Usual Protein Intake Amount and Sources of Nursing Home Residents with (Risk of) Malnutrition and Effects of an Individualized Nutritional Intervention: An enable Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Participant Characteristics
2.3. Usual Care
2.4. Individualized Intervention
2.5. Dietary Intake Assessment
2.6. Protein Intake
- Six plant-based protein sources: (1) starchy foods (e.g., rice, potatoes, noodles), (2) fruit and vegetables, (3) pulses (incl. stew with lentils or peas), (4) pastry, confectionery mainly vegetable (e.g., croissant, apple pie), (5) nuts and seeds and (6) other mainly plant-based protein sources (e.g., dumplings, beer) and
- Six animal-based protein sources: (7) meat and meat products, (8) dairy and dairy products, (9) eggs and egg products, (10) pastry, confectionery mainly animal (e.g., porridge, cream pies, pudding), (11) fish and seafood and (12) other mainly animal-based protein sources (e.g., stew with meat)
2.7. Data Analysis and Statistics
3. Results
3.1. Study Population
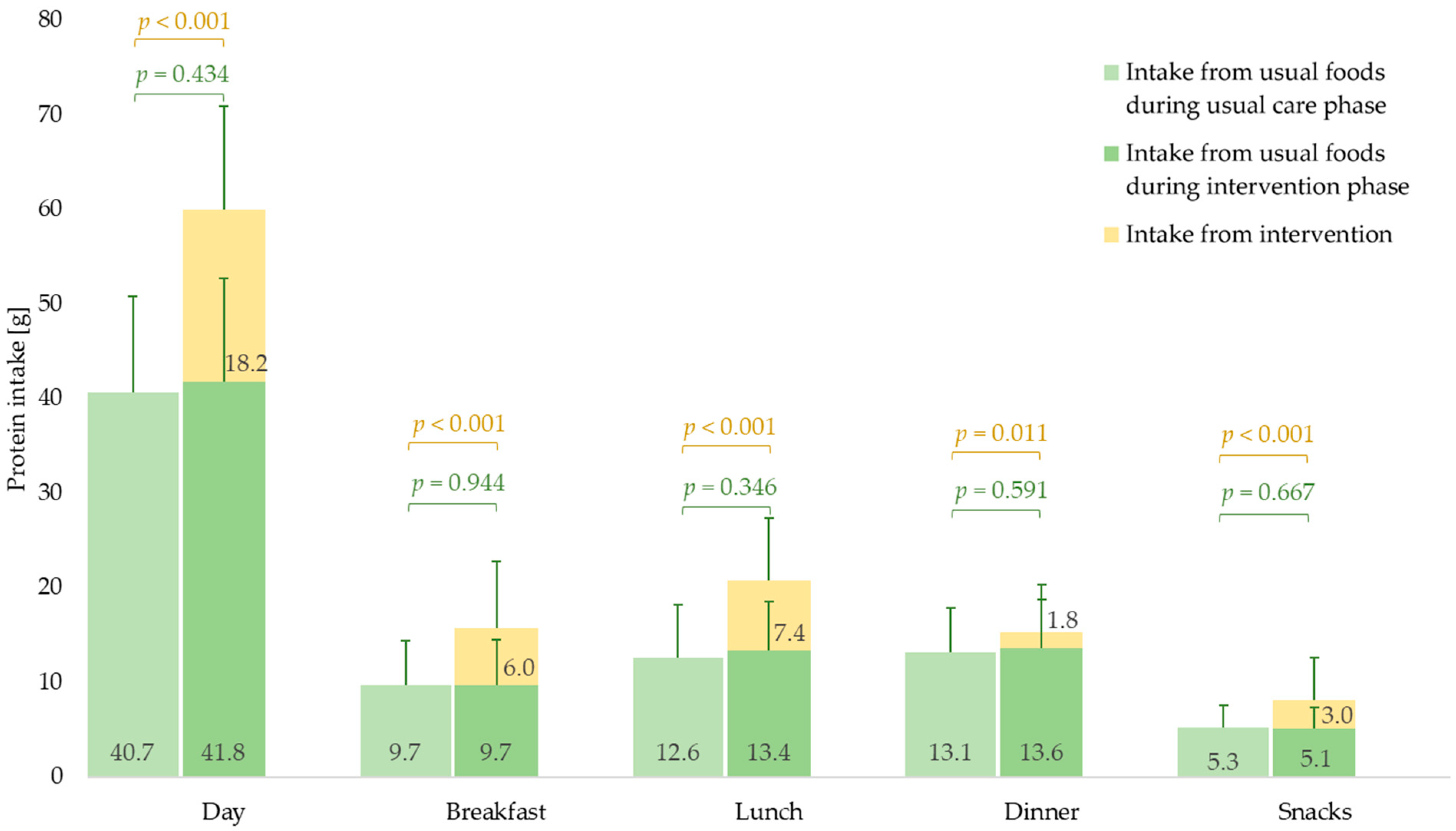
3.2. Daily and Mealtime Protein Intake Amount
3.2.1. Usual Care Phase
3.2.2. Intervention Phase
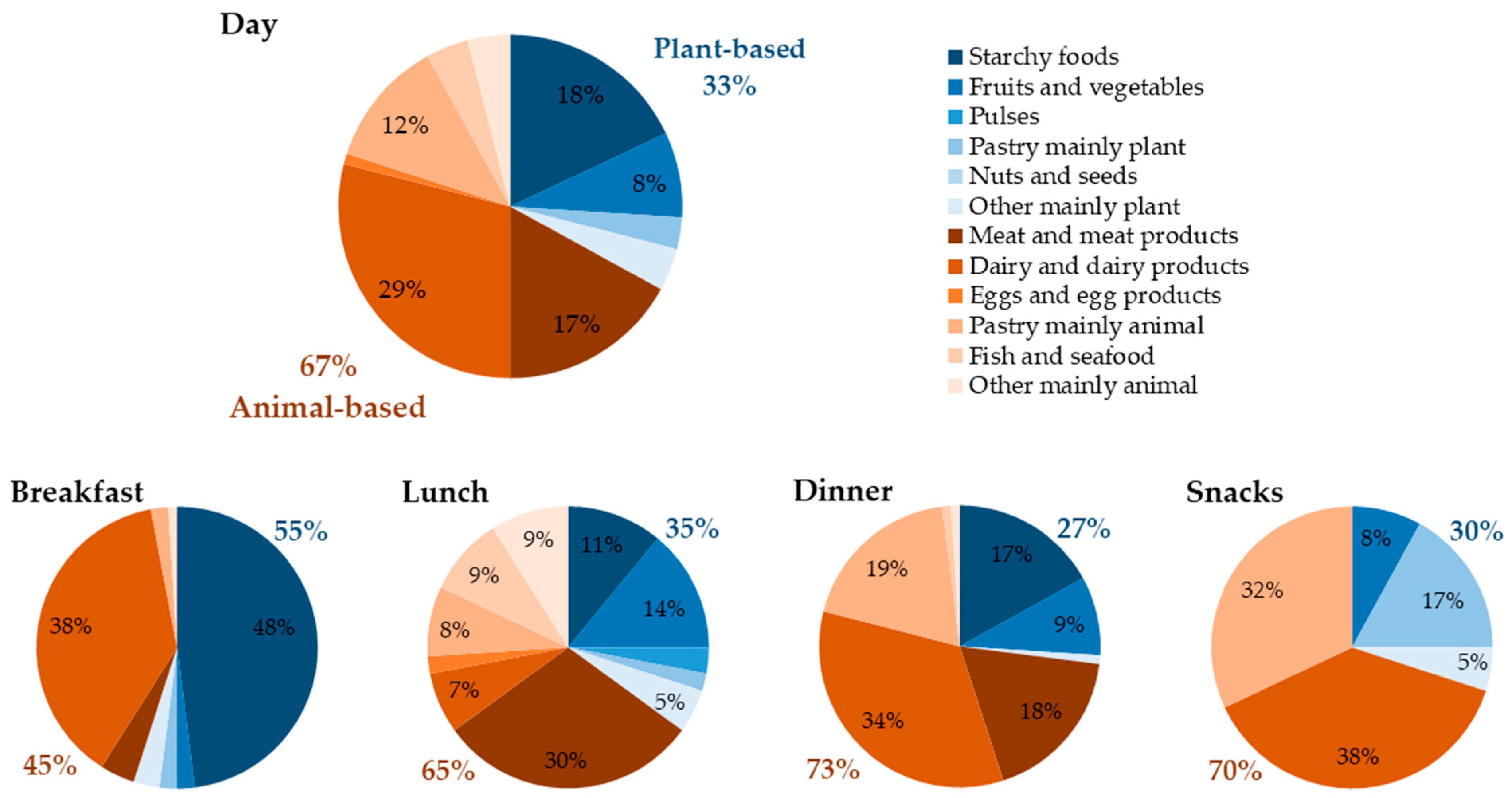
3.3. Daily and Mealtime Protein Intake by Sources
3.3.1. Usual Care Phase
3.3.2. Intervention Phase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaytan-Gonzalez, A.; Ocampo-Alfaro, M.J.; Torres-Naranjo, F.; Gonzalez-Mendoza, R.G.; Gil-Barreiro, M.; Arroniz-Rivera, M.; López-Taylor, J.R. Dietary Protein Intake Patterns and Inadequate Protein Intake in Older Adults from Four Countries. Nutrients 2020, 12, 3156. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Sisto, A.; Marzetti, E. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients 2016, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Tooze, J.A.; Garcia, K.; Visser, M.; Rubin, S.; Harris, T.B.; Newman, A.B.; Kritchevsky, S.B. Protein Intake and Mobility Limitation in Community-Dwelling Older Adults: The Health ABC Study. J. Am. Geriatr. Soc. 2017, 65, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; D’Angelo, E.; Sisto, A.; Marzetti, E. Protein Intake and Muscle Health in Old Age: From Biological Plausibility to Clinical Evidence. Nutrients 2016, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.; Bunn, D.; Copley, M.; Cowap, V.; Dickinson, A.; Gray, L.; Howe, A.; Killett, A.; Lee, J.; Li, F.; et al. Effectiveness of interventions to directly support food and drink intake in people with dementia: Systematic review and meta-analysis. BMC Geriatr. 2016, 16, 26. [Google Scholar] [CrossRef] [Green Version]
- Morilla-Herrera, J.C.; Martin-Santos, F.J.; Caro-Bautista, J.; Saucedo-Figueredo, C.; Garcia-Mayor, S.; Morales-Asencio, J.M. Effectiveness of Food-Based Fortification in Older People. A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2016, 20, 178–184. [Google Scholar] [CrossRef]
- Milne, A.C.; Potter, J.; Vivanti, A.; Avenell, A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst. Rev. 2009, CD003288. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Marzetti, E.; Picca, A.; Cesari, M.; Uchida, M.C.; Calvani, R. Protein Intake and Frailty: A Matter of Quantity, Quality, and Timing. Nutrients 2020, 12, 2915. [Google Scholar] [CrossRef]
- Hudson, J.L.; Iii, R.E.B.; Campbell, W.W. Protein Distribution and Muscle-Related Outcomes: Does the Evidence Support the Concept? Nutrients 2020, 12, 1441. [Google Scholar] [CrossRef]
- van Vliet, S.; Burd, N.A.; van Loon, L.J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [Green Version]
- Gorissen, S.H.M.; Witard, O.C. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc. Nutr. Soc. 2018, 77, 20–31. [Google Scholar] [CrossRef] [PubMed]
- DGE. Referenzwerte für die Nährstoffzufuhr, 4. aktualisierte Ausgabe ed.; Deutsche Gesellschaft für Ernährung: Bonn, Germany; Österreichische Gesellschaft für Ernährung: Vienna, Austria; Schweizerische Gesellschaft für Ernährung: Bern, Switzerland, 2018. [Google Scholar]
- EFSA. (Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for protein. EFSA J. 2021, 10, 66. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Rasmussen, B.B. Dietary protein recommendations and the prevention of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 86–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gingrich, A.; Spiegel, A.; Gradl, J.E.; Skurk, T.; Hauner, H.; Sieber, C.C.; Volkert, D.; Kiesswetter, E. Daily and per-meal animal and plant protein intake in relation to muscle mass in healthy older adults without functional limitations: An enable study. Aging Clin. Exp. Res. 2019, 31, 1271–1281. [Google Scholar] [CrossRef]
- Hengeveld, L.M.; Pelgrom, A.D.A.; Visser, M.; Boer, J.M.A.; Haveman-Nies, A.; Wijnhoven, H.A.H. Comparison of protein intake per eating occasion, food sources of protein and general characteristics between community-dwelling older adults with a low and high protein intake. Clin. Nutr. ESPEN 2019, 29, 165–174. [Google Scholar] [CrossRef]
- Tieland, M.; Borgonjen-Van den Berg, K.J.; Van Loon, L.J.; de Groot, L.C. Dietary Protein Intake in Dutch Elderly People: A Focus on Protein Sources. Nutrients 2015, 7, 9697–9706. [Google Scholar] [CrossRef]
- Tieland, M.; Beelen, J.; Laan, A.C.; Poon, S.; de Groot, L.C.; Seeman, E.; Wang, X.; Iuliano, S. An Even Distribution of Protein Intake Daily Promotes Protein Adequacy but Does Not Influence Nutritional Status in Institutionalized Elderly. J. Am. Med. Dir. Assoc. 2018, 19, 33–39. [Google Scholar] [CrossRef]
- Mila, R.; Abellana, R.; Padro, L.; Basulto, J.; Farran, A. High consumption foods and their influence on energy and protein intake in institutionalized older adults. J. Nutr. Health Aging 2012, 16, 115–122. [Google Scholar] [CrossRef] [PubMed]
- DGE. Ernährung älterer Menschen in stationären Einrichtungen. In Ernährungsbericht 2008; Deutsche Gesellschaft für Ernährung e.V.: Bonn, Germany, 2008. [Google Scholar]
- Loman, B.R.; Luo, M.; Baggs, G.E.; Mitchell, D.C.; Nelson, J.L.; Ziegler, T.R.; Deutz, N.E.; Matarese, L.E.; NOURISH Study Group. Specialized High-Protein Oral Nutrition Supplement Improves Home Nutrient Intake of Malnourished Older Adults Without Decreasing Usual Food Intake. JPEN J. Parenter. Enter. Nutr. 2019, 43, 794–802. [Google Scholar] [CrossRef] [Green Version]
- Rufenacht, U.; Ruhlin, M.; Wegmann, M.; Imoberdorf, R.; Ballmer, P.E. Nutritional counseling improves quality of life and nutrient intake in hospitalized undernourished patients. Nutrition 2010, 26, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Wouters-Wesseling, W.; Van Hooijdonk, C.; Wagenaar, L.; Bindels, J.; de Groot, L.; Van Staveren, W. The effect of a liquid nutrition supplement on body composition and physical functioning in elderly people. Clin. Nutr. 2003, 22, 371–377. [Google Scholar] [CrossRef]
- Fiatarone Singh, M.A.; Bernstein, M.A.; Ryan, A.D.; O’Neill, E.F.; Clements, K.M.; Evans, W.J. The effect of oral nutritional supplements on habitual dietary quality and quantity in frail elders. J. Nutr. Health Aging 2000, 4, 5–12. [Google Scholar] [PubMed]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Seemer, J.; Kiesswetter, E.; Blawert, A.; Fleckenstein, D.; Gloning, M.; Bader-Mittermaier, S.; Sieber, C.C.; Wurm, S.; Volkert, D. An Individualised Nutritional Intervention Concept for Nursing Home Residents with or at Risk of Malnutrition: An enable Study. Geriatrics 2021, 6, 2. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Müller, M.J.; Bosy-Westphal, A.; Klaus, S.; Kreymann, G.; Lührmann, P.M.; Neuhäuser-Berthold, M.; Noack, R.; Pirke, K.M.; Platte, P.; Selberg, O.; et al. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: Generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am. J. Clin. Nutr. 2004, 80, 1379–1390. [Google Scholar] [CrossRef]
- Van Wymelbeke, V.; Brondel, L.; Bon, F.; Martin-Pfitzenmeyer, I.; Manckoundia, P. An innovative brioche enriched in protein and energy improves the nutritional status of malnourished nursing home residents compared to oral nutritional supplement and usual breakfast: FARINE+ project. Clin. Nutr. ESPEN 2016, 15, 93–100. [Google Scholar] [CrossRef]
- Beck, A.M.; Damkjaer, K.; Beyer, N. Multifaceted nutritional intervention among nursing-home residents has a positive influence on nutrition and function. Nutrition 2008, 24, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Stange, I.; Bartram, M.; Liao, Y.; Poeschl, K.; Kolpatzik, S.; Uter, W.; Sieber, C.C.; Stehle, P.; Volkert, D. Effects of a low-volume, nutrient- and energy-dense oral nutritional supplement on nutritional and functional status: A randomized, controlled trial in nursing home residents. J. Am. Med. Dir. Assoc. 2013, 14, e621–e628. [Google Scholar] [CrossRef] [PubMed]
- Smoliner, C.; Norman, K.; Scheufele, R.; Hartig, W.; Pirlich, M.; Lochs, H. Effects of food fortification on nutritional and functional status in frail elderly nursing home residents at risk of malnutrition. Nutrition 2008, 24, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Symons, T.B.; Sheffield-Moore, M.; Wolfe, R.R.; Paddon-Jones, D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J. Am. Diet. Assoc. 2009, 109, 1582–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef] [Green Version]
- Loenneke, J.P.; Loprinzi, P.D.; Murphy, C.H.; Phillips, S.M. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin. Nutr. 2016, 35, 1506–1511. [Google Scholar] [CrossRef]
- Gaytán-González, A.; Ocampo-Alfaro, M.D.J.; Torres-Naranjo, F.; Arroniz-Rivera, M.; González-Mendoza, R.G.; Gil-Barreiro, M.; López-Taylor, J.R. The Consumption of Two or Three Meals per Day with Adequate Protein Content Is Associated with Lower Risk of Physical Disability in Mexican Adults Aged 60 Years and Older. Geriatrics 2020, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- McGrath, R.; Stastny, S.; Casperson, S.; Jahns, L.; Roemmich, J.; Hackney, K.J. Daily Protein Intake and Distribution of Daily Protein Consumed Decreases Odds for Functional Disability in Older Americans. J. Aging Health 2020, 32, 1075–1083. [Google Scholar] [CrossRef]
- Gingrich, A.; Spiegel, A.; Kob, R.; Schoene, D.; Skurk, T.; Hauner, H.; Sieber, C.C.; Volkert, D.; Kiesswetter, E. Amount, Distribution, and Quality of Protein Intake Are Not Associated with Muscle Mass, Strength, and Power in Healthy Older Adults without Functional Limitations-An enable Study. Nutrients 2017, 9, 1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.Y.; Schutzler, S.; Schrader, A.M.; Spencer, H.J.; Azhar, G.; Wolfe, R.R.; Ferrando, A.A. Protein intake distribution pattern does not affect anabolic response, lean body mass, muscle strength or function over 8 weeks in older adults: A randomized-controlled trial. Clin. Nutr. 2018, 37, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.; Lejeune, M.P.G.M.; Diepvens, K.; Nieuwenhuizen, A.; Engelen, M.P.K.J.; Deutz, N.E.P.; Azzout-Marniche, D.; Tome, D.; Westerterp, K.R. Dietary protein, metabolism, and body-weight regulation: Dose–response effects. Int. J. Obes. 2006, 30, S16–S23. [Google Scholar] [CrossRef] [Green Version]
- Olin, A.Ö.; Armyr, I.; Soop, M.; Jerström, S.; Classon, I.; Cederholm, T.; Ljunggren, G.; Ljungqvist, O. Energy-dense meals improve energy intake in elderly residents in a nursing home. Clin. Nutr. 2003, 22, 125–131. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, E.J.; Leerlooijer, J.N.; Steijns, J.M.; Tieland, M.; de Groot, L.C.; Haveman-Nies, A. Translation of a tailored nutrition and resistance exercise intervention for elderly people to a real-life setting: Adaptation process and pilot study. BMC Geriatr. 2017, 17, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verreijen, A.M.; van den Helder, J.; Streppel, M.T.; Rotteveel, I.; Heman, D.; van Dronkelaar, C.; Memelink, R.G.; Engberink, M.F.; Visser, M.; Tieland, M.; et al. SA higher protein intake at breakfast and lunch is associated with a higher total daily protein intake in older adults: A post-hoc cross-sectional analysis of four randomised controlled trials. J. Hum. Nutr. Diet. 2021, 34, 384–394. [Google Scholar] [CrossRef]
- Smeuninx, B.; Greig, C.A.; Breen, L. Amount, Source and Pattern of Dietary Protein Intake Across the Adult Lifespan: A Cross-Sectional Study. Front. Nutr. 2020, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Beelen, J.; de Roos, N.M.; de Groot, L.C. Protein Enrichment of Familiar Foods as an Innovative Strategy to Increase Protein Intake in Institutionalized Elderly. J. Nutr. Health Aging 2017, 21, 173–179. [Google Scholar] [CrossRef]
- Reinders, I.; Visser, M.; Wijnhoven, H.A.H. Two dietary advice strategies to increase protein intake among community-dwelling older adults: A feasibility study. Clin. Nutr. ESPEN 2020, 37, 157–167. [Google Scholar] [CrossRef] [Green Version]
- van Til, A.J.; Naumann, E.; Cox-Claessens, I.J.; Kremer, S.; Boelsma, E.; de van der Schueren, M.A. Effects of the daily consumption of protein enriched bread and protein enriched drinking yoghurt on the total protein intake in older adults in a rehabilitation centre: A single blind randomised controlled trial. J. Nutr. Health Aging 2015, 19, 525–530. [Google Scholar] [CrossRef]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Distribution but not amount of protein intake is associated with frailty: A cross-sectional investigation in the region of Nurnberg. Nutr. J. 2013, 12, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imamura, F.; Micha, R.; Khatibzadeh, S.; Fahimi, S.; Shi, P.; Powles, J.; Mozaffarian, D.; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Dietary quality among men and women in 187 countries in 1990 and 2010: A systematic assessment. Lancet Glob. Health 2015, 3, e132–e142. [Google Scholar] [CrossRef] [Green Version]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.H.; Oikawa, S.Y.; Phillips, S.M. Dietary Protein to Maintain Muscle Mass in Aging: A Case for Per-meal Protein Recommendations. J. Frailty Aging 2016, 5, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.K.; Skulas-Ray, A.C.; Champagne, C.M.; Kris-Etherton, P.M. Plant protein and animal proteins: Do they differentially affect cardiovascular disease risk? Adv. Nutr 2015, 6, 712–728. [Google Scholar] [CrossRef]
- Hu, F.B. Protein, body weight, and cardiovascular health. Am. J. Clin. Nutr. 2005, 82 (Suppl. 1), 242S–247S. [Google Scholar] [CrossRef]
- Pedersen, A.N.; Kondrup, J.; Borsheim, E. Health effects of protein intake in healthy adults: A systematic literature review. Food Nutr. Res. 2013, 57, 21245. [Google Scholar] [CrossRef] [Green Version]
- Foscolou, A.; Critselis, E.; Tyrovolas, S.; Chrysohoou, C.; Naumovski, N.; Sidossis, L.S.; Rallidis, L.; Matalas, A.L.; Panagiotakos, D. The association of animal and plant protein with successful ageing: A combined analysis of MEDIS and ATTICA epidemiological studies. Public Health Nutr. 2021, 24, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Stehfest, E.; Bouwman, L.; van Vuuren, D.P.; den Elzen, M.G.J.; Eickhout, B.; Kabat, P. Climate benefits of changing diet. Clim. Chang. 2009, 95, 83–102. [Google Scholar] [CrossRef]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The Role of the Anabolic Properties of Plant- versus Animal-Based Protein Sources in Supporting Muscle Mass Maintenance: A Critical Review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, M. Nutritional value of proteins from different food sources. A review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- Mentes, J.C.; Tripp-Reimer, T. Barriers and facilitators in nursing home intervention research. West. J. Nurs. Res. 2002, 24, 918–936. [Google Scholar] [CrossRef] [PubMed]
| n = 40 | n (%) | Mean ± SD/Median (IQR) | |
|---|---|---|---|
| Age (years) | 85.2 ± 7.8 | ||
| Sex, female | 30 (75.0) | ||
| Number of medications per day | 6.0 (4.0–9.5) | ||
| Barthel-Index (points) | 35.0 (7.5–60.0) | ||
| CFS (points) | 6.9 ± 0.7 | ||
| Dementia | Severe | 22 (55.0) | |
| Mild | 12 (30.0) | ||
| No | 6 (15.0) | ||
| Mobility | Bed or chair bound | 15 (37.5) | |
| Able to get out of bed/chair | 17 (42.5) | ||
| Goes out | 8 (20.0) | ||
| Body weight (kg) | 59.3 ± 10.5 | ||
| BMI (kg/m2) | 22.0 (19.7–23.9) | ||
| Energy intake (kcal/d) | 1404.3 ± 327.1 | ||
| Texture-modified meals, yes | 13 (32.5) | ||
| MNA-SF | Malnourished | 11 (27.5) | |
| Risk of malnutrition | 29 (72.5) | ||
| Usual Care Phase | Intervention Phase | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | IQR | Mean | SD | Median | IQR | p-Value 1 | ||
| Day | Starchy foods | 7.4 | 3.3 | 7.2 | 4.9–10.0 | 7.3 | 3.4 | 6.5 | 4.8–9.9 | 0.633 |
| Fruit and vegetables | 3.0 | 2.1 | 2.1 | 1.6–3.6 | 3.1 | 2.8 | 1.8 | 1.0–4.5 | 0.307 | |
| Meat and meat products | 6.9 | 4.8 | 6.4 | 3.4–8.8 | 7.1 | 4.8 | 6.0 | 4.0–10.2 | 0.414 | |
| Dairy products 2 | 12.0 | 7.5 | 9.0 | 6.4–14.2 | 11.6 | 7.3 | 10.7 | 7.0–13.3 | 0.420 | |
| Pastry mainly animal | 4.9 | 4.3 | 3.5 | 2.2–5.6 | 4.7 | 3.8 | 3.9 | 1.9–6.3 | 0.512 | |
| Protein-energy drink | 10.8 | 9.2 | 12.7 | 0.0–19.7 | ||||||
| Sweet protein cream | 5.1 | 3.4 | 5.8 | 1.9–8.9 | ||||||
| Savory protein cream | 2.2 | 2.8 | 0.4 | 0.0–4.3 | ||||||
| Breakfast | Starchy foods | 4.0 | 1.9 | 3.9 | 2.4–5.3 | 3.8 | 1.8 | 3.6 | 2.4–5.0 | 0.276 |
| Dairy products 2 | 4.2 | 4.4 | 2.3 | 1.5–5.9 | 4.5 | 4.3 | 2.6 | 1.5–6.5 | 0.330 | |
| Protein-energy drink | 6.0 | 5.8 | 5.5 | 0.0–10.0 | ||||||
| Sweet protein cream | 0.0 | 0.0 | 0.0 | 0.0–0.0 | ||||||
| Savory protein cream | 0.0 | 0.0 | 0.0 | 0.0–0.0 | ||||||
| Lunch | Starchy foods | 1.3 | 0.9 | 1.1 | 0.6–2.1 | 1.3 | 0.9 | 1.1 | 0.6–1.9 | 0.640 |
| Fruit and vegetables | 1.4 | 1.2 | 1.1 | 0.5–2.0 | 1.8 | 1.6 | 1.2 | 0.6–2.5 | 0.563 | |
| Other mainly plant | 0.7 | 0.8 | 0.4 | 0.0–1.2 | 1.2 | 1.7 | 0.5 | 0.0–2.0 | 0.149 | |
| Meat and meat products | 4.1 | 3.5 | 3.7 | 1.4–6.2 | 4.1 | 3.3 | 3.7 | 1.6–5.9 | 0.910 | |
| Dairy products 2 | 0.7 | 1.3 | 0.0 | 0.0–1.2 | 1.5 | 1.4 | 1.1 | 0.7–2.1 | <0.001 | |
| Pastry mainly animal | 0.8 | 1.1 | 0.4 | 0.0–1.3 | 0.5 | 0.5 | 0.4 | 0.1–0.8 | 0.248 | |
| Other mainly animal | 1.3 | 1.6 | 0.7 | 0.1–2.0 | 1.0 | 1.4 | 0.3 | 0.1–1.3 | 0.255 | |
| Fish and seafood | 1.3 | 2.0 | 0.0 | 0.0–2.0 | 1.4 | 1.8 | 0.6 | 0.0–2.3 | 0.328 | |
| Protein-energy drink | 1.6 | 2.6 | 0.0 | 0.0–2.5 | ||||||
| Sweet protein cream | 3.6 | 3.1 | 3.6 | 0.3–6.3 | ||||||
| Savory protein cream | 2.2 | 2.7 | 0.3 | 0.0–4.3 | ||||||
| Dinner | Starchy foods | 2.1 | 1.5 | 2.1 | 0.9–3.2 | 2.2 | 1.9 | 2.1 | 0.1–3.7 | 0.874 |
| Fruit and vegetables | 1.2 | 1.7 | 0.4 | 0.2–1.3 | 1.1 | 2.0 | 0.2 | 0.1–0.8 | 0.111 | |
| Meat and meat products | 2.4 | 2.2 | 1.8 | 0.0–4.1 | 2.6 | 2.5 | 1.8 | 0.0–4.5 | 0.357 | |
| Dairy products 2 | 4.8 | 3.5 | 4.2 | 2.4–6.4 | 3.8 | 3.9 | 3.1 | 1.6–4.8 | 0.009 | |
| Pastry mainly animal | 2.2 | 3.4 | 0.4 | 0.3–3.7 | 2.6 | 3.3 | 0.9 | 0.1–4.4 | 0.519 | |
| Protein-energy drink | 1.4 | 1.8 | 0.0 | 0.0–2.3 | ||||||
| Sweet protein cream | 0.3 | 0.7 | 0.0 | 0.0–0.2 | ||||||
| Savory protein cream | 0.1 | 0.2 | 0.0 | 0.0–0.0 | ||||||
| Snacks | Fruit and vegetables | 0.3 | 0.3 | 0.2 | 0.0–0.4 | 0.1 | 0.2 | 0.0 | 0.0–0.1 | 0.001 |
| Pastry mainly plant | 0.8 | 0.8 | 0.7 | 0.3–1.1 | 1.4 | 1.1 | 1.2 | 0.6–2.0 | 0.002 | |
| Other mainly plant | 0.3 | 0.5 | 0.2 | 0.1–0.3 | 0.3 | 0.5 | 0.2 | 0.1–0.3 | 0.700 | |
| Dairy products 2 | 2.2 | 2.1 | 1.7 | 0.9–2.8 | 1.8 | 1.6 | 1.4 | 0.8–2.3 | 0.255 | |
| Pastry mainly animal | 1.7 | 1.2 | 1.7 | 0.6–2.3 | 1.5 | 1.3 | 1.2 | 0.7–1.8 | 0.499 | |
| Protein-energy drink | 1.8 | 2.7 | 0.0 | 0.0–3.0 | ||||||
| Sweet protein cream | 1.2 | 1.5 | 0.6 | 0.0–1.9 | ||||||
| Savory protein cream | 0.0 | 0.0 | 0.0 | 0.0–0.0 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seemer, J.; Volkert, D.; Fleckenstein-Sußmann, D.; Bader-Mittermaier, S.; Sieber, C.C.; Kiesswetter, E. Usual Protein Intake Amount and Sources of Nursing Home Residents with (Risk of) Malnutrition and Effects of an Individualized Nutritional Intervention: An enable Study. Nutrients 2021, 13, 2168. https://doi.org/10.3390/nu13072168
Seemer J, Volkert D, Fleckenstein-Sußmann D, Bader-Mittermaier S, Sieber CC, Kiesswetter E. Usual Protein Intake Amount and Sources of Nursing Home Residents with (Risk of) Malnutrition and Effects of an Individualized Nutritional Intervention: An enable Study. Nutrients. 2021; 13(7):2168. https://doi.org/10.3390/nu13072168
Chicago/Turabian StyleSeemer, Johanna, Dorothee Volkert, Daniela Fleckenstein-Sußmann, Stephanie Bader-Mittermaier, Cornel Christian Sieber, and Eva Kiesswetter. 2021. "Usual Protein Intake Amount and Sources of Nursing Home Residents with (Risk of) Malnutrition and Effects of an Individualized Nutritional Intervention: An enable Study" Nutrients 13, no. 7: 2168. https://doi.org/10.3390/nu13072168
APA StyleSeemer, J., Volkert, D., Fleckenstein-Sußmann, D., Bader-Mittermaier, S., Sieber, C. C., & Kiesswetter, E. (2021). Usual Protein Intake Amount and Sources of Nursing Home Residents with (Risk of) Malnutrition and Effects of an Individualized Nutritional Intervention: An enable Study. Nutrients, 13(7), 2168. https://doi.org/10.3390/nu13072168







