fat globule membrane as a potential nutraceutical. J. Dairy Sci. 2005, 88, 2289–2294. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Sacco, R.E.; Nonnecke, B.J.; Lippolis, J.D. Bovine milk proteome: Quantitative changes in normal milk exosomes, milk fat globule membranes and whey proteomes resulting from Staphylococcus aureus mastitis. J. Proteom. 2013, 82, 141–154. [Google Scholar] [CrossRef]
- Wat, E.; Tandy, S.; Kapera, E.; Kamili, A.; Chung, R.W.; Brown, A.; Rowney, M.; Cohn, J.S. Dietary phospholipid-rich dairy milk extract reduces hepatomegaly, hepatic steatosis and hyperlipidemia in mice fed a high-fat diet. Atherosclerosis 2009, 205, 144–150. [Google Scholar] [CrossRef]
- Walzem, R.L.; Dillard, C.J.; German, J.B. Whey Components: Millennia of Evolution Create Functionalities for Mammalian Nutrition: What We Know and What We May Be Overlooking. Crit. Rev. Food Sci. Nutr. 2002, 42, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.P.; Dunn, T.N.; Oort, P.J.; Grino, M.; Adams, S.H. Inflammatory Phenotyping Identifies CD11d as a Gene Markedly Induced in White Adipose Tissue in Obese Rodents and Women. J. Nutr. 2011, 141, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Hintze, K.J.; Benninghoff, A.D.; Ward, R.E. Formulation of the Total Western Diet (TWD) as a basal diet for rodent cancer studies. J. Agric. Food Chem. 2012, 60, 6736–6742. [Google Scholar] [CrossRef] [PubMed]
- Diets, T. Teklad S-2335 Mouse Breeder Sterilizable Diet. Available online: https://www.envigo.com/resources/data-sheets/7004-datasheet-0915.pdf (accessed on 22 August 2019).
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture: Alexandria, VA, USA; U.S. Department of Health and Human Services: Rockville, MD, USA, 2020.
- Hwang, J.; Singh, N.; Long, C.; Smith, S.B. The Lentiviral System Construction for Highly Expressed Porcine Stearoyl-CoA Desaturase-1 and Functional Characterization in Stably Transduced Porcine Swine Kidney Cells. Lipids 2018, 53, 933–945. [Google Scholar] [CrossRef]
- Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jiménez-Flores, R. Sources, Production, and Clinical Treatments of Milk Fat Globule Membrane for Infant Nutrition and Well-Being. Nutrients 2020, 12, 1607. [Google Scholar] [CrossRef]
- Mehta, B.M. Chemical composition of milk and milk products. In Handbook of Food Chemistry; Cheung, P.C.K., Mehta, B.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 511–553. [Google Scholar] [CrossRef]
- Storr, M.A.; Bashashati, M.; Hirota, C.; Vemuri, V.K.; Keenan, C.M.; Duncan, M.; Lutz, B.; Mackie, K.; Makriyannis, A.; Macnaughton, W.K.; et al. Differential effects of CB(1) neutral antagonists and inverse agonists on gastrointestinal motility in mice. Neurogastroenterol. Motil. 2010, 22, 787-e223. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Vijay-Kumar, M.; Sanders, C.J.; Taylor, R.T.; Kumar, A.; Aitken, J.D.; Sitaraman, S.V.; Neish, A.S.; Uematsu, S.; Akira, S.; Williams, I.R.; et al. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Investig. 2007, 117, 3909–3921. [Google Scholar] [CrossRef]
- Ludgero-Correia, A., Jr.; Aguila, M.B.; Mandarim-de-Lacerda, C.A.; Faria, T.S. Effects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized C57BL/6 mice. Nutrition 2012, 28, 316–323. [Google Scholar] [CrossRef]
- Catta-Preta, M.; Martins, M.A.; Cunha Brunini, T.M.; Mendes-Ribeiro, A.C.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Modulation of cytokines, resistin, and distribution of adipose tissue in C57BL/6 mice by different high-fat diets. Nutrition 2012, 28, 212–219. [Google Scholar] [CrossRef]
- Alex, P.; Zachos, N.C.; Nguyen, T.; Gonzales, L.; Chen, T.E.; Conklin, L.S.; Centola, M.; Li, X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel. Dis. 2009, 15, 341–352. [Google Scholar] [CrossRef]
- Nov, O.; Shapiro, H.; Ovadia, H.; Tarnovscki, T.; Dvir, I.; Shemesh, E.; Kovsan, J.; Shelef, I.; Carmi, Y.; Voronov, E.; et al. Interleukin-1β regulates fat-liver crosstalk in obesity by auto-paracrine modulation of adipose tissue inflammation and expandability. PLoS ONE 2013, 8, e53626. [Google Scholar] [CrossRef]
- Zhang, C.; Monk, J.M.; Lu, J.T.; Zarepoor, L.; Wu, W.; Liu, R.; Pauls, K.P.; Wood, G.A.; Robinson, L.; Tsao, R.; et al. Cooked navy and black bean diets improve biomarkers of colon health and reduce inflammation during colitis. Br. J. Nutr. 2014, 111, 1549–1563. [Google Scholar] [CrossRef]
- Minamoto, Y.; Minamoto, T.; Isaiah, A.; Sattasathuchana, P.; Buono, A.; Rangachari, V.R.; McNeely, I.H.; Lidbury, J.; Steiner, J.M.; Suchodolski, J.S. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 2019, 33, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.-H.; Lee, S.-O.; Sridharan, G.; Lee, K.; Davidson, L.A.; Jayaraman, A.; Chapkin, R.S.; Alaniz, R.; Safe, S. Microbiome-Derived Tryptophan Metabolites and Their Aryl Hydrocarbon Receptor-Dependent Agonist and Antagonist Activities. Mol. Pharmacol. 2014, 85, 777–788. [Google Scholar] [CrossRef]
- Di Marzo, V.; Sepe, N.; De Petrocellis, L.; Berger, A.; Crozier, G.; Fride, E.; Mechoulam, R. Trick or treat from food endocannabinoids? Nature 1998, 396, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Jager, G.; Witkamp, R.F. The endocannabinoid system and appetite: Relevance for food reward. Nutr. Res. Rev. 2014, 27, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, T.C. Endocannabinoids in the regulation of appetite and body weight. Behav. Pharmacol. 2005, 16, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.G.; Naslain, D.; Backhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef]
- Mattes, R.D.; Engelman, K.; Shaw, L.M.; Elsohly, M.A. Cannabinoids and appetite stimulation. Pharmacol. Biochem. Behav. 1994, 49, 187–195. [Google Scholar] [CrossRef]
- Body Weight Information For C57BL/6J. Available online: https://www.jax.org/jax-mice-and-services/strain-data-sheet-pages/body-weight-chart-000664 (accessed on 1 December 2018).
- Onaivi, E.S.; Carpio, O.; Ishiguro, H.; Schanz, N.; Uhl, G.R.; Benno, R. Behavioral effects of CB2 cannabinoid receptor activation and its influence on food and alcohol consumption. Ann. N. Y. Acad. Sci. 2008, 1139, 426–433. [Google Scholar] [CrossRef]
- Zayat, M.; Lichtenberger, L.M.; Dial, E.J. Pathophysiology of LPS-induced gastrointestinal injury in the rat: Role of secretory phospholipase A2. Shock 2008, 30, 206–211. [Google Scholar] [CrossRef]
- Fink, M.P.; Antonsson, J.B.; Wang, H.; Rothschild, H.R. Increased Intestinal Permeability in Endotoxic Pigs—Mesenteric Hypoperfusion as an Etiologic Factor. Arch. Surg. 1991, 126, 211–218. [Google Scholar] [CrossRef]
- Tellez, G.; Latorre, J.D.; Kuttappan, V.A.; Kogut, M.H.; Wolfenden, A.; Hernandez-Velasco, X.; Hargis, B.M.; Bottje, W.G.; Bielke, L.R.; Faulkner, O.B. Utilization of rye as energy source affects bacterial translocation, intestinal viscosity, microbiota composition, and bone mineralization in broiler chickens. Front. Genet. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Alizadeh, M.; Lotfi Yagin, N.; Pasdar, Y.; Nachvak, S.M.; Abdollahzad, H.; Sadeghpour Tabaei, A. New insights on atherosclerosis: A cross-talk between endocannabinoid systems with gut microbiota. J. Cardiovasc. Thorac. Res. 2018, 10, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Silva, F.J.; Sanchez-Vera, I.; Suarez, J.; Serrano, A.; Fuentes, E.; Juan-Pico, P.; Nadal, A.; Rodriguez de Fonseca, F. Role of cannabinoid CB2 receptors in glucose homeostasis in rats. Eur. J. Pharmacol. 2007, 565, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiu, V.; Battistini, L.; Maccarrone, M. Endocannabinoid signalling in innate and adaptive immunity. Immunology 2015, 144, 352–364. [Google Scholar] [CrossRef]
- Montecucco, F.; Di Marzo, V.; da Silva, R.F.; Vuilleumier, N.; Capettini, L.; Lenglet, S.; Pagano, S.; Piscitelli, F.; Quintao, S.; Bertolotto, M.; et al. The activation of the cannabinoid receptor type 2 reduces neutrophilic protease-mediated vulnerability in atherosclerotic plaques. Eur. Heart J. 2012, 33, 846–856. [Google Scholar] [CrossRef]
- Cavaglieri, C.R.; Nishiyama, A.; Fernandes, L.C.; Curi, R.; Miles, E.A.; Calder, P.C. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003, 73, 1683–1690. [Google Scholar] [CrossRef]
- Whitfield-Cargile, C.M.; Cohen, N.D.; Chapkin, R.S.; Weeks, B.R.; Davidson, L.A.; Goldsby, J.S.; Hunt, C.L.; Steinmeyer, S.H.; Menon, R.; Suchodolski, J.S.; et al. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes 2016, 7, 246–261. [Google Scholar] [CrossRef]
- Guard, B.C.; Barr, J.W.; Reddivari, L.; Klemashevich, C.; Jayaraman, A.; Steiner, J.M.; Vanamala, J.; Suchodolski, J.S. Characterization of microbial dysbiosis and metabolomic changes in dogs with acute diarrhea. PLoS ONE 2015, 10, e0127259. [Google Scholar] [CrossRef]
- Cardona, M.E.; Collinder, E.; Stern, S.; Tjellström, B.; Norin, E.; Midtvedt, T. Correlation between faecal iso-butyric and iso-valeric acids in different species. Microb. Ecol. Health Dis. 2009, 17, 177–182. [Google Scholar] [CrossRef]
- Tjellstrom, B.; Stenhammar, L.; Hogberg, L.; Falth-Magnusson, K.; Magnusson, K.E.; Midtvedt, T.; Sundqvist, T.; Norin, E. Gut microflora associated characteristics in children with celiac disease. Am. J. Gastroenterol. 2005, 100, 2784–2788. [Google Scholar] [CrossRef]
- Lau, D.; Mollnau, H.; Eiserich, J.P.; Freeman, B.A.; Daiber, A.; Gehling, U.M.; Brummer, J.; Rudolph, V.; Munzel, T.; Heitzer, T.; et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc. Natl. Acad. Sci. USA 2005, 102, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Madsen, K.; Doyle, J.; Meddings, J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 2009, 58, 41–48. [Google Scholar] [CrossRef]
- Burgess, A.R.; Trujillo, A.N.; Barter, M.; Breslin, J.W. Ileal smooth muscle thickness is greater in young obese Zucker rats. FASEB J. 2017, 31, 683–685. [Google Scholar] [CrossRef]
- Stark, A.; Nyska, A.; Madar, Z. Metabolic and morphometric changes in small and large intestine in rats fed high-fiber diets. Toxicol. Pathol. 1996, 24, 166–171. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kuhl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar]
- Lalles, J.P. Intestinal alkaline phosphatase: Multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr. Rev. 2010, 68, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Bol-Schoenmakers, M.; Fiechter, D.; Raaben, W.; Hassing, I.; Bleumink, R.; Kruijswijk, D.; Maijoor, K.; Tersteeg-Zijderveld, M.; Brands, R.; Pieters, R. Intestinal alkaline phosphatase contributes to the reduction of severe intestinal epithelial damage. Eur. J. Pharmacol. 2010, 633, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Paiva, D.M.; Walk, C.L.; McElroy, A.P. Influence of dietary calcium level, calcium source, and phytase on bird performance and mineral digestibility during a natural necrotic enteritis episode. Poult. Sci. 2013, 92, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Melgar, S.; Karlsson, L.; Rehnstrom, E.; Karlsson, A.; Utkovic, H.; Jansson, L.; Michaelsson, E. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int. Immunopharmacol. 2008, 8, 836–844. [Google Scholar] [CrossRef]
- Bing, C. Is interleukin-1β a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte 2015, 4, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Owyang, A.M.; Maedler, K.; Gross, L.; Yin, J.; Esposito, L.; Shu, L.; Jadhav, J.; Domsgen, E.; Bergemann, J.; Lee, S.; et al. XOMA 052, an anti-IL-1β monoclonal antibody, improves glucose control and β-cell function in the diet-induced obesity mouse model. Endocrinology 2010, 151, 2515–2527. [Google Scholar] [CrossRef]
- Getz, G.S.; Reardon, C.A. Diet and Murine Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 242–249. [Google Scholar] [CrossRef]
- Savard, C.; Tartaglione, E.V.; Kuver, R.; Haigh, W.G.; Farrell, G.C.; Subramanian, S.; Chait, A.; Yeh, M.M.; Quinn, L.S.; Ioannou, G.N. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology 2013, 57, 81–92. [Google Scholar] [CrossRef]

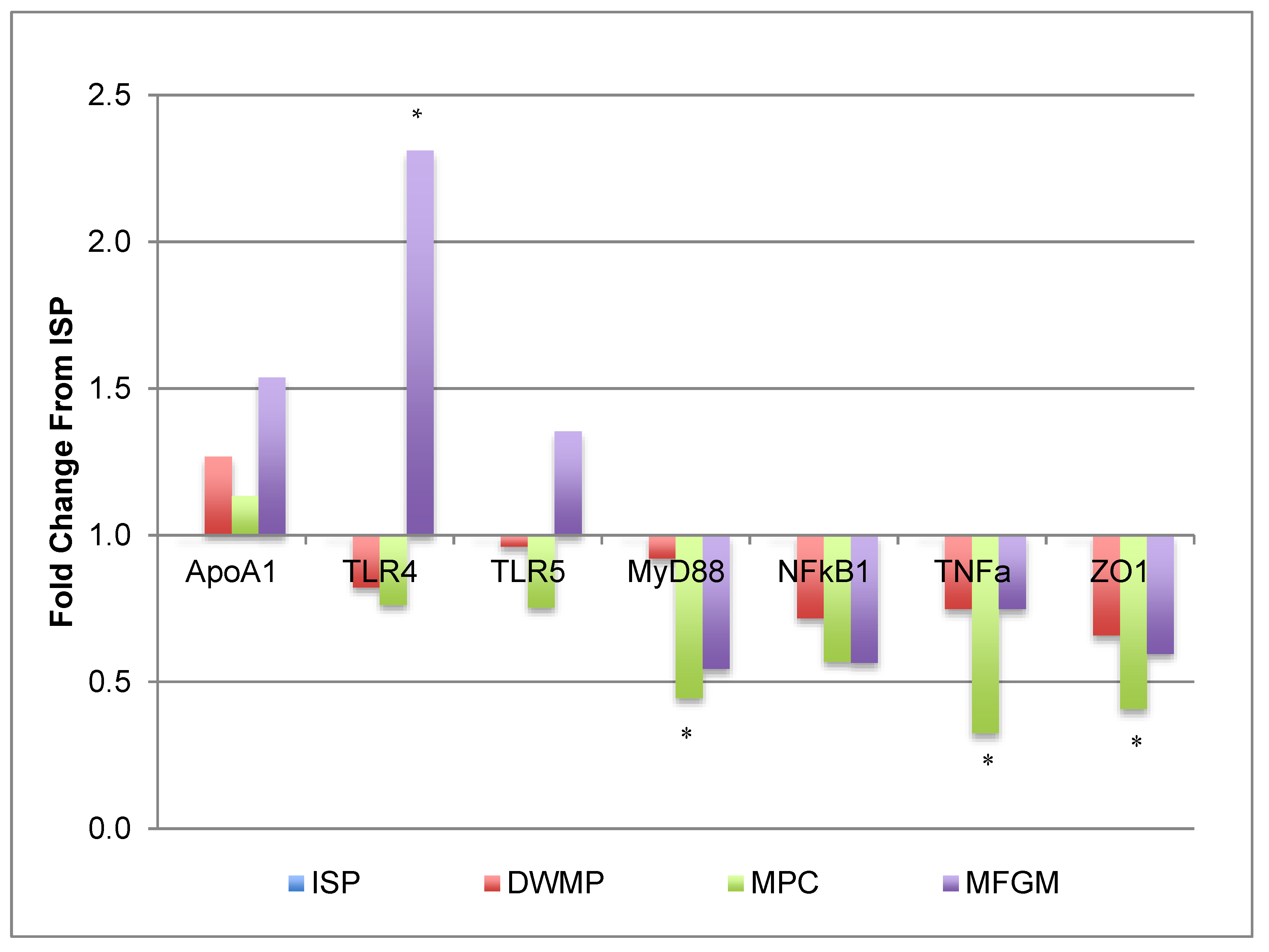
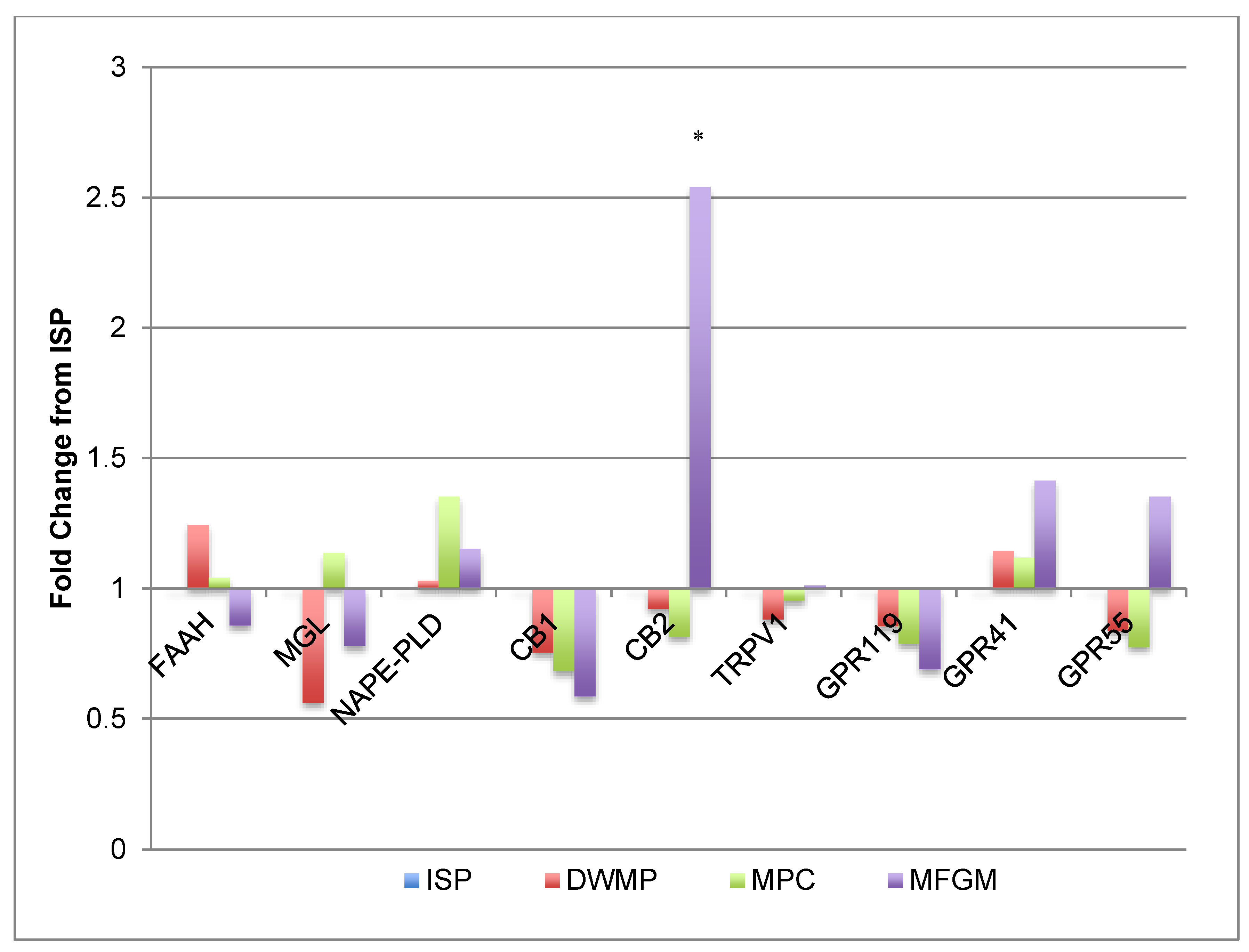
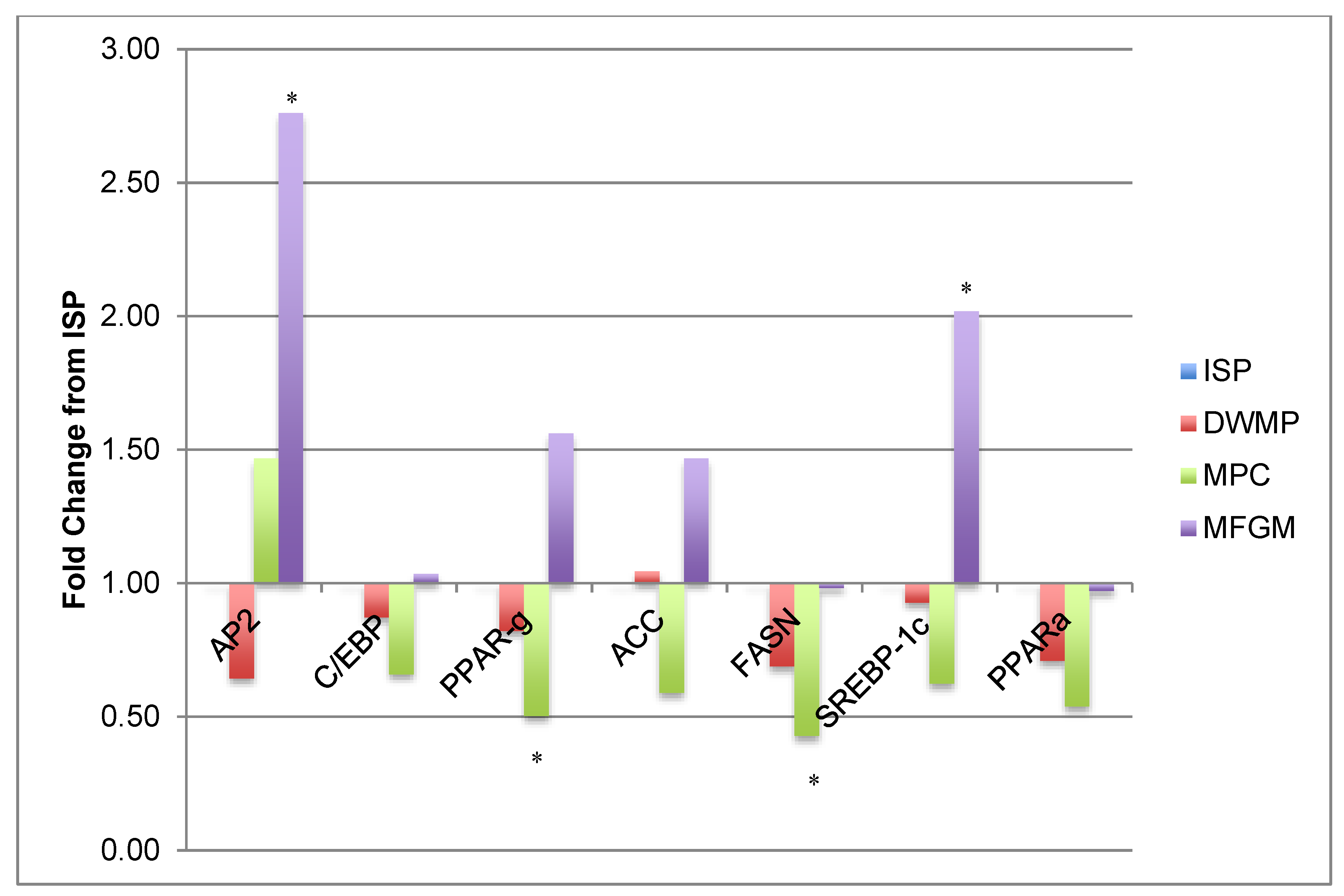


| ISP | DWMP | MPC | MFGM | |
|---|---|---|---|---|
| Protein, % by weight | 18.7 | 18.0 | 18.6 | 18.6 |
| Carbohydrate, % by weight | 46.9 | 46.4 | 47.8 | 47.1 |
| Fat, % by weight | 12.2 | 12.4 | 12.1 | 12.5 |
| Cholesterol mg/kg | 95 | 378 | 139 | 74 |
| Cholesterol (% by weight) | 0.01 | 0.04 | 0.01 | 0.01 |
| Calcium (%) | 0.37 | 0.89 | 0.55 | 0.12 |
| Ca:P | 1.37 | 3.62 | 1.49 | 1.67 |
| Protein, % kcal from | 20.1 | 19.5 | 19.9 | 19.8 |
| Carbohydrate, % kcal from | 50.4 | 50.3 | 51.1 | 50.2 |
| Fat, % kcal from | 29.5 | 30.2 | 29.1 | 30 |
| Saturated Fat, % of total fat | 33.8 | 58.6 | 35.4 | 55.5 |
| MUFA, % of total fat | 39.7 | 26.5 | 40.1 | 31.5 |
| PUFA, % of total fat | 26.5 | 14.9 | 24.5 | 12.9 |
| Kcal/g | 3.7 | 3.7 | 3.7 | 3.8 |
| ISP | DWMP | MPC | MFGM | p-Value | |
|---|---|---|---|---|---|
| Start Weight | 16.0 ± 1.24 | 16.9 ± 1.24 | 16.0 ± 1.24 | 16.7 ± 1.24 | 0.183 |
| Final Weight | 30.0 ± 1.49 b | 30.3 ± 1.49 b | 29.6 ± 1.49 b | 32.3 ± 1.49 a | 0.002 |
| Weight Gain | 13.7 ± 1.49 b | 13.9 ± 1.49 b | 13.2 ± 1.49 b | 16.0 ± 1.49 a | 0.002 |
| Total Feed Disappearance | 256 ± 7.09 ab | 267 ± 7.09 a | 252 ± 7.09 b | 263 ± 7.08 a | 0.004 |
| Feed Efficiency | 19.6 ± 1.89 a | 20.3 ± 1.89 a | 19.9 ± 1.89 a | 17.5 ± 1.89 b | 0.004 |
| ISP | DWMP | MPC | MFGM | p-Value | |
|---|---|---|---|---|---|
| Liver | 1.21 ± 0.05 ab | 1.10 ± 0.05 b | 1.08 ± 0.05 b | 1.34 ± 0.05 a | 0.000 |
| Cecum | 0.32 ± 0.02 ab | 0.39 ± 0.02 a | 0.31 ± 0.02 b | 0.28 ± 0.02 b | 0.001 |
| Retroperitoneal Fat Pad | 0.33 ± 0.06 ab | 0.27 ± 0.06 b | 0.30 ± 0.06 b | 0.36 ± 0.06 a | 0.004 |
| Gastrocnemius Muscle | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.292 |
| Spleen | 0.081 ± 0.007 | 0.078 ± 0.008 | 0.080 ± 0.007 | 0.083 ± 0.007 | 0.825 |
| Thymus (mg) | 32.3 ± 1.93 | 32.7 ± 2.09 | 34.4 ± 1.94 | 33.2 ± 2.06 | 0.868 |
| ISP | DWMP | MPC | MFGM | p-value | |
|---|---|---|---|---|---|
| Transit Time (hours) n = 5 | 3.24 ± 0.37 | 2.83 ± 0.39 | 3.14 ± 0.60 | 3.58 ± 1.42 | 0.930 |
| Endotoxin (EU/mL) n = 15 | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.875 |
| FITC-Dextran (ug/mL) n = 5 | 0.19 ± 0.02 | 0.11 ± 0.01 | 0.19 ± 0.03 | 0.15 ± 0.02 | 0.0545 |
| IgA (ug/mL) n = 15 | 161 ± 18.2 c | 212 ± 18.2 b | 204 ± 18.2 bc | 285 ± 18.2 a | 0.000 |
| IFN-γ (pg/mL) | <LOD | <LOD | 0.04 ± 0.04 | <LOD | -- |
| IL-1β (pg/mL) | 0.55 ± 0.55 | 3.59 ± 3.47 | 5.08 ± 1.75 | 3.18 ± 1.52 | 0.483 |
| IL-6 (pg/mL) | 100 ± 52.9 | 41.9 ± 23.5 | 57.6 ± 18.9 | 35.4 ± 14.7 | 0.469 |
| IL-12p70 (pg/mL) | 8.81 ± 3.66 | 7.11 ± 3.55 | 4.42 ± 1.52 | 1.84 ± 1.23 | 0.295 |
| MIP-2 (pg/mL) | 112 ± 43 | 90.8 ± 38 | 94.6 ± 26 | 96.0 ± 25 | 0.971 |
| TNF-α (pg/mL) | <LOD | <LOD | 18.2 ± 7.14 a | 4.84 ± 1.67 b | 0.007 |
| IL-10 (pg/mL) | 0.21 ± 0.21 | <LOD | <LOD | 0.35 ± 0.28 | 0.438 |
| Eotaxin (pg/mL) | 4215 ± 238 | 4107 ± 172 | 3773 ± 201 | 3780 ± 211 | 0.318 |
| MPO (uU/mL) n = 5 | 36.3 ± 10.7 a | 9.75 ± 2.59 b | 8.16 ± 1.70 b | 8.36 ± 1.23 b | 0.006 |
| ALP (mg/mL) n = 5 | 4.49 ± 0.90 | 2.65 ± 0.38 | 2.28 ± 0.26 | 2.99 ± 0.26 | 0.118 |
| ISP | DWMP | MPC | MFGM | p-Value | |
|---|---|---|---|---|---|
| Villous Height | 234 ± 27.0 | 264 ± 29.8 | 269 ± 18.9 | 262 ± 41.8 | 0.760 |
| Villous Width | 64.0 ± 3.65 | 58.9 ± 4.55 | 66.2 ± 1.48 | 71.0 ± 1.41 | 0.104 |
| Crypt Depth | 75.7 ± 3.63 | 87.9 ± 4.27 | 89.9 ± 3.90 | 86.8 ± 5.89 | 0.077 |
| Crypt Width | 27.8 ± 1.93 | 27.3 ± 0.86 | 30.3 ± 0.62 | 28.9 ± 0.80 | 0.594 |
| Smooth Muscle | 33.5 ± 2.02 | 40.5 ± 3.74 | 32.9 ± 2.06 | 31.2 ± 3.54 | 0.189 |
| ISP | DWMP | MPC | MFGM | p-Value | |
|---|---|---|---|---|---|
| Indole | 159 ± 99.3 | 239 ± 101 | 90.4 ± 100 | 252 ± 97.4 | 0.274 |
| Acetic Acid | 23.8 ± 5.15 | 17.5 ± 5.14 | 18.3 ± 5.17 | 21.7 ± 5.14 | 0.066 |
| Propionic Acid | 1.79 ± 0.42 | 1.60 ± 0.42 | 1.67 ± 0.44 | 2.37 ± 0.42 | 0.428 |
| Butyric Acid | 4.23 ± 0.32 a | 3.48 ± 0.32 a | 2.21 ± 0.33 b | 3.47 ± 0.32 a | 0.000 |
| Isobutyric Acid | 0.26 ± 0.09 | 0.22 ± 0.09 | 0.25 ± 0.10 | 0.25 ± 0.09 | 0.836 |
| Isovaleric Acid | 0.11 ± 0.04 | 0.09 ± 0.04 | 0.08 ± 0.04 | 0.11 ± 0.04 | 0.107 |
| Valeric Acid | 0.25 ± 0.09 | 0.23 ± 0.09 | 0.20 ± 0.09 | 0.30 ± 0.09 | 0.129 |
| Total SCFA | 30.4 ± 2.93 | 23.1 ± 2.93 | 23.2 ± 3.03 | 28.2 ± 2.93 | 0.209 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Price, T.R.; Baskaran, S.A.; Moncada, K.L.; Minamoto, Y.; Klemashevich, C.; Jayuraman, A.; Sucholdoski, J.S.; Tedeschi, L.O.; Steiner, J.M.; Pillai, S.D.; et al. Whole and Isolated Protein Fractions Differentially Affect Gastrointestinal Integrity Markers in C57Bl/6 Mice Fed Diets with a Moderate-Fat Content. Nutrients 2021, 13, 1251. https://doi.org/10.3390/nu13041251
Price TR, Baskaran SA, Moncada KL, Minamoto Y, Klemashevich C, Jayuraman A, Sucholdoski JS, Tedeschi LO, Steiner JM, Pillai SD, et al. Whole and Isolated Protein Fractions Differentially Affect Gastrointestinal Integrity Markers in C57Bl/6 Mice Fed Diets with a Moderate-Fat Content. Nutrients. 2021; 13(4):1251. https://doi.org/10.3390/nu13041251
Chicago/Turabian StylePrice, Tara R., Sangeetha A. Baskaran, Kristin L. Moncada, Yasushi Minamoto, Cory Klemashevich, Arul Jayuraman, Jan S. Sucholdoski, Luis O. Tedeschi, Jörg M. Steiner, Suresh D. Pillai, and et al. 2021. "Whole and Isolated Protein Fractions Differentially Affect Gastrointestinal Integrity Markers in C57Bl/6 Mice Fed Diets with a Moderate-Fat Content" Nutrients 13, no. 4: 1251. https://doi.org/10.3390/nu13041251
APA StylePrice, T. R., Baskaran, S. A., Moncada, K. L., Minamoto, Y., Klemashevich, C., Jayuraman, A., Sucholdoski, J. S., Tedeschi, L. O., Steiner, J. M., Pillai, S. D., & Walzem, R. L. (2021). Whole and Isolated Protein Fractions Differentially Affect Gastrointestinal Integrity Markers in C57Bl/6 Mice Fed Diets with a Moderate-Fat Content. Nutrients, 13(4), 1251. https://doi.org/10.3390/nu13041251









