Effects of Fermented Milk Containing Lacticaseibacillus paracasei Strain Shirota on Constipation in Patients with Depression: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Ethical Considerations and Inclusion/Exclusion Criteria
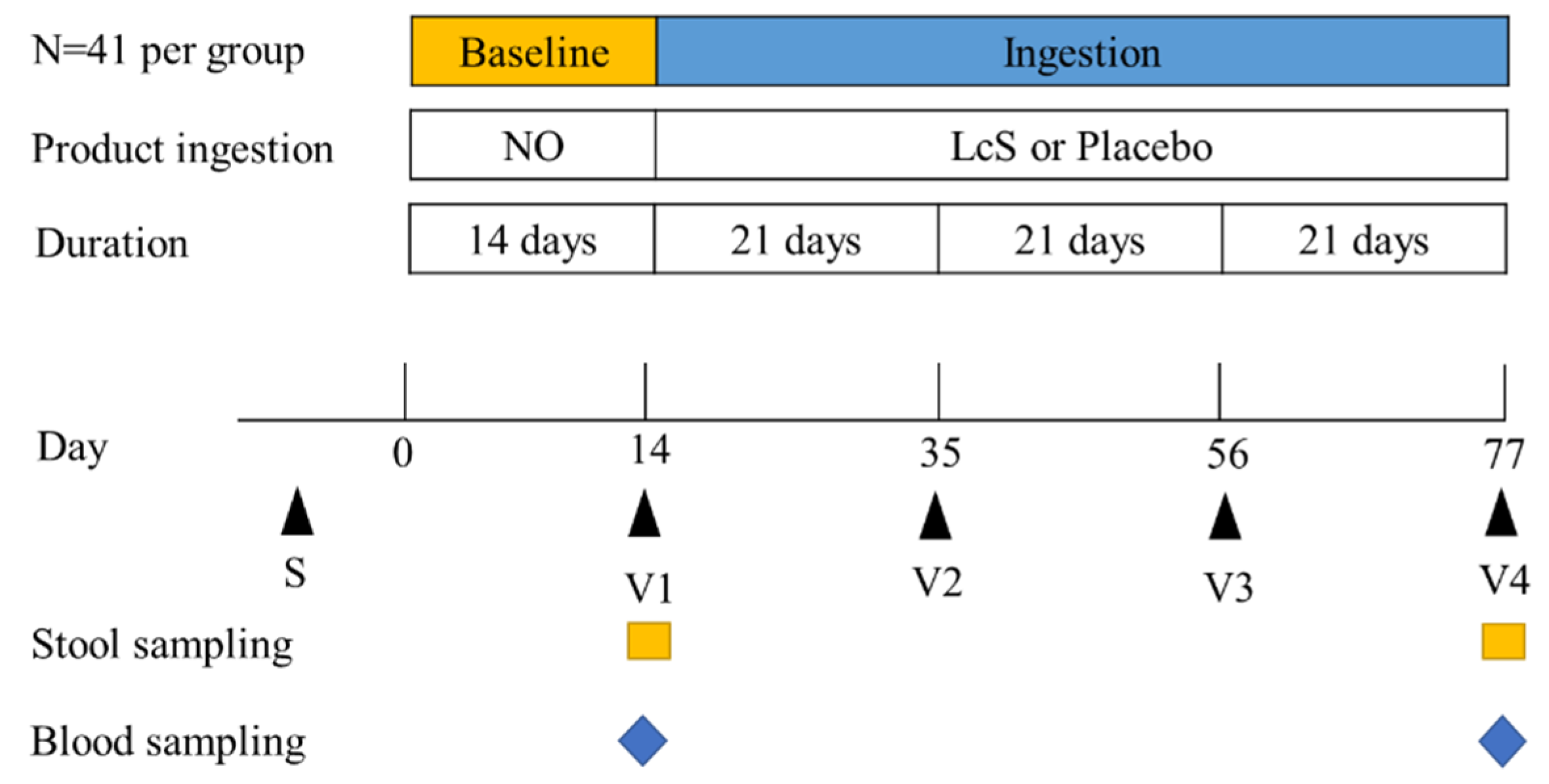
2.3. Data Collection and Study Timeline
2.4. Changes in Intestinal Microbiota Composition
2.5. Serum Parameters
2.6. Statistical Methods
3. Results
3.1. Subject Information
3.2. Constipation-Related Symptom Scores
3.3. Depression-Related Symptom Scores
3.4. Changes in Intestinal Microbiota Composition
3.5. Serum Pro-Inflammatory Cytokine Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization. Depression and Other Common Mental Disorders Global Health Estimates. 2017. Available online: http://apps.who.int/iris/bitstream/10665/254610/1/WHO-MSD-MER-2017.2-eng.pdf/ (accessed on 21 March 2017).
- Lopresti, A.L.; Hood, S.D.; Drummond, P.D. A review of lifestyle factors that contribute to important pathways associated with major depression: Diet, sleep and exercise. J. Affect. Disord. 2013, 148, 12–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, E.A.; Katon, W.J.; Jemelka, R.P.; Roy-Byrne, P.P. Comorbidity of gastrointestinal complaints, depression, and anxiety in the epidemiologic catchment area (ECA) study. Am. J. Med. 1992, 92, S26–S30. [Google Scholar] [CrossRef]
- Nellesen, D.; Chawla, A.; Oh, D.L.; Weissman, T.; Lavins, B.J.; Murray, C.W. Comorbidities in patients with irritable bowel syndrome with constipation or chronic idiopathic constipation: A review of the literature from the past decade. Postgrad. Med. 2013, 125, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Cao, J.; Xiong, N.; Zhao, X.; Jiang, J.; Zhu, L.; Wang, Z.; Sun, X.; Fang, X.; Wei, J. A Comorbidity study of functional gastrointestinal disorders in patients with major depressive disorder. J. Depress. Anxiety 2015, 4, 1. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Kunugi, H. Depressive Disorder and Gut-brain Interaction. Brain Nerve 2016, 68, 641–646. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The central nervous system and the gut microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [Green Version]
- De Palma, G.; Blennerhassett, P.; Lu, J.; Deng, Y.; Park, A.J.; Green, W.; Denou, E.; Silva, M.A.; Santacruz, A.; Sanz, Y.; et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 2015, 6, 7735. [Google Scholar] [CrossRef] [Green Version]
- Wallace, C.J.K.; Milev, R. The effects of probiotics on depressive symptoms in humans: A systematic review. Ann. Gen. Psychiatry 2017, 16, 14. [Google Scholar] [CrossRef] [Green Version]
- Koebnick, C.; Wagner, I.; Leitzmann, P.; Stern, U.; Zunft, H. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can. J. Gastroenterol. Hepatol. 2003, 17, 655–659. [Google Scholar] [CrossRef] [Green Version]
- Krammer, H.J.; von Seggern, H.; Schaumburg, J.; Neumer, F. Effect of Lactobacillus casei Shirota on colonic transit time in patients with chronic constipation. Coloproctology 2011, 33, 109–113. [Google Scholar] [CrossRef]
- Sakai, T.; Makino, H.; Ishikawa, E.; Oishi, K.; Kushiro, A. Fermented milk containing Lactobacillus casei strain Shirota reduces incidence of hard or lumpy stools in healthy population. Int. J. Food Sci. Nutr. 2011, 62, 423–430. [Google Scholar] [CrossRef]
- Tilley, L.; Keppens, K.; Kushiro, A.; Takada, T.; Sakai, T.; Vaneechoutte, M.; Degeest, B. A probiotic fermented milk drink containing Lactobacillus Casei strain Shirota improves stool consistency of subjects with hard stools. Int. J. Probiotics Prebiotic 2014, 9, 23. [Google Scholar]
- Cassani, E.; Privitera, G.; Pezzoli, G.; Pusani, C.; Madio, C.; Iorio, L.; Barichella, M. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Dietol. Gastroenterol. 2011, 57, 117–121. [Google Scholar]
- Sakai, T.; Kubota, H.; Gawad, A.; Gheyle, L.; Ramael, S.; Oishi, K. Effect of fermented milk containing Lactobacillus casei strain Shirota on constipation-related symptoms and haemorrhoids in women during puerperium. Benef. Microbes 2015, 6, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, T.; Asahara, T.; Matsumoto, K.; Takada, T.; Chonan, O.; Nakamori, K.; Nonaka, C.; Yamaji, I.; Hisamoto, T.; Sato, M.; et al. Effects of the continuous intake of a milk drink containing Lactobacillus casei strain Shirota on abdominal symptoms, fecal microbiota, and metabolites in gastrectomized subjects. Scand. J. Gastroenterol. 2014, 49, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ou, Y.; Zhao, L.; Li, Y.; Qiao, Z.; Hao, Y.; Ren, F. Differential effects of Lactobacillus casei Strain Shirota on patients with constipation regarding stool consistency in China. J. Neurogastroenterol. Motil. 2019, 25, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Suda, K.; Kawai, M.; Shimizu, K.; Kushiro, A.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef. Microbes 2016, 7, 153–156. [Google Scholar] [CrossRef]
- Takada, M.; Nishida, K.; Gondo, Y.; Kikuchi-Hayakawa, H.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Kuwano, Y.; Miyazaki, K.; et al. Beneficial effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults: A double-blind, randomised, placebo-controlled trial. Benef. Microbes 2017, 8, 153–162. [Google Scholar] [CrossRef]
- Takada, M.; Nishida, K.; Kataoka-Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2016, 28, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Kawai, M.; Kikuchi-Hayakawa, H.; Suda, K.; Ishikawa, H.; Gondo, Y.; Shimizu, K.; Matsuki, T.; et al. Fermented milk containing Lactobacillus casei Strain Shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl. Environ. Microbiol. 2016, 82, 3649–3658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Whitehead, W.E.; Palsson, O.S.; Törnblom, H.; Simrén, M. An approach to the diagnosis and management of Rome IV functional disorders of chronic constipation. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, A.T.; Rial, W.Y.; Rickels, K. Short form of depression inventory: Cross-validation. Psychol. Rep. 1974, 34, 1184–1186. [Google Scholar] [CrossRef]
- Neri, L.; Conway, P.M.; Basilisco, G. Laxative Inadequate Relief Survey (LIRS) Group. Confirmatory factor analysis of the Patient Assessment of Constipation-Symptoms (PAC-SYM) among patients with chronic constipation. Qual. Life Res. 2015, 24, 1597–1605. [Google Scholar] [CrossRef]
- Liao, X.; Song, L.; Zeng, B.; Liu, B.; Qiu, Y.; Qu, H.; Zheng, Y.; Long, M.; Zhou, H.; Wang, Y.; et al. Alteration of gut microbiota induced by DPP-4i treatment improves glucose homeostasis. EBioMedicine 2019, 44, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A.; Rex Gaskins, H.; Stumpf, R.M.; Yildirim, S.; Torralba, M. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. Isme J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, I. Placebo effect in the treatment of depression and anxiety. Front. Psychiatry 2019, 10, 407. [Google Scholar] [CrossRef] [Green Version]
- Slykerman, R.F.; Hood, F.; Wickens, K.; Thompson, J.M.D.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: A randomised double-blind placebo-controlled trial. EBioMedicine 2017, 24, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baars, T.; Berge, A.C.; Garssen, J.; Verster, J.C. Effect of raw milk consumption on perceived health, mood and immune functioning among US adults with a poor and normal health: A retrospective questionnaire based study. Complement. Ther. Med. 2019, 47, 102196. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef] [PubMed]
- Tziatzios, G.; Gkolfakis, P.; Papanikolaou, I.S. Gut microbiota dysbiosis in functional dyspepsia. Microorganisms 2020, 8, 691. [Google Scholar] [CrossRef]
- Tamura, M.; Tsushida, T.; Shinohara, K. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe 2007, 13, 32–35. [Google Scholar] [CrossRef]
- Maruo, T.; Sakamoto, M.; Ito, C.; Toda, T.; Benno, Y. Adlercreutzia equolifaciens gen. nov., sp nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int. J. Syst. Evol. Microbiol. 2008, 58, 1221–1227. [Google Scholar] [CrossRef]
- Liu, S.Q. 17—Impact of yeast and bacteria on beer appearance and flavour. In Brewing Microbiology; Hill, A.E., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 357–374. [Google Scholar]
- Hu, X.; Huang, Z. Effects of a probiotic drink containing Lactobacillus casei strain Shirota on dental plaque microbiota. J. Int. Med Res. 2019, 47, 3190–3202. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P.; Zeng, B.; Liu, M.; Chen, J.; Pan, J.; Han, Y.; Liu, Y.; Cheng, K.; Zhou, C.; Wang, H.; et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 2019, 5, eaau8317. [Google Scholar] [CrossRef] [Green Version]
- Hiippala, K.; Kainulainen, V.; Kalliomäki, M.; Arkkila, P.; Satokari, R. Mucosal prevalence and interactions with the epithelium indicate commensalism of Sutterella spp. Front. Microbiol. 2016, 7, 1706. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.L.; Hornig, M.; Parekh, T.; Lipkin, W.I. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. mBio 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2014, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic review of gut microbiota and major depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Malus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Bluthé, R.-M.; Michaud, B.; Poli, V.; Dantzer, R. Role of IL-6 in cytokine-induced sickness behavior: A study with IL-6 deficient mice. Physiol. Behav. 2000, 70, 367–373. [Google Scholar] [CrossRef]







| Groups | Placebo | LcS | |
|---|---|---|---|
| Age (years) | 49.7 ± 9.6 | 45.8 ± 12.3 | |
| Weight (kg) | 66.0 ± 11.8 | 66.0 ± 10.7 | |
| BMI a (kg/m2) | 25.0 ± 4.6 | 24.2 ± 3.4 | |
| HAMD b (score) | 17.1 ± 4.4 | 16.4 ± 4.8 | |
| Gender (n) | Female | 20 | 24 |
| Male | 11 | 14 | |
| Item | Placebo (V1) | LcS (V1) | Placebo (V4) | LcS (V4) | p-Value a | |
|---|---|---|---|---|---|---|
| Abdominal symptoms | 1. Discomfort in abdomen | 1.03 ± 0.98 | 1.05 ± 0.84 | 0.65 ± 0.71 # | 0.45 ± 0.65 * | 0.171 |
| 2. Pain in abdomen | 0.81 ± 0.83 | 0.92 ± 0.85 | 0.48 ± 0.68 | 0.34 ± 0.53 * | ||
| 3. Bloating in abdomen | 0.90 ± 0.87 | 1.18 ± 0.95 | 0.71 ± 0.78 | 0.50 ± 0.65 * | ||
| 4. Stomach cramps | 0.74 ± 0.86 | 0.66 ± 0.97 | 0.58 ± 0.92 | 0.34 ± 0.53 * | ||
| Rectal symptoms | 5. Painful bowel movement | 0.90 ± 1.08 | 1.18 ± 1.27 | 0.61 ± 0.72 | 0.47 ± 0.73 * | 0.145 |
| 6. Rectal burning during or after a bowel movement | 1.23 ± 1.18 | 1.21 ± 1.19 | 0.58 ± 0.67 # | 0.42 ± 0.68 * | ||
| 7. Rectal tearing or bleeding after a bowel movement | 1.32 ± 1.30 | 1.11 ± 1.16 | 0.58 ± 0.76 # | 0.29 ± 0.65 *& | ||
| Stool symptoms | 8. Incomplete bowel movement, like you didn’t “finish” | 1.32 ± 1.17 | 1.26 ± 0.92 | 1.00 ± 1.06 | 0.55 ± 0.65 * | 0.033 |
| 9. Bowel movement that were too hard | 1.77 ± 1.56 | 1.53 ± 1.31 | 0.90 ± 0.94 # | 0.71 ± 0.80 * | ||
| 10. Bowel movement that were too small | 1.55 ± 1.43 | 1.45 ± 1.41 | 0.84 ± 0.82 # | 0.50 ± 0.65 * | ||
| 11. Straining or squeezing to try to pass bowel movements | 1.94 ± 1.39 | 1.58 ± 1.24 | 1.16 ± 0.86 # | 0.76 ± 0.63 *& | ||
| 12. Feeling like you had to pass a bowel movement you couldn’t | 1.48 ± 1.34 | 1.26 ± 1.18 | 0.87 ± 0.92 # | 0.53 ± 0.69 * | ||
| Total scores | 15.00 ± 10.39 | 14.39 ± 9.67 | 8.97 ± 7.87 # | 5.87 ± 6.05 * | 0.055 | |
| Item | Placebo (V1) | LcS (V1) | Placebo (V4) | LcS (V4) | |
|---|---|---|---|---|---|
| BDI | Mean ± SD | 14.00 ± 5.53 | 13.16 ± 7.24 | 7.39 ± 7.57 # | 4.82 ± 6.42 * |
| Not depressed individual (%) | 3 (9.7) | 7 (18.4) | 17 (54.8) | 24 (63.2) | |
| Depressed individual (%) | 28 (90.3) | 29 (81.6) | 14 (45.2) | 14 (36.8) | |
| Mild individual (%) | 2 (6.5) | 2 (5.3) | 0 (0) | 4 (10.5) | |
| Moderate individual (%) | 15 (48.4) | 16 (42.1) | 8 (25.8) | 7 (18.4) | |
| Severe individual (%) | 11 (35.4) | 13 (34.2) | 6 (19.4) | 3 (7.9) | |
| HAMD | Mean ± SD | 17.13 ± 4.43 | 16.39 ± 4.84 | 5.84 ± 6.14 # | 4.13 ± 2.86 * |
| Not depressed individual (%) | 0 (0.0) | 0 (0.0) | 26 (83.9) | 34 (89.5) | |
| Depressed individual (%) | 31 (100.0) | 38 (100.0) | 5 (16.1) | 4 (10.5) | |
| Mild individual (%) | 19 (61.3) | 25 (65.8) | 4 (12.9) | 4 (10.5) | |
| Moderate individual (%) | 10 (32.3) | 11 (28.9) | 0 (0.0) | 0 (0.0) | |
| Severe individual (%) | 2 (6.4) | 2 (5.3) | 1 (3.2) | 0 (0.0) | |
| Item | Placebo (V1) | LcS (V1) | Placebo (V4) | LcS (V4) |
|---|---|---|---|---|
| IL-1β (pg/mL) | 24.73 ± 0.59 | 24.88 ± 1.26 | 15.33 ± 5.31 # | 15.67 ± 6.97 * |
| IL-6 (pg/mL) | 7.56 ± 2.28 | 7.68 ± 1.97 | 8.39 ± 3.58 | 6.82 ± 1.21 *& |
| TNF-α (pg/mL) | 19.26 ± 2.09 | 21.16 ± 3.70 | 16.22 ± 1.67 # | 17.19 ± 3.06 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Chen, S.; Zhang, M.; Ren, F.; Ren, Y.; Li, Y.; Liu, N.; Zhang, Y.; Zhang, Q.; Wang, R. Effects of Fermented Milk Containing Lacticaseibacillus paracasei Strain Shirota on Constipation in Patients with Depression: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2238. https://doi.org/10.3390/nu13072238
Zhang X, Chen S, Zhang M, Ren F, Ren Y, Li Y, Liu N, Zhang Y, Zhang Q, Wang R. Effects of Fermented Milk Containing Lacticaseibacillus paracasei Strain Shirota on Constipation in Patients with Depression: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2021; 13(7):2238. https://doi.org/10.3390/nu13072238
Chicago/Turabian StyleZhang, Xiaomei, Shanbin Chen, Ming Zhang, Fazheng Ren, Yimei Ren, Yixuan Li, Ning Liu, Yan Zhang, Qi Zhang, and Ran Wang. 2021. "Effects of Fermented Milk Containing Lacticaseibacillus paracasei Strain Shirota on Constipation in Patients with Depression: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 13, no. 7: 2238. https://doi.org/10.3390/nu13072238






