Healthy Lifestyle Intervention and Weight Loss Improve Cardiovascular Dysfunction in Children with Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Intervention
2.3. Anthropometric and Biochemical Variables
2.4. Echocardiographic and Vascular Assessment
2.5. Statistical Analysis
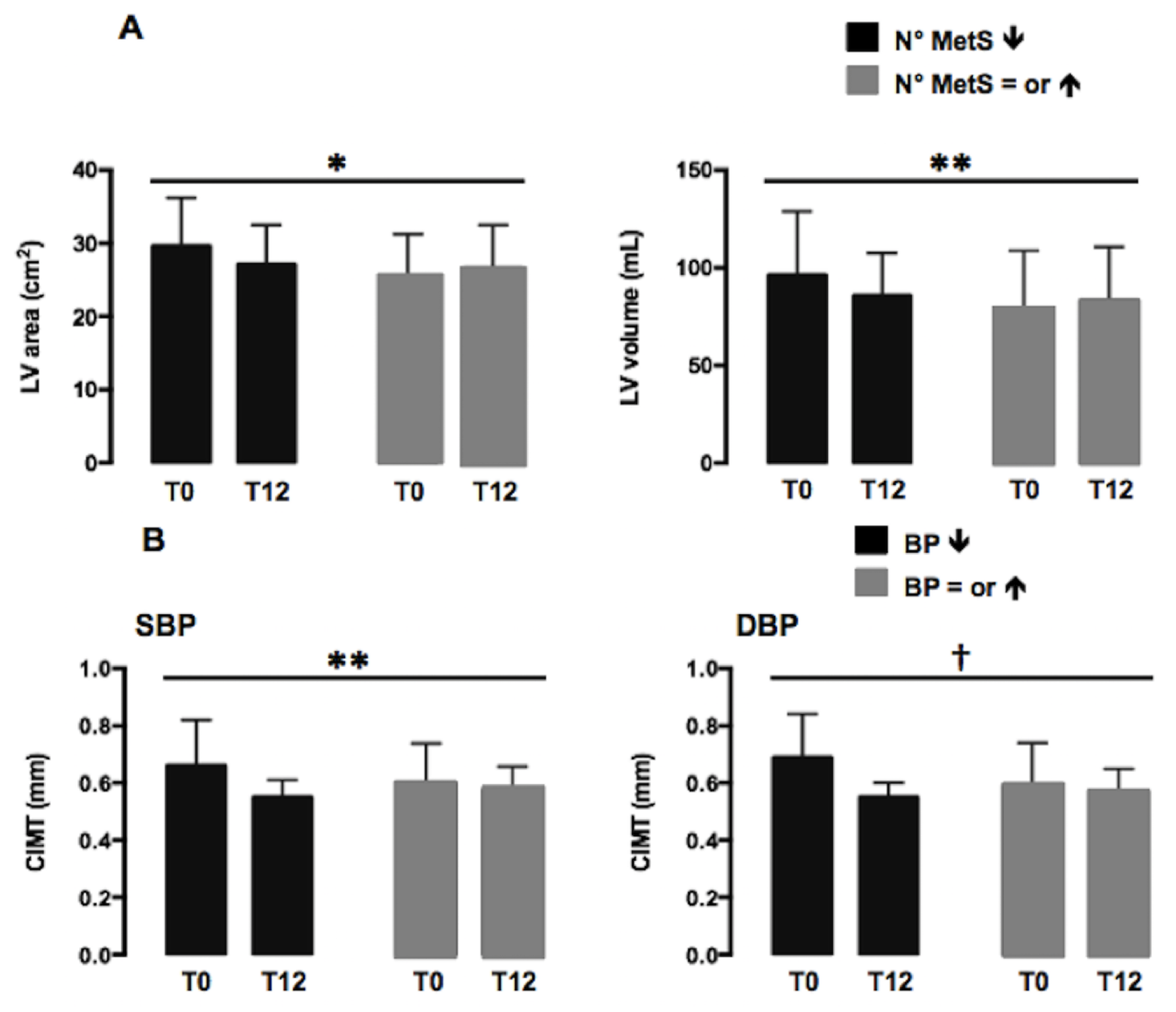
3. Results
Echocardiographic and Vascular Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebbeling, C.B.; Pawlak, D.B. Childhood obesity: Public-health crisis, common sense cure. Lancet 2002, 360, 473–482. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Coxson, P.; Pletcher, M.J.; Lightwood, J.; Goldman, L. Adolescent overweight and future adult coronary heart disease. N. Engl. J. Med. 2007, 357, 2371–2379. [Google Scholar] [CrossRef]
- Twig, G.; Yaniv, G.; Levine, H.; Leiba, A.; Goldberger, N.; Derazne, E.; Shor, D.B.-A.; Tzur, D.; Afek, A.; Shamiss, A.; et al. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. N. Engl. J. Med. 2016, 374, 2430–2440. [Google Scholar] [CrossRef]
- Genoni, G.; Menegon, V.; Secco, G.G.; Sonzini, M.; Martelli, M.; Castagno, M.; Ricotti, R.; Monzani, A.; Aronici, M.; Grossini, E.; et al. Insulin resistance, serum uric acid and metabolic syndrome are linked to cardiovascular dysfunction in pediatric obesity. Int. J. Cardiol. 2017, 249, 366–371. [Google Scholar] [CrossRef]
- Talib, A.; Roebroek, Y.G.M.; Paulus, G.F.; van Loo, K.; Winkens, B.; Bouvy, N.D.; van Heurn, E.L.W.E. Left Ventricular Geometrical Changes in Severely Obese Adolescents: Prevalence, Determinants, and Clinical Implications. Pediatr. Cardiol. 2021, 42, 331–339. [Google Scholar] [CrossRef]
- Cote, A.T.; Harris, K.C.; Panagiotopoulos, C.; Sandor, G.G.; Devlin, A.M. Childhood obesity and cardiovascular dysfunction. J. Am. Coll. Cardiol. 2013, 62, 1309–1319. [Google Scholar] [CrossRef]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef]
- Società Italiana di Nutrizione Umana. Livelli di Assunzione Raccomandati di Energia e Nutrienti per la Popolazione Italiana (LARN), 4th ed.; Società Italiana di Nutrizione Umana: Milan, Italy, 2014. [Google Scholar]
- Arós, F.; Estruch, R. Mediterranean diet and cardiovascular prevention. Rev. Esp. Cardiol. 2013, 66, 771–774. [Google Scholar] [CrossRef]
- Ayer, J.; Charakida, M.; Deanfield, J.E.; Celermajer, D.S. Lifetime risk: Childhood obesity and cardiovascular risk. Eur. Heart J. 2015, 36, 1371–1376. [Google Scholar] [CrossRef]
- Lee, S.C.; Daimon, M.; Di Tullio, M.R.; Homma, S.; Hasegawa, T.; Han Chiou, S.; Nakao, T.; Hirokawa, M.; Mizuno, Y.; Yatomi, Y.; et al. Beneficial effect of body weight control on left ventricular diastolic function in the general population: An analysis of longitudinal data from a health check-up clinic. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 136–142. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.L.; Goran, M.I. The metabolic syndrome in children and adolescents. Curr. Diabetes Rep. 2004, 4, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Lacombe, F.; Dart, A.; Dewar, E.; Jennings, G.; Cameron, J.; Laufer, E. Arterial elastic properties in man: A comparison of echo-Doppler indices of aortic stiffness. Eur. Heart J. 1992, 13, 1040–1045. [Google Scholar] [CrossRef]
- Anderson, T.J.; Charbonneau, F.; Title, L.M.; Buithieu, J.; Rose, M.R.; Conradson, H.; Hildebrand, K.; Fung, M.; Verma, S.; Lonn, E.M. Microvascular function predicts cardiovascular events in primary prevention: Long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation 2011, 123, 163–169. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H.; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism; American Heart Association, Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, Metabolism. Circulation 2006, 113, 898–918. [Google Scholar]
- Diabetes Prevention Program Research Group; Nathan, D.M.; Barrett-Connor, E.; Crandall, J.P.; Edelstein, S.L.; Goldberg, R.B.; Horton, E.S. Long-term Effects of Lifestyle Intervention or Metformin on Diabetes Development and Microvascular Complications: The DPP Outcomes Study. Lancet Diabetes Endocrinol. 2015, 3, 866–875. [Google Scholar]
- Reinehr, T. Lifestyle intervention in childhood obesity: Changes and challenges. Nat. Rev. Endocrinol. 2013, 9, 607–614. [Google Scholar] [CrossRef]
- Ho, M.; Garnett, S.P.; Baur, L.; Burrows, T.; Stewart, L.; Neve, M.; Collins, C. Effectiveness of lifestyle interventions in child obesity: Systematic review with meta-analysis. Pediatrics 2012, 130, e1647–e1671. [Google Scholar] [CrossRef]
- Reinehr, T.; Kleber, M.; Toschke, A.M. Lifestyle intervention in obese children is associated with a decrease of the metabolic syndrome prevalence. Atherosclerosis 2009, 207, 174–180. [Google Scholar] [CrossRef]
- Ippisch, H.M.; Inge, T.H.; Daniels, S.R.; Wang, B.; Khoury, P.R.; Witt, S.A.; Glascock, B.J.; Garcia, V.F.; Kimball, T.R. Reversibility of cardiac abnormalities in morbidly obese adolescents. J. Am. Coll. Cardiol. 2008, 51, 1342–1348. [Google Scholar] [CrossRef]
- Naylor, L.H.; Watts, K.; Sharpe, J.A.; Jones, T.W.; Davis, E.A.; Thompson, A.; George, K.; Ramsay, J.M.; O’Driscoll, G.; Green, D.J. Resistance training and diastolic myocardial tissue velocities in obese children. Med. Sci. Sports Exerc. 2008, 40, 2027–2032. [Google Scholar] [CrossRef] [PubMed]
- Ingul, C.B.; Dias, K.A.; Tjonna, A.E.; Follestad, T.; Hosseini, M.S.; Timilsina, A.S.; Hollekim-Strand, S.M.; Ro, T.B.; Davies, P.S.; Cain, P.A.; et al. Effect of High Intensity Interval Training on Cardiac Function in Children with Obesity: A Randomised Controlled Trial. Prog. Cardiovasc. Dis. 2018, 61, 214–221. [Google Scholar] [CrossRef]
- Zeybek, C.; Çelebi, A.; Aktuglu-Zeybek, C.; Onal, H.; Yalcin, Y.; Erdem, A.; Akdeniz, C.; Imanov, E.; Altay, S.; Aydın, A. The effect of low-carbohydrate diet on left ventricular diastolic function in obese children. Pediatr. Int. 2010, 52, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Floody, P.; Alvarez, C.; Caamaño-Navarrete, F.; Jerez-Mayorga, D.; Latorre-Román, P. Influence of Mediterranean diet adherence, physical activity patterns, and weight status on cardiovascular response to cardiorespiratory fitness test in Chilean school children. Nutrition 2020, 71, 110621. [Google Scholar] [CrossRef] [PubMed]
- Aggoun, Y.; Farpour-Lambert, N.J.; Marchand, L.M.; Golay, E.; Maggio, A.B.; Beghetti, M. Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressure. Eur. Heart J. 2008, 29, 792–799. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, S.; Peto, R.; Collins, R.; Godwin, J.; Cutler, J.; Sorlie, P.; Abbott, R.; Neaton, J.; Dyer, A.; Stamler, J. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet 1990, 335, 765–774. [Google Scholar] [CrossRef]
- Farpour-Lambert, N.J.; Aggoun, Y.; Marchand, L.M.; Martin, X.E.; Herrmann, F.R.; Beghetti, M. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J. Am. Coll. Cardiol. 2009, 54, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Kavey, R.E.W.; St Pierre, J.; McCrindle, B.W. Incorporating Risk Stratification into the Practice of Pediatric Preventive Cardiology. Can. J. Cardiol. 2020, 36, 1417–1428. [Google Scholar] [CrossRef]

| WL n = 28 | NWL n = 27 | p Value | |
|---|---|---|---|
| Age | 11.2 ± 2.7 | 11.4 ± 2.8 | ns |
| Female | 13(44.8%) | 13(48.1%) | ns |
| Prepubertal | 11(40.7%) | 9(31.0%) | ns |
| Height (cm) | 149.5 ± 13.7 | 151.6 ± 17.0 | ns |
| Height (SDS) | 1.0 ± 1.1 | 1.1 ± 1.2 | ns |
| Weight (kg) | 67.3 ± 21.0 | 75.2 ± 25.4 | ns |
| BMI (kg/m2) | 29.3 ± 5.2 | 31.8 ± 5.7 | ns |
| BMI z-score | 2.23 ± 0.51 | 2.50 ± 0.57 | ns |
| Waist (cm) | 88.9 ± 14.2 | 95.1 ± 13.7 | ns |
| SBP (mmHg) | 123.3 ± 18.2 | 126.0 ± 15.9 | ns |
| DBP (mmHg) | 78.0 ± 11.8 | 77.7 ± 10.0 | ns |
| WL n = 28 | NWL n = 27 | |||||
|---|---|---|---|---|---|---|
| T0 | T12 | p Value | T0 | T12 | p Value | |
| HR (b/min) | 81.1 ± 12.8 | 73.8 ± 10.3 | 0.01 | 85.8 ± 10.7 | 84.7 ± 12.7 | ns |
| EF (%) | 69.8 ± 7.9 | 71.1 ± 7.9 | ns | 70.0 ± 9.9 | 69.4 ± 6.8 | ns |
| FS (%) | 39.7 ± 6.3 | 40.5 ± 4.6 | ns | 42.9 ± 7.4 | 40.1 ± 5.7 | ns |
| LVEDD (mm) | 45.4 ± 6.0 | 47.1 ± 6.6 | ns | 46.1 ± 7.7 | 47.0 ± 5.1 | 0.04 |
| LVEDD z-score | −0.65 ± 0.91 | −0.24 ± 0.91 | ns | −1.35 ± 1.28 | −0.94 ± 1.19 | ns |
| LVESD (mm) | 27.5 ± 4.8 | 28.0 ± 5.1 | ns | 28.2 ± 7.1 | 28.4 ± 3.4 | ns |
| LVESD z-score | −0.74 ± 1.07 | −0.52 ± 1.13 | ns | −1.16 ± 1.13 | −1.03 ± 1.24 | ns |
| IVSD (mm) | 7.6 ± 1.6 | 7.6 ± 1.7 | ns | 7.7 ± 2.0 | 8.2 ± 1.9 | ns |
| IVSD z-score | −0.28 ± 0.94 | −0.49 ± 0.80 | ns | −0.09 ± 1.05 | 0.30 ± 1.06 | ns |
| LVPWD (mm) | 7.5 ± 1.8 | 7.5 ± 1.2 | ns | 8.5 ± 2.8 | 7.7 ± 2.1 | ns |
| LVPWD z-score | 0.20 ± 0.20 | 0.31 ± 1.00 | ns | 0.46 ± 0.95 | −0.25 ± 1.08 | 0.004 |
| LAD (mm) | 32.0 ± 5.2 | 33.0 ± 4.9 | ns | 33.7 ± 6.1 | 33.4 ± 5.4 | ns |
| LAD z-score | 1.12 ± 1.25 | 0.83 ± 1.22 | ns | 1.26 ± 1.00 | 1.54 ± 0.85 | ns |
| Ao (mm) | 24.0 ± 3.2 | 25.0 ± 3.8 | ns | 2.5 ± 0.5 | 2.65 ± 0.41 | ns |
| LA/Ao ratio | 1.40 ± 0.21 | 1.34 ± 0.22 | ns | 1.35 ± 0.22 | 1.30 ± 0.19 | ns |
| LV mass (g) | 113.1 ± 47.2 | 123.2 ± 40.9 | ns | 131.7 ± 85.1 | 121.0 ± 53.2 | ns |
| LVmass index (g/m2) | 66.8 ± 18.3 | 73.9 ± 17.8 | ns | 72.0 ± 30.9 | 64.8 ± 17.5 | ns |
| LV mass z-score | −0.001 ± 1.479 | 0.11 ± 1.17 | ns | 0.26 ± 1.49 | −0.15 ± 1.07 | ns |
| RWT | 0.33 ± 0.07 | 0.33 ± 0.07 | ns | 0.37 ± 0.09 | 0.32 ± 0.08 | 0.017 |
| LV area (cm2) | 26.5 ± 6.0 | 26.9 ± 5.7 | ns | 26.9 ± 7.2 | 26.5 ± 5.0 | ns |
| LV volume (mL) | 83.0 ± 27.3 | 84.8 ± 26.9 | ns | 86.3 ± 36.9 | 83.5 ± 23.4 | ns |
| LA area (cm2) | 12.9 ± 3.5 | 14.4 ± 2.8 | 0.04 | 13.9 ± 4.6 | 13.9 ± 3.1 | ns |
| LA volume (mL) | 32.5 ± 13.9 | 37.2 ± 12.5 | ns | 31.6 ± 12.8 | 34.9 ± 12.2 | ns |
| Mitral E (cm/s) | 1.02 ± 0.20 | 1.12 ± 0.18 | 0.001 | 1.04 ± 0.24 | 1.21 ± 0.59 | 0.007 |
| Mitral A (cm/s) | 0.55 ± 0.14 | 0.56 ± 0.11 | ns | 0.60 ± 0.13 | 0.65 ± 0.23 | ns |
| Mitral E/A ratio | 1.91 ± 0.43 | 2.02 ± 0.44 | ns | 1.7 ± 0.44 | 1.8 ± 0.47 | ns |
| CIMT (mm) | 0.62 ± 0.17 | 0.52 ± 0.09 | 0.010 | 0.61 ± 0.11 | 0.55 ± 0.05 | 0.014 |
| AoDd (mm) | 10.0 ± 1.6 | 10.8 ± 1.7 | 0.031 | 10.8 ± 2.4 | 11.2 ± 1.6 | ns |
| AoDs (mm) | 12.5 ± 1.9 | 13.1 ± 1.5 | ns | 13.2 ± 2.3 | 13.5 ± 1.8 | ns |
| Aortic Strain, S | 0.25 ± 0.10 | 0.23 ± 0.12 | ns | 0.24 ± 0.15 | 0.21 ± 0.11 | ns |
| Ep (mmHg) | 201.8 ± 128.3 | 249.1 ± 161.5 | ns | 478.7 ± 1140.1 | 253.4 ± 149.2 | ns |
| Ep* | 2.7 ± 1.8 | 3.4 ± 2.2 | ns | 6.3 ± 15.5 | 3.0 ± 1.8 | ns |
| BAD basal (mm) | 3.40 ± 0.74 | 3.49 ± 0.54 | ns | 3.53 ± 0.73 | 3.44 ± 0.57 | ns |
| BAD after (mm) | 3.48 ± 0.68 | 3.38 ± 0.88 | ns | 3.70 ± 0.75 | 3.48 ± 0.94 | ns |
| FMD% | 3.4 ± 14.6 | 0.9 ± 10.5 | ns | 6.5 ± 18.4 | 1.8 ± 24.6 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genoni, G.; Menegon, V.; Monzani, A.; Archero, F.; Tagliaferri, F.; Mancioppi, V.; Peri, C.; Bellone, S.; Prodam, F. Healthy Lifestyle Intervention and Weight Loss Improve Cardiovascular Dysfunction in Children with Obesity. Nutrients 2021, 13, 1301. https://doi.org/10.3390/nu13041301
Genoni G, Menegon V, Monzani A, Archero F, Tagliaferri F, Mancioppi V, Peri C, Bellone S, Prodam F. Healthy Lifestyle Intervention and Weight Loss Improve Cardiovascular Dysfunction in Children with Obesity. Nutrients. 2021; 13(4):1301. https://doi.org/10.3390/nu13041301
Chicago/Turabian StyleGenoni, Giulia, Veronica Menegon, Alice Monzani, Francesca Archero, Francesco Tagliaferri, Valentina Mancioppi, Caterina Peri, Simonetta Bellone, and Flavia Prodam. 2021. "Healthy Lifestyle Intervention and Weight Loss Improve Cardiovascular Dysfunction in Children with Obesity" Nutrients 13, no. 4: 1301. https://doi.org/10.3390/nu13041301
APA StyleGenoni, G., Menegon, V., Monzani, A., Archero, F., Tagliaferri, F., Mancioppi, V., Peri, C., Bellone, S., & Prodam, F. (2021). Healthy Lifestyle Intervention and Weight Loss Improve Cardiovascular Dysfunction in Children with Obesity. Nutrients, 13(4), 1301. https://doi.org/10.3390/nu13041301







