Pre-Exercise Carbohydrate or Protein Ingestion Influences Substrate Oxidation but Not Performance or Hunger Compared with Cycling in the Fasted State
Abstract
:1. Introduction
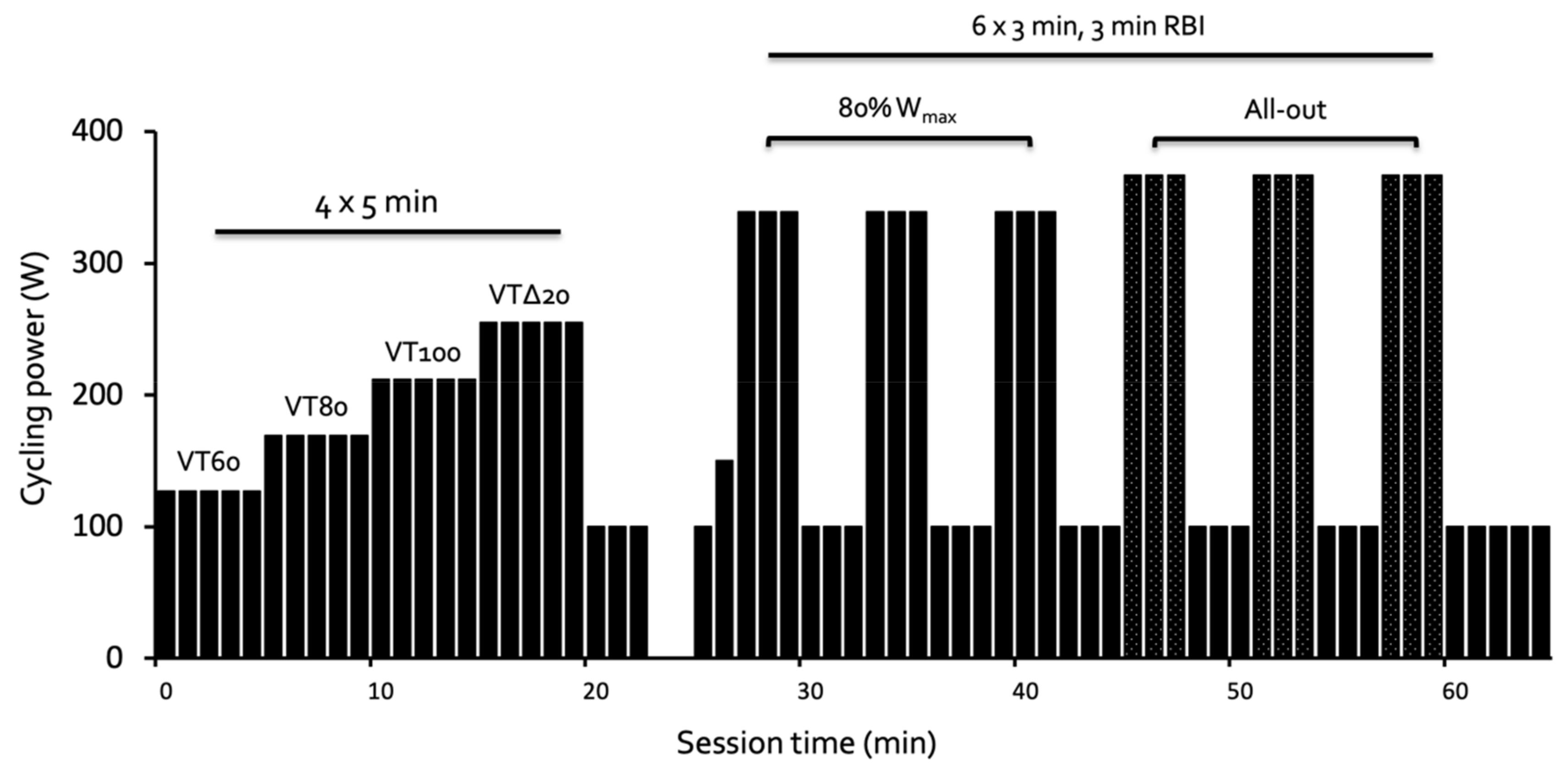
2. Materials and Methods
3. Results
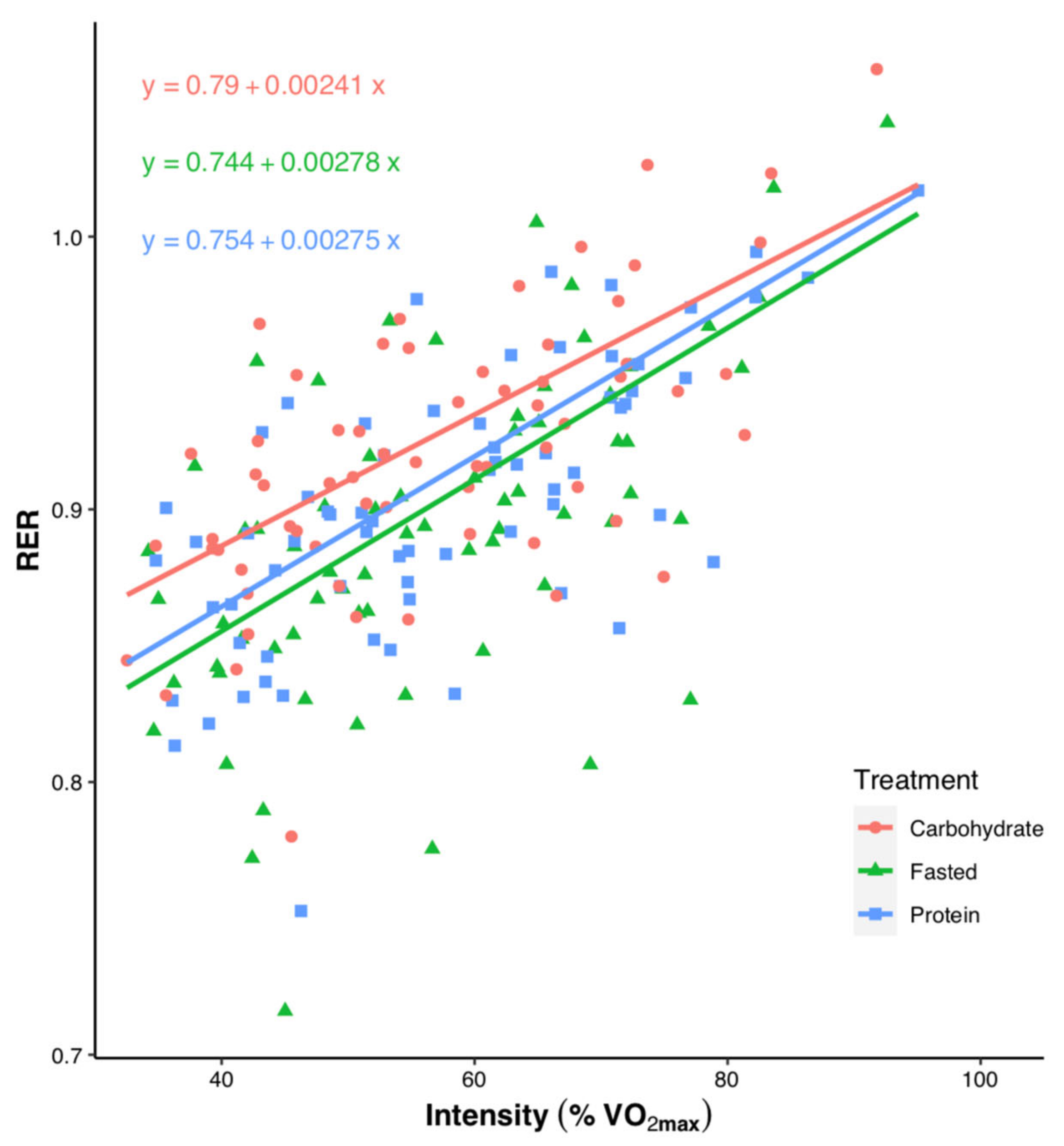
3.1. Submaximal Exercise
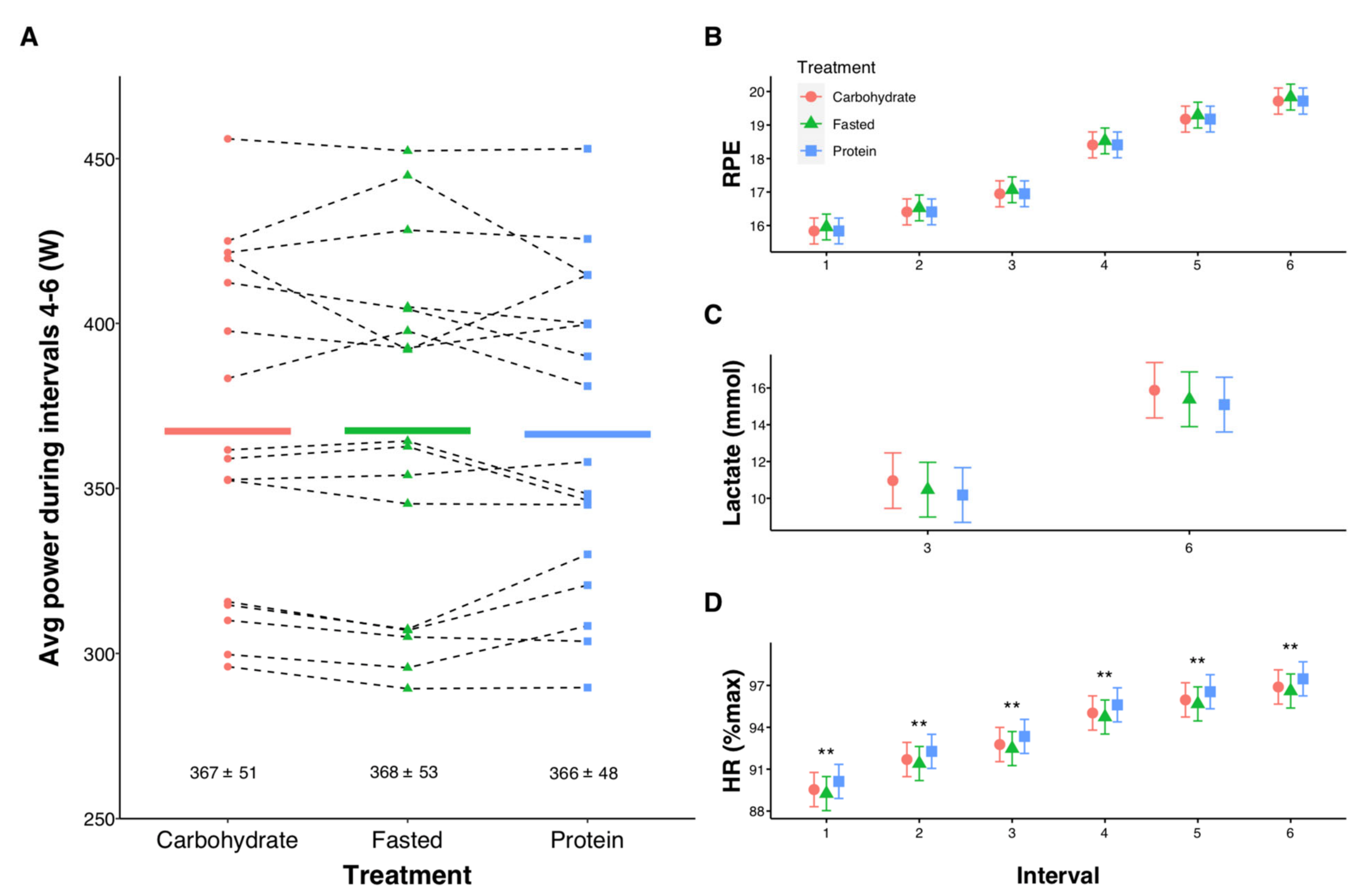
3.2. High-Intensity Exercise
3.3. Pre-Post Exercise
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rothschild, J.; Kilding, A.E.; Plews, D.J. What Should I Eat before Exercise? Pre-Exercise Nutrition and the Response to Endurance Exercise: Current Prospective and Future Directions. Nutrients 2020, 12, 3473. [Google Scholar] [CrossRef] [PubMed]
- Impey, S.G.; Hearris, M.A.; Hammond, K.M.; Bartlett, J.D.; Louis, J.; Close, G.L.; Morton, J.P. Fuel for the work required: A theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Med. 2018, 48, 1031–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, A.F.; Costa, R.R.; Macedo, R.C.O.; Coconcelli, L.; Kruel, L.F.M. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: A systematic review and meta-analysis. Br. J. Nutr. 2016, 116, 1153–1164. [Google Scholar] [CrossRef] [Green Version]
- Burke, L.M.; Hawley, J.A.; Jeukendrup, A.; Morton, J.P.; Stellingwerff, T.; Maughan, R.J. Toward a Common Understanding of Diet-Exercise Strategies to Manipulate Fuel Availability for Training and Competition Preparation in Endurance Sport. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 451–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothschild, J.A.; Kilding, A.E.; Plews, D.J. Pre-Exercise Nutrition Habits and Beliefs of Endurance Athletes Vary by Sex, Competitive Level, and Diet. J. Am. Coll. Nutr. 2020. [Google Scholar] [CrossRef]
- Rothschild, J.; Kilding, A.E.; Plews, D.J. Prevalence and Determinants of Fasted Training in Endurance Athletes: A Survey Analysis. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 345–356. [Google Scholar] [CrossRef]
- Impey, S.G.; Smith, D.; Robinson, A.L.; Owens, D.J.; Bartlett, J.D.; Smith, K.; Limb, M.; Tang, J.; Fraser, W.D.; Close, G.L. Leucine-enriched protein feeding does not impair exercise-induced free fatty acid availability and lipid oxidation: Beneficial implications for training in carbohydrate-restricted states. Amino Acids 2015, 47, 407–416. [Google Scholar] [CrossRef]
- Taylor, C.; Bartlett, J.D.; van de Graaf, C.S.; Louhelainen, J.; Coyne, V.; Iqbal, Z.; MacLaren, D.P.; Gregson, W.; Close, G.L.; Morton, J.P. Protein ingestion does not impair exercise-induced AMPK signalling when in a glycogen-depleted state: Implications for train-low compete-high. Eur. J. Appl. Physiol. 2013, 113, 1457–1468. [Google Scholar] [CrossRef]
- Gieske, B.T.; Stecker, R.A.; Smith, C.R.; Witherbee, K.E.; Harty, P.S.; Wildman, R.; Kerksick, C.M. Metabolic impact of protein feeding prior to moderate-intensity treadmill exercise in a fasted state: A pilot study. J. Int. Soc. Sports Nutr. 2018, 15, 56. [Google Scholar] [CrossRef] [Green Version]
- Rowlands, D.S.; Hopkins, W.G. Effect of high-fat, high-carbohydrate, and high-protein meals on metabolism and performance during endurance cycling. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 318–335. [Google Scholar] [CrossRef]
- Willcutts, K.F.; Wilcox, A.; Grunewald, K. Energy metabolism during exercise at different time intervals following a meal. Int. J. Sports Med. 1988, 9, 240–243. [Google Scholar] [CrossRef] [Green Version]
- Aird, T.P.; Davies, R.W.; Carson, B.P. Effects of fasted vs. fed-state exercise on performance and post-exercise metabolism: A systematic review and meta-analysis. Scand J. Med. Sci. Sports 2018, 28, 1476–1493. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S. What is best practice for training intensity and duration distribution in endurance athletes? Int. J. Sports Physiol. Perform. 2010, 5, 276–291. [Google Scholar] [CrossRef]
- Little, J.P.; Chilibeck, P.D.; Ciona, D.; Forbes, S.; Rees, H.; Vandenberg, A.; Zello, G.A. Effect of low- and high-glycemic-index meals on metabolism and performance during high-intensity, intermittent exercise. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 447–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terada, T.; Toghi Eshghi, S.R.; Liubaoerjijin, Y.; Kennedy, M.; Myette-Cote, E.; Fletcher, K.; Boule, N.G. Overnight fasting compromises exercise intensity and volume during sprint interval training but improves high-intensity aerobic endurance. J. Sports Med. Phys. Fit. 2019, 59, 357–365. [Google Scholar] [CrossRef]
- Astorino, T.A.; Sherrick, S.; Mariscal, M.; Jimenez, V.C.; Stetson, K.; Courtney, D. No effect of meal intake on physiological or perceptual responses to self-selected high intensity interval exercise (HIIE). Biol. Sport 2019, 36, 225. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Redox basis of exercise physiology. Redox Biol. 2020, 35, 101499. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Theodorou, A.A.; Paschalis, V.; Veskoukis, A.S.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Kyparos, A.; Nikolaidis, M.G. Adaptations to endurance training depend on exercise-induced oxidative stress: Exploiting redox interindividual variability. Acta Physiol. 2018, 222. [Google Scholar] [CrossRef]
- Gregersen, S.; Samocha-Bonet, D.; Heilbronn, L.K.; Campbell, L.V. Inflammatory and oxidative stress responses to high-carbohydrate and high-fat meals in healthy humans. J. Nutr. Metab. 2012, 2012, 238056. [Google Scholar] [CrossRef]
- Draganidis, D.; Karagounis, L.G.; Athanailidis, I.; Chatzinikolaou, A.; Jamurtas, A.Z.; Fatouros, I.G. Inflammaging and skeletal muscle: Can protein intake make a difference? J. Nutr. 2016, 146, 1940–1952. [Google Scholar] [CrossRef]
- McAnulty, S.R.; McAnulty, L.S.; Morrow, J.D.; Nieman, D.C.; Owens, J.T.; Carper, C.M. Influence of carbohydrate, intense exercise, and rest intervals on hormonal and oxidative changes. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 478–490. [Google Scholar] [CrossRef]
- Achten, J.; Jeukendrup, A.E. The effect of pre-exercise carbohydrate feedings on the intensity that elicits maximal fat oxidation. J. Sports Sci. 2003, 21, 1017–1025. [Google Scholar] [CrossRef]
- Enevoldsen, L.; Simonsen, L.; Macdonald, I.; Bülow, J. The combined effects of exercise and food intake on adipose tissue and splanchnic metabolism. J. Physiol. 2004, 561, 871–882. [Google Scholar] [CrossRef]
- Davis, J.A.; Whipp, B.J.; Lamarra, N.; Huntsman, D.J.; Frank, M.H.; Wasserman, K. Effect of ramp slope on determination of aerobic parameters from the ramp exercise test. Med. Sci. Sports Exerc. 1982, 14, 339–343. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.D.; Coutts, A.J.; Kelly, V.; McGuigan, M.R.; Cormack, S.J. Neuromuscular, endocrine, and perceptual fatigue responses during different length between-match microcycles in professional rugby league players. Int. J. Sports Physiol. Perform. 2010, 5, 367–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Lansley, K.E.; Dimenna, F.J.; Bailey, S.J.; Jones, A.M. A ‘new’ method to normalise exercise intensity. Int. J. Sports Med. 2011, 32, 535–541. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int. J. Sports Med. 2005, 26 (Suppl. 1), S28–S37. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Foster, C.; Florhaug, J.A.; Franklin, J.; Gottschall, L.; Hrovatin, L.A.; Parker, S.; Doleshal, P.; Dodge, C. A new approach to monitoring exercise training. J. Strength Cond. Res. 2001, 15, 109–115. [Google Scholar] [PubMed]
- Nikolaidis, M.G.; Kyparos, A.; Spanou, C.; Paschalis, V.; Theodorou, A.A.; Panayiotou, G.; Grivas, G.V.; Zafeiridis, A.; Dipla, K.; Vrabas, I.S. Aging is not a barrier to muscle and redox adaptations: Applying the repeated eccentric exercise model. Exp. Gerontol. 2013, 48, 734–743. [Google Scholar] [CrossRef]
- Westfall, J.; Kenny, D.A.; Judd, C.M. Statistical power and optimal design in experiments in which samples of participants respond to samples of stimuli. J. Exp. Psychol. Gen. 2014, 143, 2020–2045. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: Hillside, NJ, USA, 1988. [Google Scholar]
- Oliveira, C.L.P.; Boule, N.G.; Berg, A.; Sharma, A.M.; Elliott, S.A.; Siervo, M.; Ghosh, S.; Prado, C.M. Consumption of a High-Protein Meal Replacement Leads to Higher Fat Oxidation, Suppression of Hunger, and Improved Metabolic Profile After an Exercise Session. Nutrients 2021, 13, 155. [Google Scholar] [CrossRef]
- Kang, J.; Raines, E.; Rosenberg, J.; Ratamess, N.; Naclerio, F.; Faigenbaum, A. Metabolic responses during postprandial exercise. Res. Sports Med. 2013, 21, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Diet induced thermogenesis. Nutr. Metab. 2004, 1, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crovetti, R.; Porrini, M.; Santangelo, A.; Testolin, G. The influence of thermic effect of food on satiety. Eur. J. Clin. Nutr. 1998, 52, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Welle, S.; Lilavivathana, U.; Campbell, R.G. Increased plasma norepinephrine concentrations and metabolic rates following glucose ingestion in man. Metabolism 1980, 29, 806–809. [Google Scholar] [CrossRef]
- Hulston, C.J.; Venables, M.C.; Mann, C.H.; Martin, C.; Philp, A.; Baar, K.; Jeukendrup, A.E. Training with low muscle glycogen enhances fat metabolism in well-trained cyclists. Med. Sci. Sports Exerc. 2010, 42, 2046–2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, S.C.; Areta, J.L.; Bird, S.R.; Coffey, V.G.; Burke, L.M.; Desbrow, B.; Karagounis, L.G.; Hawley, J.A. Caffeine ingestion and cycling power output in a low or normal muscle glycogen state. Med. Sci. Sports Exerc. 2013, 45, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Knapik, J.J.; Meredith, C.N.; Jones, B.H.; Suek, L.; Young, V.R.; Evans, W.J. Influence of fasting on carbohydrate and fat metabolism during rest and exercise in men. J. Appl. Physiol. 1988, 64, 1923–1929. [Google Scholar] [CrossRef]
- Galloway, S.D.; Lott, M.J.; Toulouse, L.C. Preexercise carbohydrate feeding and high-intensity exercise capacity: Effects of timing of intake and carbohydrate concentration. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Khong, T.K.; Selvanayagam, V.S.; Hamzah, S.H.; Yusof, A. Effect of quantity and quality of pre-exercise carbohydrate meals on central fatigue. J. Appl. Physiol. 2018, 125, 1021–1029. [Google Scholar] [CrossRef]
- AbuMoh’d, M.F.; Matalqah, L.; Al-Abdulla, Z. Effects of Oral Branched-Chain Amino Acids (BCAAs) Intake on Muscular and Central Fatigue during an Incremental Exercise. J. Hum. Kinet. 2020, 72, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, S.F.; Islam, H.; Hazell, T.J. The emerging role of lactate as a mediator of exercise-induced appetite suppression. Am. J. Physiol. Endocrinol. Metab. 2020, 314, E814–E819. [Google Scholar] [CrossRef] [PubMed]
- Deighton, K.; Barry, R.; Connon, C.E.; Stensel, D.J. Appetite, gut hormone and energy intake responses to low volume sprint interval and traditional endurance exercise. Eur. J. Appl. Physiol. 2013, 113, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Deighton, K.; Zahra, J.C.; Stensel, D.J. Appetite, energy intake and resting metabolic responses to 60 min treadmill running performed in a fasted versus a postprandial state. Appetite 2012, 58, 946–954. [Google Scholar] [CrossRef]
- Rehrer, N.J.; van Kemenade, M.; Meester, W.; Brouns, F.; Saris, W.H. Gastrointestinal complaints in relation to dietary intake in triathletes. Int. J. Sport Nutr. 1992, 2, 48–59. [Google Scholar] [CrossRef]
- Snipe, R.M.J.; Khoo, A.; Kitic, C.M.; Gibson, P.R.; Costa, R.J.S. Carbohydrate and protein intake during exertional heat stress ameliorates intestinal epithelial injury and small intestine permeability. Appl. Physiol. Nutr. Metab. 2017, 42, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, A.S.; Barrack, M.T.; Nattiv, A.; Fredericson, M. Parallels with the Female Athlete Triad in Male Athletes. Sports Med. 2016, 46, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Deutz, R.C.; Benardot, D.; Martin, D.E.; Cody, M.M. Relationship between energy deficits and body composition in elite female gymnasts and runners. Med. Sci. Sports Exerc. 2000, 32, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Olguin, C.; Renani, L.B.; Arab-Ceschia, L.; Raun, S.H.; Bhatia, A.; Li, Z.; Knudsen, J.R.; Holmdahl, R.; Jensen, T.E. Adaptations to high-intensity interval training in skeletal muscle require NADPH oxidase 2. Redox Biol. 2019, 24, 101188. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, J.A.; Bishop, D.J. Effects of Dietary Supplements on Adaptations to Endurance Training. Sports Med. 2020, 50, 25–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaidis, M.G.; Kyparos, A.; Vrabas, I.S. F2-isoprostane formation, measurement and interpretation: The role of exercise. Prog Lipid Res. 2011, 50, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Kyparos, A.; Paschalis, V.; Theodorou, A.A.; Panayiotou, G.; Zafeiridis, A.; Dipla, K.; Nikolaidis, M.G.; Vrabas, I.S. Reductive stress after exercise: The issue of redox individuality. Redox Biol. 2014, 2, 520–528. [Google Scholar] [CrossRef] [Green Version]
- Braakhuis, A.J.; Hopkins, W.G.; Lowe, T.E. Effect of dietary antioxidants, training, and performance correlates on antioxidant status in competitive rowers. Int. J. Sports Physiol. Perform. 2013, 8, 565–572. [Google Scholar] [CrossRef]
- Coyle, E.F.; Coggan, A.R.; Hemmert, M.K.; Lowe, R.C.; Walters, T.J. Substrate usage during prolonged exercise following a preexercise meal. J. Appl. Physiol. 1985, 59, 429–433. [Google Scholar] [CrossRef]
- Moseley, L.; Lancaster, G.I.; Jeukendrup, A.E. Effects of timing of pre-exercise ingestion of carbohydrate on subsequent metabolism and cycling performance. Eur. J. Appl. Physiol. 2003, 88, 453–458. [Google Scholar] [CrossRef]
- Pritchett, K.; Bishop, P.; Pritchett, R.; Kovacs, M.; Davis, J.; Casaru, C.; Green, M. Effects of timing of pre-exercise nutrient intake on glucose responses and intermittent cycling performance. S. Afr. J. Sports Med. 2008, 20, 86–90. [Google Scholar] [CrossRef]
- Smith, G.J.; Rhodes, E.C.; Langill, R.H. The effect of pre-exercise glucose ingestion on performance during prolonged swimming. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 136–144. [Google Scholar] [CrossRef]
- Sherman, W.M.; Brodowicz, G.; Wright, D.A.; Allen, W.K.; Simonsen, J.; Dernbach, A. Effects of 4 h preexercise carbohydrate feedings on cycling performance. Med. Sci. Sports Exerc. 1989, 21, 598–604. [Google Scholar] [CrossRef]
- Jentjens, R.L.; Cale, C.; Gutch, C.; Jeukendrup, A.E. Effects of pre-exercise ingestion of differing amounts of carbohydrate on subsequent metabolism and cycling performance. Eur. J. Appl. Physiol. 2003, 88, 444–452. [Google Scholar] [CrossRef]
- Mears, S.A.; Dickinson, K.; Bergin-Taylor, K.; Dee, R.; Kay, J.; James, L.J. Perception of Breakfast Ingestion Enhances High-Intensity Cycling Performance. Int. J. Sports Physiol. Perform. 2018, 13, 504–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, V.R.; Hopkins, W.G.; Hawley, J.A.; Burke, L.M. Placebo effect of carbohydrate feedings during a 40-km cycling time trial. Med. Sci. Sports Exerc. 2000, 32, 1642–1647. [Google Scholar] [CrossRef]
- Hulston, C.J.; Jeukendrup, A.E. No placebo effect from carbohydrate intake during prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Riddell, M.C.; Partington, S.L.; Stupka, N.; Armstrong, D.; Rennie, C.; Tarnopolsky, M.A. Substrate utilization during exercise performed with and without glucose ingestion in female and male endurance trained athletes. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 407–421. [Google Scholar] [CrossRef] [Green Version]
- Tobias, I.S.; Lazauskas, K.K.; Siu, J.; Costa, P.B.; Coburn, J.W.; Galpin, A.J. Sex and fiber type independently influence AMPK, TBC1D1, and TBC1D4 at rest and during recovery from high-intensity exercise in humans. J. Appl. Physiol. 2020, 128, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Hetlelid, K.J.; Plews, D.J.; Herold, E.; Laursen, P.B.; Seiler, S. Rethinking the role of fat oxidation: Substrate utilisation during high-intensity interval training in well-trained and recreationally trained runners. BMJ Open Sport Exerc. Med. 2015, 1, e000047. [Google Scholar] [CrossRef] [Green Version]
- Rowlands, D.S.; Hopkins, W.G. Effects of high-fat and high-carbohydrate diets on metabolism and performance in cycling. Metabolism 2002, 51, 678–690. [Google Scholar] [CrossRef]
- McKenzie, S.; Phillips, S.M.; Carter, S.L.; Lowther, S.; Gibala, M.J.; Tarnopolsky, M.A. Endurance exercise training attenuates leucine oxidation and BCOAD activation during exercise in humans. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E580–E587. [Google Scholar] [CrossRef] [Green Version]
- Bowtell, J.L.; Leese, G.P.; Smith, K.; Watt, P.W.; Nevill, A.; Rooyackers, O.; Wagenmakers, A.J.; Rennie, M.J. Modulation of whole body protein metabolism, during and after exercise, by variation of dietary protein. J. Appl. Physiol. 1998, 85, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Lemon, P.W.; Mullin, J.P. Effect of initial muscle glycogen levels on protein catabolism during exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 48, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.S.; Holm, L.; Svart, M.V.; Hjelholt, A.J.; Bengtsen, M.B.; Dollerup, O.L.; Dalgaard, L.B.; Vendelbo, M.H.; van Hall, G.; Moller, N.; et al. Effects of protein intake prior to carbohydrate-restricted endurance exercise: A randomized crossover trial. J. Int. Soc. Sports Nutr. 2020, 17, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Contrast | Intensity | Heart Rate | Gross Efficiency | RPE | RER | Fat Oxidation (g/min) | Carbohydrate Oxidation (g/min) |
|---|---|---|---|---|---|---|---|
| CARB-FASTED | VT60 | Small | Trivial | Trivial | Large | Large | Medium |
| ES = 0.34 | ES = −0.15 | ES = 0.08 | ES = 1.29 | ES = −0.85 | ES = 0.69 | ||
| [0.07, 0.61] | [−0.32, 0.02] | [−0.23, 0.39] | [0.69, 1.89] | [−1.46, −0.25] | [0.18, 1.21] | ||
| CARB-PROTEIN | VT60 | Trivial | Trivial | Trivial | Large | Medium | Small |
| ES = −0.06 | ES = 0.11 | ES = 0.15 | ES = 0.80 | ES = −0.54 | ES = 0.37 | ||
| [−0.37, 0.25] | [−0.06, 0.28] | [−0.16, 0.45] | [0.28, 1.33] | [−1, −0.07] | [−0.1, 0.84] | ||
| FASTED-PROTEIN | VT60 | Small | Small | Trivial | Small | Small | Small |
| ES = −0.4 | ES = 0.26 | ES = 0.07 | ES = −0.49 | ES = 0.32 | ES = −0.33 | ||
| [−0.55, −0.24] | [0.09, 0.42] | [−0.23, 0.37] | [−1.03, 0.06] | [−0.2, 0.84] | [−0.82, 0.17] | ||
| CARB-FASTED | VT80 | Small | Trivial | Trivial | Large | Large | Medium |
| ES = 0.34 | ES = −0.15 | ES = 0.08 | ES = 0.95 | ES = −0.82 | ES = 0.67 | ||
| [0.07, 0.61] | [−0.32, 0.02] | [−0.23, 0.39] | [0.36, 1.54] | [−1.43, −0.22] | [0.16, 1.19] | ||
| CARB-PROTEIN | VT80 | Trivial | Trivial | Trivial | Medium | Small | Small |
| ES = −0.06 | ES = 0.11 | ES = 0.15 | ES = 0.50 | ES = −0.47 | ES = 0.31 | ||
| [−0.37, 0.25] | [−0.06, 0.28] | [−0.16, 0.45] | [−0.02, 1.02] | [−0.94, −0.01] | [−0.16, 0.77] | ||
| FASTED-PROTEIN | VT80 | Small | Small | Trivial | Small | Small | Small |
| ES = −0.4 | ES = 0.26 | ES = 0.07 | ES = −0.45 | ES = 0.35 | ES = −0.37 | ||
| [−0.55, −0.24] | [0.09, 0.42] | [−0.23, 0.37] | [−0.99, 0.09] | [−0.17, 0.87] | [−0.86, 0.13] | ||
| CARB-FASTED | VT100 | Small | Trivial | Trivial | Medium | Medium | Medium |
| ES = 0.34 | ES = −0.15 | ES = 0.08 | ES = 0.71 | ES = −0.75 | ES = 0.66 | ||
| [0.07, 0.61] | [−0.32, 0.02] | [−0.23, 0.39] | [0.13, 1.3] | [−1.35, −0.15] | [0.14, 1.17] | ||
| CARB-PROTEIN | VT100 | Trivial | Trivial | Trivial | Trivial | Small | Trivial |
| ES = −0.06 | ES = 0.11 | ES = 0.15 | ES = 0.19 | ES = −0.26 | ES = 0.13 | ||
| [−0.37, 0.25] | [−0.06, 0.28] | [−0.16, 0.45] | [−0.33, 0.7] | [−0.73, 0.2] | [−0.34, 0.59] | ||
| FASTED-PROTEIN | VT100 | Small | Small | Trivial | Medium | Small | Medium |
| ES = −0.4 | ES = 0.26 | ES = 0.07 | ES = −0.53 | ES = 0.49 | ES = −0.53 | ||
| [−0.55, −0.24] | [0.09, 0.42] | [−0.23, 0.37] | [−1.07, 0.01] | [−0.03, 1.01] | [−1.02, −0.03] | ||
| CARB-FASTED | VTΔ20 | Small | Trivial | Trivial | Small | Medium | Medium |
| ES = 0.34 | ES = −0.15 | ES = 0.08 | ES = 0.44 | ES = −0.52 | ES = 0.53 | ||
| [0.07, 0.61] | [−0.32, 0.02] | [−0.23, 0.39] | [−0.14, 1.03] | [−1.13, 0.09] | [0, 1.05] | ||
| CARB-PROTEIN | VTΔ20 | Trivial | Trivial | Trivial | Trivial | Trivial | Trivial |
| ES = −0.06 | ES = 0.11 | ES = 0.15 | ES = 0.14 | ES = −0.11 | ES = 0.07 | ||
| [−0.37, 0.25] | [−0.06, 0.28] | [−0.16, 0.45] | [−0.37, 0.66] | [−0.58, 0.36] | [−0.4, 0.54] | ||
| FASTED-PROTEIN | VTΔ20 | Small | Small | Trivial | Small | Small | Small |
| ES = −0.4 | ES = 0.26 | ES = 0.07 | ES = −0.30 | ES = 0.41 | ES = −0.46 | ||
| [−0.55, −0.24] | [0.09, 0.42] | [−0.23, 0.37] | [−0.84, 0.24] | [−0.11, 0.94] | [−0.96, 0.04] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rothschild, J.A.; Kilding, A.E.; Broome, S.C.; Stewart, T.; Cronin, J.B.; Plews, D.J. Pre-Exercise Carbohydrate or Protein Ingestion Influences Substrate Oxidation but Not Performance or Hunger Compared with Cycling in the Fasted State. Nutrients 2021, 13, 1291. https://doi.org/10.3390/nu13041291
Rothschild JA, Kilding AE, Broome SC, Stewart T, Cronin JB, Plews DJ. Pre-Exercise Carbohydrate or Protein Ingestion Influences Substrate Oxidation but Not Performance or Hunger Compared with Cycling in the Fasted State. Nutrients. 2021; 13(4):1291. https://doi.org/10.3390/nu13041291
Chicago/Turabian StyleRothschild, Jeffrey A., Andrew E. Kilding, Sophie C. Broome, Tom Stewart, John B. Cronin, and Daniel J. Plews. 2021. "Pre-Exercise Carbohydrate or Protein Ingestion Influences Substrate Oxidation but Not Performance or Hunger Compared with Cycling in the Fasted State" Nutrients 13, no. 4: 1291. https://doi.org/10.3390/nu13041291
APA StyleRothschild, J. A., Kilding, A. E., Broome, S. C., Stewart, T., Cronin, J. B., & Plews, D. J. (2021). Pre-Exercise Carbohydrate or Protein Ingestion Influences Substrate Oxidation but Not Performance or Hunger Compared with Cycling in the Fasted State. Nutrients, 13(4), 1291. https://doi.org/10.3390/nu13041291







