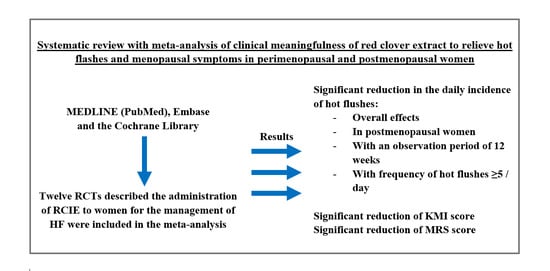
Evaluation of Clinical Meaningfulness of Red Clover (Trifolium pratense L.) Extract to Relieve Hot Flushes and Menopausal Symptoms in Peri- and Post-Menopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
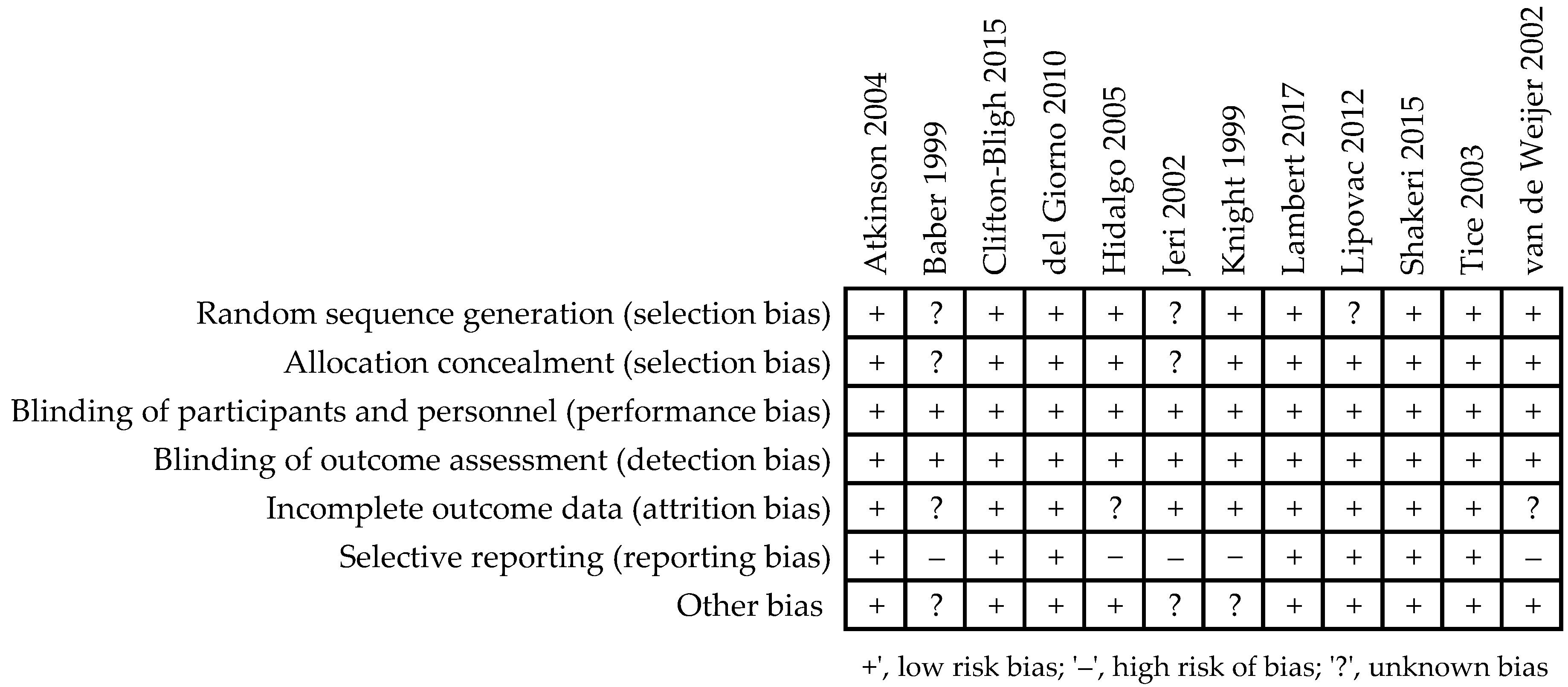
2.4. Quality Assessment and Bias Risk of the Trials
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Included Trials
3.2. Assessment of the Methodological Quality of Trials
3.3. Systematic Review and Meta-Analysis
3.3.1. Daily Hot Flushes Frequency
3.3.2. Rating of Menopausal Complaints Using Instruments to Measure Intensity of Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thurston, R.C. Vasomotor symptoms: Natural history, physiology, and links with cardiovascular health. Climacteric 2018, 21, 96–100. [Google Scholar] [CrossRef]
- Monteleone, P.; Mascagni, G.; Giannini, A.; Genazzani, A.R.; Simoncini, T. Symptoms of menopause—Global prevalence, physiology and implications. Nat. Rev. Endocrinol. 2018, 14, 199–215. [Google Scholar] [CrossRef]
- Avis, N.E.; Crawford, S.L.; Greendale, G.; Bromberger, J.T.; Everson-Rose, S.A.; Gold, E.B.; Hess, R.; Joffe, H.; Kravitz, H.M.; Tepper, P.G.; et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern. Med. 2015, 175, 531–539. [Google Scholar] [CrossRef]
- Freeman, E.W.; Sherif, K. Prevalence of hot flushes and night sweats around the world: A systematic review. Climacteric 2007, 10, 197–214. [Google Scholar] [CrossRef]
- Gold, E.B.; Colvin, A.; Avis, N.; Bromberger, J.; Greendale, G.A.; Powell, L.; Sternfeld, B.; Matthews, K. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of women’s health across the nation. Am. J. Public Health 2006, 96, 1226–1235. [Google Scholar] [CrossRef]
- Duffy, O.K.; Iversen, L.; Hannaford, P.C. Factors associated with reporting classic menopausal symptoms differ. Climacteric 2013, 16, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Herber-Gast, G.-C.M.; Mishra, G.D.; van der Schouw, Y.T.; Brown, W.J.; Dobson, A.J. Risk factors for night sweats and hot flushes in midlife: Results from a prospective cohort study. Menopause 2013, 20, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-F.; Pandeya, N.; Dobson, A.J.; Kuh, D.; Brunner, E.J.; Crawford, S.L.; Avis, N.E.; Gold, E.B.; Mitchell, E.S.; Woods, N.F.; et al. The role of sleep difficulties in the vasomotor menopausal symptoms and depressed mood relationships: An international pooled analysis of eight studies in the InterLACE consortium. Psychol. Med. 2018, 48, 2550–2561. [Google Scholar] [CrossRef] [PubMed]
- Geukes, M.; van Aalst, M.P.; Robroek, S.J.; Laven, J.S.E.; Oosterhof, H. The impact of menopause on work ability in women with severe menopausal symptoms. Maturitas 2016, 90, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossmanith, W.G.; Ruebberdt, W. What causes hot flushes? The neuroendocrine origin of vasomotor symptoms in the menopause. Gynecol. Endocrinol. 2009, 25, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.R. Menopausal hot flashes: Mechanisms, endocrinology, treatment. J. Steroid Biochem. Mol. Biol. 2014, 142, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, M.; Teng, Y.; Shao, H.; Wu, P.; Mills, E.J. Knowledge, perceptions and information about hormone therapy (HT) among menopausal women: A systematic review and meta-synthesis. PLoS ONE 2011, 6, e24661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marjoribanks, J.; Farquhar, C.; Roberts, H.; Lethaby, A.; Lee, J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst. Rev. 2017, 1, CD004143. [Google Scholar] [CrossRef] [PubMed]
- French, L.M.; Smith, M.A.; Holtrop, J.S.; Holmes-Rovner, M. Hormone therapy after the Women’s Health Initiative: A qualitative study. BMC Fam. Pract. 2006, 7, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schonberg, M.A.; Davis, R.B.; Wee, C.C. After the women’s health initiative: Decision making and trust of women taking hormone therapy. Women’s Health Issues 2005, 15, 187–195. [Google Scholar] [CrossRef]
- Nedrow, A.; Miller, J.; Walker, M.; Nygren, P.; Huffman, L.H.; Nelson, H.D. Complementary and alternative therapies for the management of menopause-related symptoms: A systematic evidence review. Arch. Intern. Med. 2006, 166, 1453–1465. [Google Scholar] [CrossRef]
- Wong, V.C.; Lim, C.E.; Luo, X.; Wong, W.S. Current alternative and complementary therapies used in menopause. Gynecol. Endocrinol. 2009, 25, 166–174. [Google Scholar] [CrossRef]
- Mintziori, G.; Lambrinoudaki, I.; Goulis, D.G.; Ceausu, I.; Depypere, H.; Erel, C.T.; Pérez-López, F.R.; Schenck-Gustafsson, K.; Simoncini, T.; Tremollieres, F.; et al. EMAS position statement: Non-hormonal management of menopausal vasomotor symptoms. Maturitas 2015, 81, 410–413. [Google Scholar] [CrossRef]
- Booth, N.L.; Piersen, C.E.; Banuvar, S.; Geller, S.E.; Shulman, L.P.; Farnsworth, N.R. Clinical studies of red clover (Trifolium pratense) dietary supplements in menopause: A literature review. Menopause 2006, 13, 251–264. [Google Scholar] [CrossRef]
- Lemežienė, N.; Padarauskas, A.; Butkutė, B.; Cesevičienė, J.; Taujenis, L.; Norkevičienė, E. The concentration of isoflavones in red clover (Trifolium pratense L.) at flowering stage. Zemdirb.-Agric. 2015, 102, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Tolleson, W.H.; Doerge, D.R.; Churchwell, M.I.; Marques, M.M.; Roberts, D.W. Metabolism of biochanin A and formononetin by human liver microsomes in vitro. J. Agric. Food Chem. 2002, 50, 4783–4790. [Google Scholar] [CrossRef]
- Liu, J.; Burdette, J.E.; Xu, H.; Gu, C.; van Breemen, R.B.; Bhat, K.P.; Booth, N.; Constantinou, A.I.; Pezzuto, J.M.; Fong, H.H.; et al. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J. Agric. Food Chem. 2001, 49, 2472–2479. [Google Scholar] [CrossRef]
- Morito, K.; Aomori, T.; Hirose, T.; Kinjo, J.; Hasegawa, J.; Ogawa, S.; Inoue, S.; Muramatsu, M.; Masamune, Y. Interaction of phy-toestrogens with estrogen receptors alpha and beta (II). Biol. Pharm. Bull. 2002, 25, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Welch, V.; Petticrew, M.; Petkovic, J.; Moher, D.; Waters, E.; White, H.; Tugwell, P.; Atun, R.; Awasthi, S.; Barbour, V.; et al. Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): Explanation and elaboration. J. Clin. Epidemiol. 2016, 70, 68–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupperman, H.S.; Blatt, M.H.; Wiesbader, H.; Filler, W. Comparative clinical evaluation of estrogenic preparations by the meno-pausal and amenorrheal indices. J. Clin. Endocrinol. Metab. 1953, 13, 688–703. [Google Scholar] [CrossRef]
- Greene, J.G. A factor analytic study of climacteric symptoms. J. Psychosom. Res. 1976, 20, 425–430. [Google Scholar] [CrossRef]
- Schneider, H.P.; Heinemann, L.A.; Rosemeier, H.P.; Potthoff, P.; Behre, H.M. The Menopause Rating Scale (MRS): Reliability of scores of menopausal complaints. Climacteric 2000, 3, 59–64. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 2011, 343, 5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Follmann, O.; Elliott, P.; Suh, I.; Cutler, J. Variance imputation for overviews of clinical trials with continuous response. J. Clin. Epidemiol. 1999, 45, 769–773. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Deeks, J.J. Selecting studies and collecting data. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration; John Wiley & Sons: Chichester, UK, 2008; pp. 172–178. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.C.; Howes, J.B.; Eden, J.A. The effect of Promensil, an isoflavone extract, on menopausal symptoms. Climacteric 1999, 2, 79–84. [Google Scholar] [CrossRef]
- Baber, R.J.; Templeman, C.; Morton, T.; Kelly, G.E.; West, L. Randomized placebo-controlled trial of an isoflavone supplement and menopausal symptoms in women. Climacteric 1999, 2, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Jeri, A.R. The use of an isoflavone supplement to relieve hot flashes. Female Patient 2002, 27, 35–37. [Google Scholar]
- van de Weijer, P.H.; Barentsen, R. Isoflavones from red clover (Promensil) significantly reduce menopausal hot flush symptoms compared with placebo. Maturitas 2002, 42, 187–193. [Google Scholar] [CrossRef]
- Tice, J.A.; Ettinger, B.; Ensrud, K.; Wallace, R.; Blackwell, T.; Cummings, S.R. Phytoestrogen supplements for the treatment of hot flashes: The isoflavone clover extract (ICE) study: A randomized controlled trial. JAMA 2003, 290, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, C.; Warren, R.M.L.; Sala, E.; Dowsett, M.; Dunning, A.M.; Healey, C.S.; Runswick, S.; Day, N.E.; Bingham, S.A. Red clover-derived isoflavones and mammographic breast density: A double-blind, randomized, placebo-controlled trial [ISRCTN42940165]. Breast Cancer Res. 2004, 6, R170–R179. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, L.A.; Chedraui, P.A.; Morocho, N.; Ross, S.; Miguel, G.S. The effect of red clover isoflavones on menopausal symptoms, lipids and vaginal cytology in menopausal women: A randomized, double-blind, placebo-controlled study. Gynecol. Endocrinol. 2005, 21, 257–264. [Google Scholar] [CrossRef]
- del Giorno, C.; Fonseca, A.M.; Bagnoli, V.R.; Assis, J.S.; Soares, J.M., Jr.; Baracat, E.C. Effects of Trifolium pratense on the climacteric and sexual symptoms in postmenopause women. Rev. Assoc. Med. Bras. 2010, 56, 558–562. [Google Scholar]
- Lipovac, M.; Chedraui, P.; Gruenhut, C.; Gocan, A.; Kurz, C.; Neuber, B.; Imhof, M. The effect of red clover isoflavone supplementation over vasomotor and menopausal symptoms in postmenopausal women. Gynecol. Endocrinol. 2012, 28, 203–207. [Google Scholar] [CrossRef]
- Clifton-Bligh, P.B.; Nery, M.L.; Cliftonbligh, R.J.; Visvalingam, S.; Fulcher, G.R.; Byth, K.; Baber, R. Red clover isoflavones enriched with formononetin lower serum LDL cholesterol—A randomized, double-blind, placebo-controlled study. Eur. J. Clin. Nutr. 2015, 69, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Shakeri, F.; Taavoni, S.; Goushegir, A.; Haghani, H. Effectiveness of red clover in alleviating menopausal symptoms: A 12-week randomized, controlled trial. Climacteric 2015, 18, 568–573. [Google Scholar] [CrossRef]
- Lambert, M.N.T.; Thorup, A.C.; Hansen, E.S.S.; Jeppesen, P.B. Combined red clover isoflavones and probiotics potently reduce menopausal vasomotor symptoms. PLoS ONE 2017, 12, e0176590. [Google Scholar] [CrossRef] [Green Version]
- Myers, S.P.; Vigar, V. Effects of a standardised extract of Trifolium pratense (Promensil) at a dosage of 80 mg in the treatment of menopausal hot flushes: A systematic review and meta-analysis. Phytomedicine 2017, 24, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Coon, J.T.; Pittler, M.H.; Ernst, E. Trifolium pratense isoflavones in the treatment of menopausal hot flushes: A systematic review and meta-analysis. Phytomedicine 2007, 14, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfarpour, M.; Sadeghi, R.; Roudsari, R.L.; Khorsand, I.; Khadivzadeh, T.; Muoio, B. Red clover for treatment of hot flashes and menopausal symptoms: A systematic review and meta-analysis. J. Obstet. Gynaecol 2016, 36, 301. [Google Scholar] [CrossRef] [PubMed]
- Lethaby, A.; Marjoribanks, J.; Kronenberg, F.; Roberts, H.; Eden, J.; Brown, J. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst. Rev. 2013, 12, CD001395. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; Vesco, K.K.; Haney, E.; Fu, R.; Nedrow, A.; Miller, J.; Nicolaidis, C.; Walker, M.; Humphrey, L. Nonhormonal therapies for menopausal hot flashes: Systematic review and meta-analysis. JAMA 2006, 295, 2057–2071. [Google Scholar] [CrossRef]
- Franco, O.H.; Chowdhury, R.; Troup, J.; Voortman, T.; Kunutsor, S.; Kavousi, M.; Oliver-Williams, C.; Muka, T. Use of plant-based therapies and menopausal symptoms: A systematic review and meta-analysis. JAMA 2016, 315, 2554–2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, E.E.; Ensrud, K.E.; Macdonald, R.; Wilt, T.J. Phytoestrogens for treatment of menopausal symptoms: A systematic review. Obstet. Gynecol. 2004, 104, 824–836. [Google Scholar] [CrossRef]
- Gartoulla, P.; Han, M.M. Red clover extract for alleviating hot flushes in postmenopausal women: A meta-analysis. Maturitas 2014, 79, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, L.; Wu, J.; Dong, L.; Lv, Y.; Zheng, Q. Quantitative analysis of placebo response and factors associated with menopausal hot flashes. Menopause 2017, 24, 932–937. [Google Scholar] [CrossRef] [PubMed]




| First Author Pub. Data (Ref.) Country | Design Follow-up Period | Sample Size: Randomized/Analyzed | Participants Age, y (Range) Trial Inclusion Criteria | Intervention: Isoflavone Daily Dose | Baseline Hot Flush Frequency/d | Baseline Menopausal Score |
|---|---|---|---|---|---|---|
| Knight 1999 [33] Australia | Placebo controlled 3-arm parallel trial 1 wk placebo run-in/12 wk follow-up | 37/37 | 54.6 ± 3.6 (40–65) Healthy postmenopausal women, bilateral oophorectomy or amenorrhea ≥ 6 mo, FSH > 40 mIU/mL, HF > 3/d | RCG “a”; 160 mga RCG “b”: 40 mgb PG: placebo | RCG “a”: 9.0 ± 5.2 RCG “b”: 6.9 ± 2.1 PG: 8.6 ± 4.6 | GCS RCG “a”: 19.9 ± 4.4 RCG “b”: 19.9 ± 10.6 PG: 18.5 ± 11.4 |
| Baber 1999 [34] Australia | Placebo controlled crossover trial 90 d active phase/ 7 d washout | 51/51 | 54.0 ± 4.1 (45–65) Healthy postmenopausal women, age of menopause 50.0 ± 3.6 y, FSH > 30 mIU/mL, HF > 3/d | RCG: 40 mgb PG: placebo | RCG: 6.2 ± 2.7 PG: 6.4 ± 2.6 | GCS RCG: 10.9 ± 6.5 PG: 12.3 ± 9.0 |
| Jeri 2002 [35] Peru | Placebo controlled parallel trial 16 wk follow-up | 30/30 | 51.0 ± 3.5 (<60) Healthy postmenopausal women, amenorrhea ≥ 12 mo, FSH > 30 mIU/mL, HF ≥ 5/d | RCG: 40 mgb PG: placebo | RCG: 7.0 ± 1.9 PG: 5.7 ± 1.6 | - - |
| van de Weijer 2002 [36] Netherlands | Placebo controlled parallel trial 4 wk placebo run-in/ 12 wk follow-up | 30/26 | 53.4 ± 6.3 (49–65) Healthy postmenopausal women, amenorrhea ≥ 12 mo, BMI 26.1 ± 4.2, HF ≥ 5/d | RCG: 80 mgc PG: placebo | RCG: 5.43 ± 2.6 PG: 5.6 ± 5.0 | GCS RCG: 12.5 ± 11.2 PG: 13.8 ± 9.5 |
| Tice 2003 [37] United States | Placebo controlled 3-arm parallel trial 2 wk placebo run-in/12 wk follow-up | 252/252 | 52.3 ± 3.1 (45–60) Healthy peri- and post-menopausal women, 3.3 ± 4.5 ysm, FSH > 30 mIU/mL, BMI 26.1 ± 4.9, HF ≥ 35/wk | RCG “a”: 80 mgc RCG “b”: 57 mgd PG: placebo | RCG “a”: 8.5 ± 5.8 RCG “b”: 8.1 ± 3.0 PG: 7.8 ± 2.4 | - - - |
| Atkinson 2004 [38] United Kingdom | Placebo controlled parallel trial 12 mo follow-up | 205/99 | 52.2 ± 4.8 (49–65) Healthy peri- and post-menopausal women, FSH > 30 mIU/mL, BMI 25.3 ± 3.7, HF > 3/d | RCG: 40 mge PG: placebo | RCG: 2.1 ± 2.7 PG: 2.5 ± 3.0 | GCS RCG: 4.3 ± 4.3 PG: 4.3 ± 4.3 |
| Hidalgo 2005 [39] Ecuador | Placebo controlled crossover trial 90 d active phase/ 7 d washout | 60/53 | 51.3 ± 3.5 (>40) Healthy postmenopausal women, amenorrhea ≥ 12 mo, FSH > 35 mIU/ml, BMI 26.6 ± 3.9,KMI score ≥ 15 | RCG: 80 mgc PG: placebo | - - | KMI RCG: 27.2 ± 7.7 PG: 27.2 ± 7.7 |
| del Giorno 2010 [40] Brazil | Placebo controlled parallel trial 12 mo follow-up | 120/100 | 55.5 ± 4.9 (45–65) Healthy peri- and post-menopausal women, amenorrhea ≥ 12 mo, FSH > 30 mIU/mL, BMI 28.8 ± 5.4 | RCG: 40 mg PG: placebo | - - | KMI RCG: 25.3 ± 10.2 PG: 25.1 ± 9.0 |
| Lipovac 2012 [41] Austria | Placebo controlled crossover trial 90 d active phase/ 7 d washout | 113/109 | 54.1 ± 7.0 (>40) Healthy postmenopausal women, amenorrhea ≥ 12 mo, FSH > 35 mIU/mL, BMI 24.7 ± 3.9, HF > 5/d, KMI score ≥ 15/wk | RCG: 80 mgc CG: placebo | RCG: 11.7 ± 4.8 PG: 11.0 ± 5.1 | KMIRCG: 32.5 ± 10.0 PG: 34.3 ± 10.4 |
| Clifton-Bligh 2015 [42] Australia | Placebo controlled parallel trial 1 mo placebo run-in/ 2 y follow-up | 147/103 | 54.4 ± 3.9 Healthy postmenopausal women, amenorrhea ≥ 12 mo, FSH > 30 mIU/mL, BMI 24.8 ± 4.3 | RCG: 57 mgd PG: placebo | - - | GCS RCG: 8.9 ± 7.3 PG: 11.0 ± 8.0 |
| Shakeri 2015 [43] Iran | Placebo controlled parallel trial 12 wk follow-up | 72/71 | 54.8 ± 2.8 (50–59) Healthy postmenopausal women, 1.85 ± 0.9 ysm, BMD 21.1 ± 1.9 | RCG: 80 mgc PG: placebo | - - | MRS RCG: 20.4 ± 6.3 PG: 20.8 ± 6.2 |
| Lambert 2017 [44] Denmark | Placebo controlled parallel trial 12 wk follow-up | 62/59 | 52.5 ± 3.5 (40–65) Healthy perimenopausal women, FSH ≥ 35 mIU/mL, BMI 25.7 ± 4.3, HF > 5/d | RCG: 37.1 mgf CG: placebo | RCG: 9.5 ± 6.4 PG: 8.6 ± 6.9 | GCS RCG: 18.6 ± 12.3 PG: 20.8 ± 2.3 |
| Variables | n | Simple Size | WMD (95% CI) | p | I2 (%) |
|---|---|---|---|---|---|
| Overall effects | 10 | 751 | −1.73 (−3.28, −0.18) | 0.0292 | 87.34 |
| Menopausal status | |||||
| Postmenopausal | 7 | 315 | −2.68 (−4.72, −0.63) | 0.0105 | 71.44 |
| Peri- and post-menopausal | 3 | 436 | 0.01 (−0.55, 0.58) | 0.9594 | 0.00 |
| Follow-up period | |||||
| 12 weeks | 9 | 652 | −1.95 (−3.61, −0.30) | 0.0206 | 81.33 |
| 12 months | 1 | 99 | 0.20 (−0.58, 0.98) | 0.6149 | (-) |
| Frequency of hot flushes | |||||
| ≥5/day | 6 | 552 | −2.56 (−4.49, −0.62) | 0.0096 | 87.67 |
| ≥3/day | 4 | 199 | 0.21 (−0.53, 0.96) | 0.5761 | 0.00 |
| Isoflavones dose | |||||
| <80 mg/day | 6 | 431 | −0.88 (−2.34, 0.58) | 0.2370 | 76.83 |
| ≥80 mg/day | 4 | 320 | −2.80 (−6.35, 0.74) | 0.1210 | 86.61 |
| Dominant of isoflavones | |||||
| Biochanin A | 8 | 524 | −1.79 (−3.60, 0.02) | 0.0520 | 85.78 |
| Formononetin | 2 | 227 | −1.14 (−4.13, 1.84) | 0.4519 | 73.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanadys, W.; Barańska, A.; Błaszczuk, A.; Polz-Dacewicz, M.; Drop, B.; Kanecki, K.; Malm, M. Evaluation of Clinical Meaningfulness of Red Clover (Trifolium pratense L.) Extract to Relieve Hot Flushes and Menopausal Symptoms in Peri- and Post-Menopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 1258. https://doi.org/10.3390/nu13041258
Kanadys W, Barańska A, Błaszczuk A, Polz-Dacewicz M, Drop B, Kanecki K, Malm M. Evaluation of Clinical Meaningfulness of Red Clover (Trifolium pratense L.) Extract to Relieve Hot Flushes and Menopausal Symptoms in Peri- and Post-Menopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2021; 13(4):1258. https://doi.org/10.3390/nu13041258
Chicago/Turabian StyleKanadys, Wiesław, Agnieszka Barańska, Agata Błaszczuk, Małgorzata Polz-Dacewicz, Bartłomiej Drop, Krzysztof Kanecki, and Maria Malm. 2021. "Evaluation of Clinical Meaningfulness of Red Clover (Trifolium pratense L.) Extract to Relieve Hot Flushes and Menopausal Symptoms in Peri- and Post-Menopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 13, no. 4: 1258. https://doi.org/10.3390/nu13041258
APA StyleKanadys, W., Barańska, A., Błaszczuk, A., Polz-Dacewicz, M., Drop, B., Kanecki, K., & Malm, M. (2021). Evaluation of Clinical Meaningfulness of Red Clover (Trifolium pratense L.) Extract to Relieve Hot Flushes and Menopausal Symptoms in Peri- and Post-Menopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 13(4), 1258. https://doi.org/10.3390/nu13041258