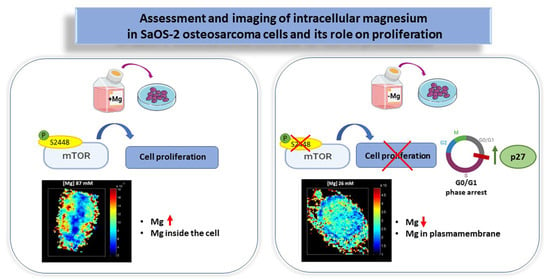
Assessment and Imaging of Intracellular Magnesium in SaOS-2 Osteosarcoma Cells and Its Role in Proliferation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Flow Cytometric Assays
2.4. Lactate Dehydrogenase Assay
2.5. Western Blotting
2.6. Magnesium Determination by DCHQ5 Spectrofluorimetric Assay
2.7. Synchrotron Based X-ray Microscopy
2.8. Statistical Analysis
3. Results
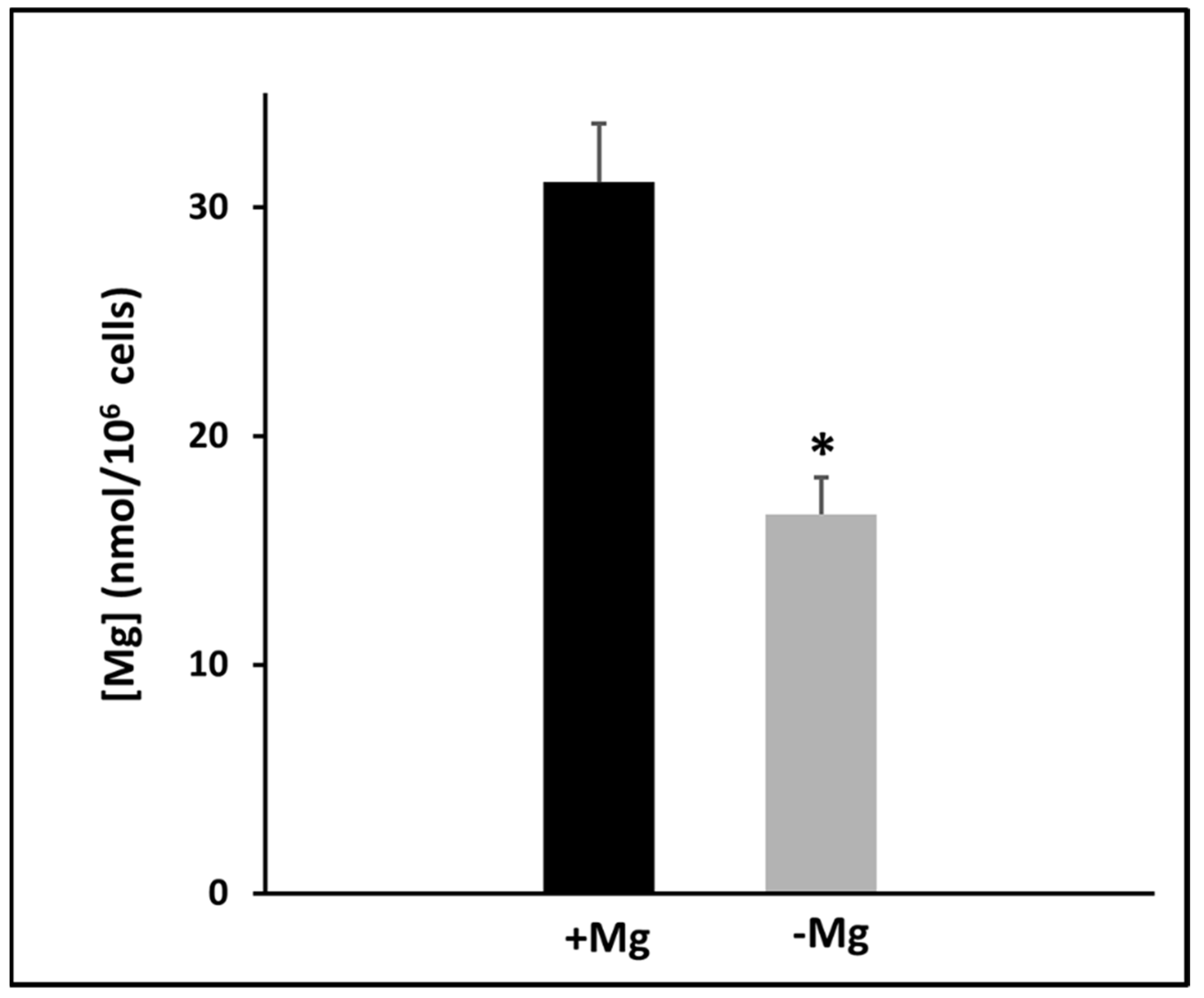
3.1. Effects of Magnesium Deprivation on Intracellular Magnesium Content
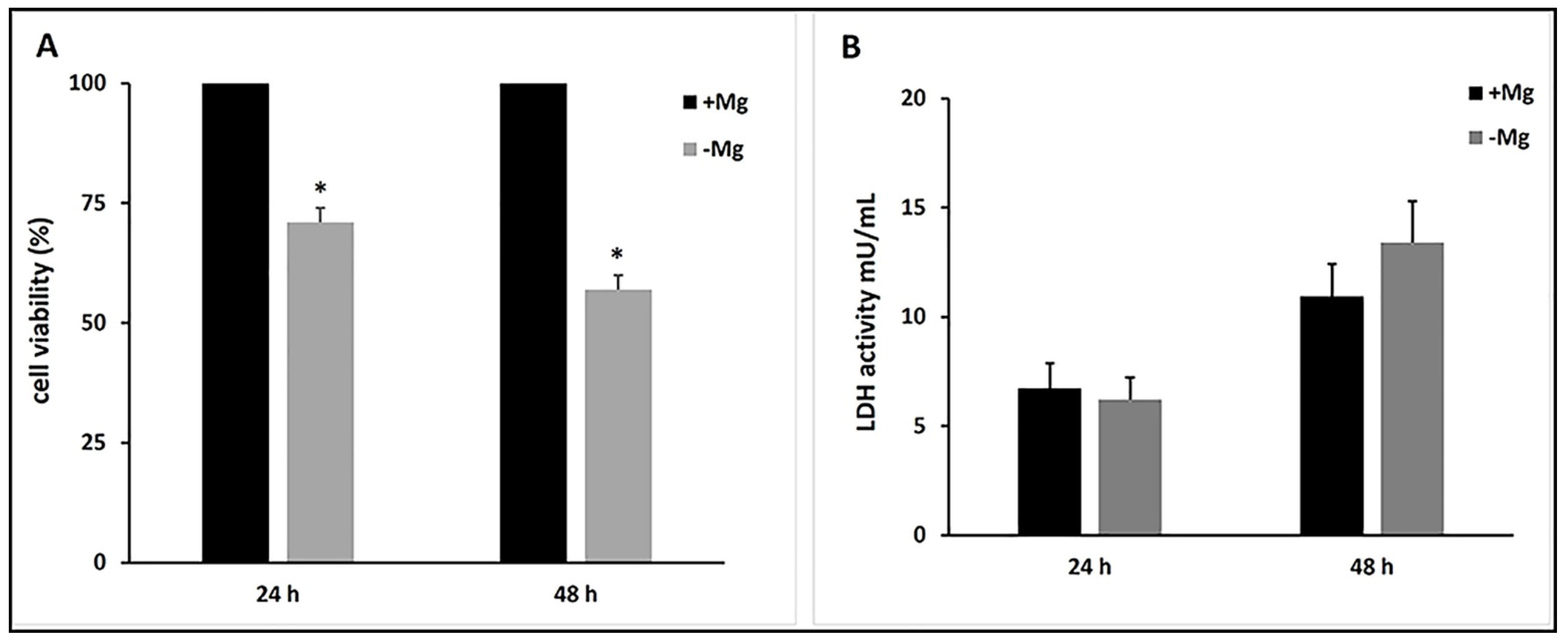
3.2. Effects of Magnesium Deficiency on Cell Growth and Cell Death
3.3. Effects of Magnesium Deficiency on Cell Cycle Progression
3.4. Effects of Magnesium Deficiency on mTOR Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Johnson, S. The Multifaceted and Widespread Pathology of Magnesium Deficiency. Med. Hypotheses 2001, 56, 163–170. [Google Scholar] [CrossRef]
- NHANES Questionnaires, Datasets, and Related Documentation. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2005 (accessed on 6 March 2021).
- Rubin, H. Magnesium: The Missing Element in Molecular Views of Cell Proliferation Control. Bioessays 2005, 27, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Iotti, S.; Frassineti, C.; Sabatini, A.; Vacca, A.; Barbiroli, B. Quantitative Mathematical Expressions for Accurate in Vivo Assessment of Cytosolic [ADP] and ΔG of ATP Hydrolysis in the Human Brain and Skeletal Muscle. Biochim. Biophys. Acta (BBA) Bioenerg. 2005, 1708, 164–177. [Google Scholar] [CrossRef][Green Version]
- Feeney, K.A.; Hansen, L.L.; Putker, M.; Olivares-Yañez, C.; Day, J.; Eades, L.J.; Larrondo, L.F.; Hoyle, N.P.; O’Neill, J.S.; van Ooijen, G. Daily Magnesium Fluxes Regulate Cellular Timekeeping and Energy Balance. Nature 2016, 532, 375–379. [Google Scholar] [CrossRef]
- Barbiroli, B.; Iotti, S.; Cortelli, P.; Martinelli, P.; Lodi, R.; Carelli, V.; Montagna, P. Low Brain Intracellular Free Magnesium in Mitochondrial Cytopathies. J. Cereb. Blood Flow Metab. 1999, 19, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Zieve, F.J.; Freude, K.A.; Zieve, L. Effects of Magnesium Deficiency on Protein and Nucleic Acid Synthesis in Vivo. J. Nutr. 1977, 107, 2178–2188. [Google Scholar] [CrossRef]
- Mushegian, A.A. A Ribosomal Strategy for Magnesium Deficiency. Sci. Signal. 2016, 9, ec269. [Google Scholar] [CrossRef]
- Rubin, H. The Logic of the Membrane, Magnesium, Mitosis (MMM) Model for the Regulation of Animal Cell Proliferation. Arch. Biochem. Biophys. 2007, 458, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Cittadini, A.R.M.; Maier, J.A.M. Magnesium and Tumors: Ally or Foe? Cancer Treat. Rev. 2009, 35, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Runnels, L.W.; Yue, L.; Clapham, D.E. TRP-PLIK, a Bifunctional Protein with Kinase and Ion Channel Activities. Science 2001, 291, 1043–1047. [Google Scholar] [CrossRef]
- Zou, Z.-G.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. TRPM7, Magnesium, and Signaling. Int. J. Mol. Sci. 2019, 20, 1877. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Chiu, H.-F.; Tsai, S.-S.; Wu, T.-N.; Chang, C.-C. Magnesium and Calcium in Drinking Water and the Risk of Death from Esophageal Cancer. Magnes. Res. 2002, 15, 215–222. [Google Scholar] [PubMed]
- Zhong, G.-C.; Peng, Y.; Wang, K.; Wan, L.; Wu, Y.-Q.-L.; Hao, F.-B.; Hu, J.-J.; Gu, H.-T. Magnesium Intake and Primary Liver Cancer Incidence and Mortality in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int. J. Cancer 2020, 147, 1577–1586. [Google Scholar] [CrossRef]
- Wolf, F.I.; Trapani, V. Magnesium and Its Transporters in Cancer: A Novel Paradigm in Tumour Development. Clin. Sci. 2012, 123, 417–427. [Google Scholar] [CrossRef]
- Gaffney-Stomberg, E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar] [CrossRef]
- Erem, S.; Atfi, A.; Razzaque, M.S. Anabolic Effects of Vitamin D and Magnesium in Aging Bone. J. Steroid Biochem. Mol. Biol. 2019, 193, 105400. [Google Scholar] [CrossRef]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.A.M.; Grande, A.; Frassineti, C. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3. Int. J. Mol. Sci. 2019, 20, 385. [Google Scholar] [CrossRef] [PubMed]
- Picone, G.; Cappadone, C.; Pasini, A.; Lovecchio, J.; Cortesi, M.; Farruggia, G.; Lombardo, M.; Gianoncelli, A.; Mancini, L.; Ralf, H.M.; et al. Analysis of Intracellular Magnesium and Mineral Depositions during Osteogenic Commitment of 3D Cultured Saos2 Cells. Int. J. Mol. Sci. 2020, 21, 2368. [Google Scholar] [CrossRef] [PubMed]
- Sargenti, A.; Farruggia, G.; Zaccheroni, N.; Marraccini, C.; Sgarzi, M.; Cappadone, C.; Malucelli, E.; Procopio, A.; Prodi, L.; Lombardo, M.; et al. Synthesis of a Highly Mg2+-Selective Fluorescent Probe and Its Application to Quantifying and Imaging Total Intracellular Magnesium. Nat. Protoc. 2017, 12, 461–471. [Google Scholar] [CrossRef]
- Andreani, A.; Burnelli, S.; Granaiola, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Varoli, L.; Farruggia, G.; Stefanelli, C.; et al. Synthesis and Antitumor Activity of Guanylhydrazones from 6-(2,4-Dichloro-5-Nitrophenyl)Imidazo[2,1-b]Thiazoles and 6-Pyridylimidazo[2,1-b]Thiazoles. J. Med. Chem. 2006, 49, 7897–7901. [Google Scholar] [CrossRef] [PubMed]
- Malucelli, E.; Procopio, A.; Fratini, M.; Gianoncelli, A.; Notargiacomo, A.; Merolle, L.; Sargenti, A.; Castiglioni, S.; Cappadone, C.; Farruggia, G.; et al. Single Cell versus Large Population Analysis: Cell Variability in Elemental Intracellular Concentration and Distribution. Anal. Bioanal. Chem. 2018, 410, 337–348. [Google Scholar] [CrossRef]
- Malucelli, E.; Iotti, S.; Gianoncelli, A.; Fratini, M.; Merolle, L.; Notargiacomo, A.; Marraccini, C.; Sargenti, A.; Cappadone, C.; Farruggia, G.; et al. Quantitative Chemical Imaging of the Intracellular Spatial Distribution of Fundamental Elements and Light Metals in Single Cells. Anal. Chem. 2014, 86, 5108–5115. [Google Scholar] [CrossRef] [PubMed]
- Gianoncelli, A.; Kourousias, G.; Merolle, L.; Altissimo, M.; Bianco, A. Current Status of the TwinMic Beamline at Elettra: A Soft X-Ray Transmission and Emission Microscopy Station. J. Synchrotron Radiat. 2016, 23, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Gianoncelli, A.; Kourousias, G.; Stolfa, A.; Kaulich, B. Recent Developments at the TwinMic Beamline at ELETTRA: An 8 SDD Detector Setup for Low Energy X-Ray Fluorescence. J. Phys. Conf. Ser. 2013, 425, 182001. [Google Scholar] [CrossRef]
- Gianoncelli, A.; Morrison, G.R.; Kaulich, B.; Bacescu, D.; Kovac, J. Scanning Transmission X-Ray Microscopy with a Configurable Detector. Appl. Phys. Lett. 2006, 89, 251117. [Google Scholar] [CrossRef]
- Picone, G.; Cappadone, C.; Farruggia, G.; Malucelli, E.; Iotti, S. The Assessment of Intracellular Magnesium: Different Strategies to Answer Different Questions. Magnes. Res. 2020, 33, 1–11. [Google Scholar] [CrossRef]
- Rubin, H. Central Roles of Mg2+ and MgATP2− in the Regulation of Protein Synthesis and Cell Proliferation: Significance for Neoplastic Transformation. Adv. Cancer Res. 2005, 93, 1–58. [Google Scholar] [CrossRef]
- Ikari, A.; Sawada, H.; Sanada, A.; Tonegawa, C.; Yamazaki, Y.; Sugatani, J. Magnesium Deficiency Suppresses Cell Cycle Progression Mediated by Increase in Transcriptional Activity of P21(Cip1) and P27(Kip1) in Renal Epithelial NRK-52E Cells. J. Cell Biochem. 2011, 112, 3563–3572. [Google Scholar] [CrossRef]
- Wolf, F.I.; Trapani, V. Cell (Patho)Physiology of Magnesium. Clin. Sci. 2008, 114, 27–35. [Google Scholar] [CrossRef]
- Shamloo, B.; Usluer, S. P21 in Cancer Research. Cancers 2019, 11, 1178. [Google Scholar] [CrossRef]
- Rosner, M.; Siegel, N.; Valli, A.; Fuchs, C.; Hengstschläger, M. MTOR Phosphorylated at S2448 Binds to Raptor and Rictor. Amino Acids 2010, 38, 223–228. [Google Scholar] [CrossRef]
- Kuma, A.; Mizushima, N. Physiological Role of Autophagy as an Intracellular Recycling System: With an Emphasis on Nutrient Metabolism. Semin. Cell Dev. Biol. 2010, 21, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed]
- Fourrier, C.; Bryksin, V.; Hattersley, K.; Hein, L.K.; Bensalem, J.; Sargeant, T.J. Comparison of Chloroquine-like Molecules for Lysosomal Inhibition and Measurement of Autophagic Flux in the Brain. Biochem. Biophys. Res. Commun. 2021, 534, 107–113. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Maier, J.A.M. Magnesium and Cancer: A Dangerous Liason. Magnes. Res. 2011, 24, 92–100. [Google Scholar] [CrossRef]
- Romani, A.M.P. Magnesium in Health and Disease. Met. Ions Life Sci. 2013, 13, 49–79. [Google Scholar] [CrossRef]
- Malucelli, E.; Fratini, M.; Notargiacomo, A.; Gianoncelli, A.; Merolle, L.; Sargenti, A.; Cappadone, C.; Farruggia, G.; Lagomarsino, S.; Iotti, S. Where Is It and How Much? Mapping and Quantifying Elements in Single Cells. Analyst 2016, 141, 5221–5235. [Google Scholar] [CrossRef]
- Covacci, V.; Bruzzese, N.; Sgambato, A.; Di Francesco, A.; Russo, M.A.; Wolf, F.I.; Cittadini, A. Magnesium Restriction Induces Granulocytic Differentiation and Expression of P27Kip1 in Human Leukemic HL-60 Cells. J. Cell Biochem. 1998, 70, 313–322. [Google Scholar] [CrossRef]
- Sgambato, A.; Wolf, F.I.; Faraglia, B.; Cittadini, A. Magnesium Depletion Causes Growth Inhibition, Reduced Expression of Cyclin D1, and Increased Expression of P27KIP1 in Normal but Not in Transformed Mammary Epithelial Cells. J. Cell. Physiol. 1999, 180, 245–254. [Google Scholar] [CrossRef]
- Parrales, A.; López, E.; Lee-Rivera, I.; López-Colomé, A.M. ERK1/2-Dependent Activation of MTOR/MTORC1/P70S6K Regulates Thrombin-Induced RPE Cell Proliferation. Cell Signal. 2013, 25, 829–838. [Google Scholar] [CrossRef]
- Qu, X.; Jin, F.; Hao, Y.; Zhu, Z.; Li, H.; Tang, T.; Dai, K. Nonlinear Association between Magnesium Intake and the Risk of Colorectal Cancer. Eur. J. Gastroenterol. Hepatol. 2013, 25, 309–318. [Google Scholar] [CrossRef]
- Du, M.; Luo, H.; Blumberg, J.B.; Rogers, G.; Chen, F.; Ruan, M.; Shan, Z.; Biever, E.; Zhang, F.F. Dietary Supplement Use among Adult Cancer Survivors in the United States. J. Nutr. 2020, 150, 1499–1508. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Nowak, A.; Pietrzyk, N.; Podsędek, A. Viburnum Opulus L. Juice Phenolic Compounds Influence Osteogenic Differentiation in Human Osteosarcoma Saos-2 Cells. Int. J. Mol. Sci. 2020, 21, 4909. [Google Scholar] [CrossRef]
- Belluci, M.M.; Giro, G.; del Barrio, R.A.L.; Pereira, R.M.R.; Marcantonio, E.; Orrico, S.R.P. Effects of Magnesium Intake Deficiency on Bone Metabolism and Bone Tissue around Osseointegrated Implants: Effects of Magnesium Intake Deficiency on Bone Tissue. Clin. Oral Implants Res. 2011, 22, 716–721. [Google Scholar] [CrossRef]
- Capozzi, A.; Scambia, G.; Lello, S. Calcium, Vitamin D, Vitamin K2, and Magnesium Supplementation and Skeletal Health. Maturitas 2020, 140, 55–63. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappadone, C.; Malucelli, E.; Zini, M.; Farruggia, G.; Picone, G.; Gianoncelli, A.; Notargiacomo, A.; Fratini, M.; Pignatti, C.; Iotti, S.; et al. Assessment and Imaging of Intracellular Magnesium in SaOS-2 Osteosarcoma Cells and Its Role in Proliferation. Nutrients 2021, 13, 1376. https://doi.org/10.3390/nu13041376
Cappadone C, Malucelli E, Zini M, Farruggia G, Picone G, Gianoncelli A, Notargiacomo A, Fratini M, Pignatti C, Iotti S, et al. Assessment and Imaging of Intracellular Magnesium in SaOS-2 Osteosarcoma Cells and Its Role in Proliferation. Nutrients. 2021; 13(4):1376. https://doi.org/10.3390/nu13041376
Chicago/Turabian StyleCappadone, Concettina, Emil Malucelli, Maddalena Zini, Giovanna Farruggia, Giovanna Picone, Alessandra Gianoncelli, Andrea Notargiacomo, Michela Fratini, Carla Pignatti, Stefano Iotti, and et al. 2021. "Assessment and Imaging of Intracellular Magnesium in SaOS-2 Osteosarcoma Cells and Its Role in Proliferation" Nutrients 13, no. 4: 1376. https://doi.org/10.3390/nu13041376
APA StyleCappadone, C., Malucelli, E., Zini, M., Farruggia, G., Picone, G., Gianoncelli, A., Notargiacomo, A., Fratini, M., Pignatti, C., Iotti, S., & Stefanelli, C. (2021). Assessment and Imaging of Intracellular Magnesium in SaOS-2 Osteosarcoma Cells and Its Role in Proliferation. Nutrients, 13(4), 1376. https://doi.org/10.3390/nu13041376