Evaluation of Dietary Assessment Tools Used in Bariatric Population
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
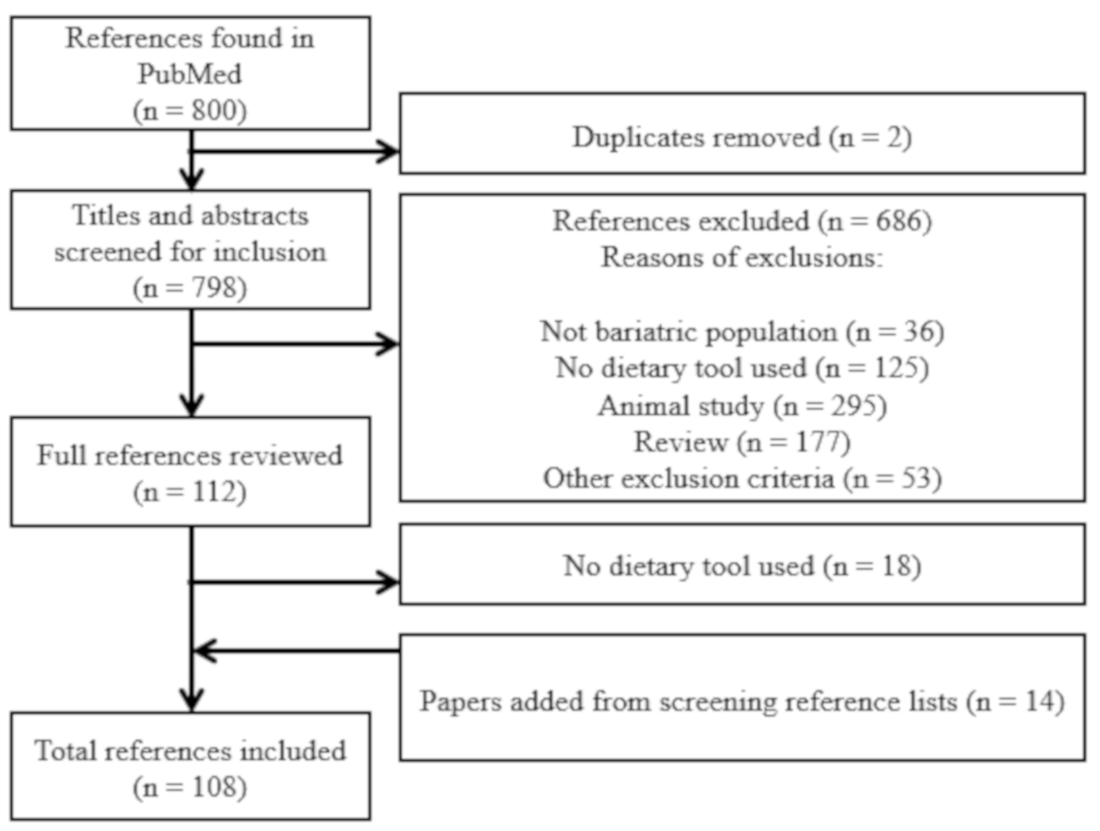
2.2. Selection of Studies
2.3. Data Extraction
3. Results
3.1. General Overview
3.2. Validation of Dietary Assessment Tools in Bariatric Population
3.2.1. Food Records (FR)
3.2.2. 24-h Dietary Recall (24HR)
3.2.3. FFQ
3.2.4. Questionnaires
3.2.5. Other Dietary Assessment Methods
3.2.6. Mixed Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wharton, S.; Lau, D.C.; Vallis, M.; Sharma, A.M.; Biertho, L.; Campbell-Scherer, D.; Adamo, K.; Alberga, A.; Bell, R.; Boulé, N.; et al. Obesity in adults: A clinical practice guideline. Can. Med. Assoc. J. 2020, 192, E875–E891. [Google Scholar] [CrossRef]
- Twells, L.K.; Gregory, D.M.; Reddigan, J.; Midodzi, W.K. Current and predicted prevalence of obesity in Canada: A trend analysis. CMAJ Open 2014, 2, E18–E26. [Google Scholar] [CrossRef] [Green Version]
- Elder, K.A.; Wolfe, B.M. Bariatric Surgery: A Review of Procedures and Outcomes. Gastroenterology 2007, 132, 2253–2271. [Google Scholar] [CrossRef] [PubMed]
- Stanford Medicine. Stanford Health Care. Types of Gastric Bypass. 2020. Available online: https://stanfordhealthcare.org/medical-treatments/g/gastric-bypass-surgery/types.html (accessed on 14 September 2020).
- Poirier, P.; Cornier, M.-A.; Mazzone, T.; Stiles, S.; Cummings, S.; Klein, S.; McCullough, P.A.; Fielding, C.R.; Franklin, B.A. Bariatric Surgery and Cardiovascular Risk Factors. Circulation 2011, 123, 1683–1701. [Google Scholar] [CrossRef]
- De Lima, K.V.G.; Costa, M.J.D.C.; Gonçalves, M.D.C.R.; De Sousa, B.S. Deficiências de micronutrientes no pré-operatório de cirurgia bariátrica. Arq. Bras. Cir. Dig. 2013, 26, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Guyot, E.; Dougkas, A.; Robert, M.; Nazare, J.-A.; Iceta, S.; Disse, E. Food Preferences and Their Perceived Changes Before and After Bariatric Surgery: A Cross-sectional Study. Obes. Surg. 2021, 31, 3075–3082. [Google Scholar] [CrossRef]
- Lafrenière, J.; Laramée, C.; Robitaille, J.; Lamarche, B.; Lemieux, S. Relative validity of a web-based, self-administered, 24-h dietary recall to evaluate adherence to Canadian dietary guidelines. Nutrition 2019, 57, 252–256. [Google Scholar] [CrossRef]
- Kirkpatrick, S.I.; Subar, A.F.; Douglass, D.; Zimmerman, T.P.; Thompson, F.E.; Kahle, L.L.; George, S.M.; Dodd, K.W.; Potischman, N. Performance of the Automated Self-Administered 24-hour Recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. Am. J. Clin. Nutr. 2014, 100, 233–240. [Google Scholar] [CrossRef]
- Brassard, D.; Lemieux, S.; Charest, A.; Lapointe, A.; Couture, P.; Labonté, M.È.; Lamarche, B. Comparing Interviewer-Administered and Web-Based Food Frequency Questionnaires to Predict Energy Requirements in Adults. Nutrients 2018, 10, 1292. [Google Scholar] [CrossRef] [Green Version]
- Kristal, A.R.; Kolar, A.S.; Fisher, J.L.; Plascak, J.J.; Stumbo, P.J.; Weiss, R.; Paskett, E.D. Evaluation of Web-Based, Self-Administered, Graphical Food Frequency Questionnaire. J. Acad. Nutr. Diet. 2014, 114, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Thompson, F.E.; Kirkpatrick, S.I.; Subar, A.F.; Reedy, J.; Schap, T.E.; Wilson, M.M.; Krebs-Smith, S.M. The National Cancer Institute’s Dietary Assessment Primer: A Resource for Diet Research. J. Acad. Nutr. Diet. 2015, 115, 1986–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institutes of Health NCI. Dietary Assessment Primer. Available online: https://dietassessmentprimer.cancer.gov/ (accessed on 28 January 2021).
- Lupoli, R.; Lembo, E.; Saldalamacchia, G.; Avola, C.K.; Angrisani, L.; Capaldo, B. Bariatric surgery and long-term nutritional issues. World J. Diabetes 2017, 8, 464–474. [Google Scholar] [CrossRef]
- Bal, B.S.; Finelli, F.C.; Shope, T.R.; Koch, T.R. Nutritional deficiencies after bariatric surgery. Nat. Rev. Endocrinol. 2012, 8, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Al Assal, K.; Prifti, E.; Belda, E.; Sala, P.; Clément, K.; Dao, M.-C.; Doré, J.; Levenez, F.; Taddei, C.R.; Fonseca, D.C.; et al. Gut Microbiota Profile of Obese Diabetic Women Submitted to Roux-en-Y Gastric Bypass and Its Association with Food Intake and Postoperative Diabetes Remission. Nutrients 2020, 12, 278. [Google Scholar] [CrossRef] [Green Version]
- Al-Ozairi, E.; Alawadhi, M.M.; Al Kandari, J.; Taghadom, E.; Abdullah, M.; Le Roux, C.W. Photo-Assisted Dietary Method Improves Estimates of Dietary Intake Among People with Sleeve Gastrectomy. Obes. Surg. 2019, 29, 1602–1606. [Google Scholar] [CrossRef]
- Amundsen, T.; Strømmen, M.; Martins, C. Suboptimal Weight Loss and Weight Regain after Gastric Bypass Surgery—Postoperative Status of Energy Intake, Eating Behavior, Physical Activity, and Psychometrics. Obes. Surg. 2017, 27, 1316–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, T.; Larsen, U. Dietary outcome in obese patients treated with a gastroplasty program. Am. J. Clin. Nutr. 1989, 50, 1328–1340. [Google Scholar] [CrossRef]
- Anderson, W.A.; Greene, G.W.; Forse, R.A.; Apovian, C.M.; Istfan, N.W. Weight Loss and Health Outcomes in African Americans and Whites After Gastric Bypass Surgery. Obesity 2007, 15, 1455–1463. [Google Scholar] [CrossRef]
- Andreu, A.; Moizé, V.; Rodríguez, L.; Flores, L.; Vidal, J. Protein Intake, Body Composition, and Protein Status Following Bariatric Surgery. Obes. Surg. 2010, 20, 1509–1515. [Google Scholar] [CrossRef]
- Anthone, G.J.; Lord, R.V.N.; DeMeester, T.R.; Crookes, P.F. The Duodenal Switch Operation for the Treatment of Morbid Obesity. Ann. Surg. 2003, 238, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Verger, E.; Bounaix, C.; Dao, M.C.; Oppert, J.-M.; Bouillot, J.-L.; Chevallier, J.-M.; Clément, K. Nutritional and Protein Deficiencies in the Short Term following Both Gastric Bypass and Gastric Banding. PLoS ONE 2016, 11, e0149588. [Google Scholar] [CrossRef] [Green Version]
- Solé, C.B.; Rugerio, M.F.Z.; Corvinos, J.F.; Díez-Noguera, A.; Cambras, T.; Izquierdo-Pulido, M. Sleeve gastrectomy in patients with severe obesity restores circadian rhythms and their relationship with sleep pattern. Chrono Int. 2021, 38, 565–575. [Google Scholar] [CrossRef]
- Bavaresco, M.; Paganini, S.; Lima, T.P., Jr.; Salgado, W.; Ceneviva, R.; Dos Santos, J.E.; Nonino-Borges, C.B. Nutritional Course of Patients Submitted to Bariatric Surgery. Obes. Surg. 2008, 20, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Benaiges, D.; Parri, A.; Subirana, I.; Pedro-Botet, J.; Villatoro, M.; Ramon, J.M.; Climent, E.; Le Roux, J.A.F.; Goday, A. Most of qualitative dietary changes observed one year post-bariatric surgery can be achieved with a preoperative dietary intervention. Endocrinol. Diabetes Nutr. 2020, 67, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Bobbioni-Harsch, E.; Huber, O.; Morel, P.; Chassot, G.; Lehmann, T.; Volery, M.; Chliamovitch, E.; Muggler, C.; Golay, A. Factors influencing energy intake and body weight loss after gastric bypass. Eur. J. Clin. Nutr. 2002, 56, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Brolin, R.E.; Robertson, L.B.; Kenler, H.A.; Cody, R.P. Weight Loss and Dietary Intake After Vertical Banded Gastroplasty and Roux-en-Y Gastric Bypass. Ann. Surg. 1994, 220, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Buzga, M.; Zavadilová, V.; Holéczy, P.; Švagera, Z.; Svorc, P.; Foltys, A.; Zonča, P. Dietary intake and ghrelin and leptin changes after sleeve gastrectomy. Videosurg. Other Miniinvasive Tech. 2014, 9, 554–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco, F.; Papapietro, K.; Csendes, A.; Salazar, G.; Echenique, C.; Lisboa, C.; Díaz, E.; Rojas, J. Changes in Resting Energy Expenditure and Body Composition after Weight Loss following Roux-en-Y Gastric Bypass. Obes. Surg. 2007, 17, 608–616. [Google Scholar] [CrossRef]
- Carrasco, F.; Rojas, P.; Csendes, A.; Codoceo, J.; Inostroza, J.; Basfi-Fer, K.; Papapietro, K.; Watkins, G.; Rojas, J.; Ruz, M. Changes in ghrelin concentrations one year after resective and non-resective gastric bypass: Associations with weight loss and energy and macronutrient intakes. Nutrition 2012, 28, 757–761. [Google Scholar] [CrossRef]
- Carvalho, A.C.; Mota, M.C.; Marot, L.; Mattar, L.A.; de Sousa, J.A.G.; Araújo, A.C.T.; Assis, C.T.D.C.; Crispim, C.A. Circadian Misalignment Is Negatively Associated with the Anthropometric, Metabolic and Food Intake Outcomes of Bariatric Patients 6 Months After Surgery. Obes. Surg. 2021, 31, 159–169. [Google Scholar] [CrossRef]
- Casagrande, D.S.; Repetto, G.; Mottin, C.C.; Schneider, R.; Rizzolli, J.; Moretto, M.; Padoin, A.V.; Schaan, B. Bone Mineral Density and Nutritional Profile in Morbidly Obese Women. Obes. Surg. 2010, 20, 1372–1379. [Google Scholar] [CrossRef]
- Chou, J.-J.; Lee, W.-J.; Almalki, O.; Chen, J.-C.; Tsai, P.-L.; Yang, S.-H. Dietary Intake and Weight Changes 5 Years After Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2017, 27, 3240–3246. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, I.; Raparelli, L.; Guarnacci, L.; Paone, E.; Del Genio, G.; Le Roux, C.W.; Silecchia, G. Food Intake and Changes in Eating Behavior After Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2016, 26, 2059–2067. [Google Scholar] [CrossRef]
- Cooper, P.; Brearley, L.; Jamieson, A.; Ball, M. Nutritional consequences of modified vertical gastroplasty in obese subjects. Int. J. Obes. 1999, 23, 382–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvath, J.D.C.; De Castro, M.L.D.; Kops, N.; Malinoski, N.K.; Friedman, R. Obesity coexists with malnutrition? Adequacy of food consumption by severely obese patients to dietary reference intake recommendations. Nutr. Hosp. 2014, 29, 292–299. [Google Scholar] [CrossRef]
- Coupaye, M.; Rivière, P.; Breuil, M.C.; Castel, B.; Bogard, C.; Dupré, T.; Flamant, M.; Msika, S.; LeDoux, S. Comparison of Nutritional Status During the First Year After Sleeve Gastrectomy and Roux-en-Y Gastric Bypass. Obes. Surg. 2014, 24, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.C.A.; De Arvelos, L.R.; Felix, G.P.; De Souza, D.N.P.; Neto, M.B.; Resende, E.S.; Penha-Silva, N. Evolution of nutritional, hematologic and biochemical changes in obese women during 8 weeks after Roux-en-Y gastric bypasss. Nutr. Hosp. 2012, 27, 1134–1140. [Google Scholar] [CrossRef]
- Dagan, S.S.; Zelber-Sagi, S.; Webb, M.; Keidar, A.; Raziel, A.; Sakran, N.; Goitein, D.; Shibolet, O. Nutritional Status Prior to Laparoscopic Sleeve Gastrectomy Surgery. Obes. Surg. 2016, 26, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Dagan, S.S.; Keidar, A.; Raziel, A.; Sakran, N.; Goitein, D.; Shibolet, O.; Zelber-Sagi, S. Do Bariatric Patients Follow Dietary and Lifestyle Recommendations during the First Postoperative Year? Obes. Surg. 2017, 27, 2258–2271. [Google Scholar] [CrossRef]
- Davies, N.; O’Sullivan, J.M.; Plank, L.D.; Murphy, R. Gut Microbial Predictors of Type 2 Diabetes Remission Following Bariatric Surgery. Obes. Surg. 2020, 30, 3536–3548. [Google Scholar] [CrossRef]
- Da Silva, M.M.; Sala, P.C.; Cardinelli, C.S.; Torrinhas, R.S.; Waitzberg, D.L. Comparison of Virtual Nutri Plus® and Dietpro 5i® software systems for the assessment of nutrient intake before and after Roux-en-Y gastric bypass. Clinics 2014, 69, 714–722. [Google Scholar] [CrossRef]
- Da Silva, F.B.L.; Gomes, D.L.; de Carvalho, K.M.B. Poor diet quality and postoperative time are independent risk factors for weight regain after Roux-en-Y gastric bypass. Nutrition 2016, 32, 1250–1253. [Google Scholar] [CrossRef]
- Dias, M.C.G.; Ribeiro, A.G.; Scabim, V.M.; Faintuch, J.; Zilberstein, B.; Gama-Rodrigues, J.J. Dietary intake of female bariatric patients after anti-obesity gastroplasty. Clinics 2006, 61, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Duffey, B.G.; Pedro, R.N.; Kriedberg, C.; Weiland, D.; Melquist, J.; Ikramuddin, S.; Kellogg, T.; Makhlouf, A.A.; Monga, M. Lithogenic Risk Factors in the Morbidly Obese Population. J. Urol. 2008, 179, 1401–1406. [Google Scholar] [CrossRef]
- El Labban, S.; Safadi, B.; Olabi, A. The Effect of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy Surgery on Dietary Intake, Food Preferences, and Gastrointestinal Symptoms in Post-Surgical Morbidly Obese Lebanese Subjects: A Cross-Sectional Pilot Study. Obes. Surg. 2015, 25, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Ernst, B.; Thurnheer, M.; Wilms, B.; Schultes, B. Differential Changes in Dietary Habits after Gastric Bypass Versus Gastric Banding Operations. Obes. Surg. 2008, 19, 274–280. [Google Scholar] [CrossRef]
- Faria, S.L.; Faria, O.P.; Cardeal, M.D.A. Comparison of weight loss, food consumption and frequency of vomiting among Roux-en-Y gastric bypass patients with or without constriction ring. Arq. Bras. Cir. Dig. 2014, 27, 43–46. [Google Scholar] [CrossRef] [Green Version]
- Farias, G.; Silva, R.M.O.; Da Silva, P.P.P.; Vilela, R.M.; Bettini, S.C.; Dâmaso, A.R.; Netto, B.D.M. Impact of dietary patterns according to NOVA food groups: 2 y after Roux-en-Y gastric bypass surgery. Nutrition 2020, 74, 110746. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Tolone, S.; Gravina, A.G.; Patrone, V.; Romano, M.; Tuccillo, C.; Mozzillo, A.L.; Amoroso, V.; Misso, G.; et al. Gastrointestinal Hormones, Intestinal Microbiota and Metabolic Homeostasis in Obese Patients: Effect of Bariatric Surgery. In Vivo 2016, 30, 321–330. [Google Scholar]
- Forbes, R.; Gasevic, D.; Watson, E.M.; Ziegler, T.R.; Lin, E.; Burgess, J.R.; Gletsu-Miller, N. Essential Fatty Acid Plasma Profiles Following Gastric Bypass and Adjusted Gastric Banding Bariatric Surgeries. Obes. Surg. 2015, 26, 1237–1246. [Google Scholar] [CrossRef]
- Freeth, A.; Prajuabpansri, P.; Victory, J.M.; Jenkins, P. Assessment of Selenium in Roux-en-Y Gastric Bypass and Gastric Banding Surgery. Obes. Surg. 2012, 22, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.A.; Overs, S.E.; Zarshenas, N.; Walton, K.L.; Jorgensen, J.O. Food tolerance and diet quality following adjustable gastric banding, sleeve gastrectomy and Roux-en-Y gastric bypass. Obes. Res. Clin. Pr. 2014, 8, e183–e191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furet, J.-P.; Kong, L.-C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.-L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential Adaptation of Human Gut Microbiota to Bariatric Surgery-Induced Weight Loss: Links With Metabolic and Low-Grade Inflammation Markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [Green Version]
- Furtado, M.C.M.B.; Vermeulen, K.M.; Bellot, P.E.N.R.; Godoy, C.M.D.A.; Coelho, D.; De Godoy, E.P.; De Oliveira, A.M.G.; Campos, J.M. Evaluation of factors that may influence in the insufficient weight loss in patients after two years of Roux-en-Y gastric bypass. Nutr. Hosp. 2018, 35, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Gesquiere, I.; Foulon, V.; Augustijns, P.; Gils, A.; Lannoo, M.; Van Der Schueren, B.; Matthys, C. Micronutrient intake, from diet and supplements, and association with status markers in pre- and post-RYGB patients. Clin. Nutr. 2017, 36, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, J.C.; Nicoletti, C.F.; Pinhel, M.A.D.S.; Oliveira, B.; Júnior, W.S.; Marchini, J.S.; Nonino, C.B. Pregnancy After Roux en Y Gastric Bypass: Nutritional and Biochemical Aspects. Obes. Surg. 2017, 27, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Giusti, V.; Theytaz, F.; Di Vetta, V.; Clarisse, M.; Suter, M.; Tappy, L. Energy and macronutrient intake after gastric bypass for morbid obesity: A 3-y observational study focused on protein consumption. Am. J. Clin. Nutr. 2015, 103, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Gobato, R.C.; Chaves, D.F.S.; Chaim, E.A. Micronutrient and physiologic parameters before and 6 months after RYGB. Surg. Obes. Relat. Dis. 2014, 10, 944–951. [Google Scholar] [CrossRef]
- Gobato, R.C.; Cazzo, E.; Baltieri, L.; Modena, D.; Chaim, E.A. Food Intolerance 1 Year After Banded Roux-En-Y Gastric Bypass. Obes. Surg. 2018, 29, 485–491. [Google Scholar] [CrossRef]
- Golpaie, A.; Tajik, N.; Masoudkabir, F.; Karbaschian, Z.; Talebpour, M.; Hosseini, M.; Hosseinzadeh-Attar, M.J. Short-term effect of weight loss through restrictive bariatric surgery on serum levels of vaspin in morbidly obese subjects. Eur. Cytokine Netw. 2011, 22, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golzarand, M.; Toolabi, K.; Djafarian, K. Changes in Body Composition, Dietary Intake, and Substrate Oxidation in Patients Underwent Laparoscopic Roux-en-Y Gastric Bypass and Laparoscopic Sleeve Gastrectomy: A Comparative Prospective Study. Obes. Surg. 2018, 29, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska-Mierzyńska, M.; Ostrowska, L.; Hady, H.R.; Dadan, J. Assessment of dietary habits, nutritional status and blood biochemical parameters in patients prepared for bariatric surgery: A preliminary study. Videosurg. Other Miniinvasive Tech. 2012, 3, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.K.; Andersen, L.F.; Hofsø, D.; Aasheim, E.T.; Holven, K.B.; Sandbu, R.; Røislien, J.; Hjelmesæth, J. Dietary changes in obese patients undergoing gastric bypass or lifestyle intervention: A clinical trial. Br. J. Nutr. 2012, 110, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanerva, N.; Larsson, I.; Peltonen, M.; Lindroos, A.-K.; Carlsson, L.M. Sociodemographic and lifestyle factors as determinants of energy intake and macronutrient composition: A 10-year follow-up after bariatric surgery. Surg. Obes. Relat. Dis. 2017, 13, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Kops, N.L.; Horvath, J.D.C.; de Castro, M.L.D.; Friedman, R. Anthropometric and lipid profile of individuals with severe obesity carrying the fatty acid–binding protein–2 Thr54 allele. Nutrition 2017, 41, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kruseman, M.; Leimgruber, A.; Zumbach, F.; Golay, A. Dietary, Weight, and Psychological Changes among Patients with Obesity, 8 Years after Gastric Bypass. J. Am. Diet. Assoc. 2010, 110, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Laurenius, A.; Larsson, I.; Melanson, K.J.; Lindroos, A.K.; Lönroth, H.; Bosaeus, I.; Olbers, T. Decreased energy density and changes in food selection following Roux-en-Y gastric bypass. Eur. J. Clin. Nutr. 2013, 67, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.L.; Kelly, E.D.O.; Faria, O.P.; Ito, M.K. Snack-Eating Patients Experience Lesser Weight Loss after Roux-En-Y Gastric Bypass Surgery. Obes. Surg. 2009, 19, 1293–1296. [Google Scholar] [CrossRef]
- Le Roux, C.W.; Bueter, M.; Theis, N.; Werling, M.; Ashrafian, H.; Löwenstein, C.; Athanasiou, T.; Bloom, S.R.; Spector, A.C.; Olbers, T.; et al. Gastric bypass reduces fat intake and preference. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R1057–R1066. [Google Scholar] [CrossRef] [Green Version]
- LeDoux, S.; Sami, O.; Breuil, M.-C.; Delapierre, M.; Calabrese, D.; Msika, S.; Coupaye, M. Relevance of Self-reported Behavioral Changes Before Bariatric Surgery to Predict Success After Surgery. Obes. Surg. 2016, 27, 1453–1459. [Google Scholar] [CrossRef]
- Magno, F.C.C.M.; Da Silva, M.S.; Cohen, L.; d’Abreu Sarmento, L.; Rosado, E.L.; Carneiro, J.R.I. Nutritional profile of patients in a multidisciplinary treatment program for severe obesity and preoperative bariatric surgery. Arq. Bras. Cir. Dig. 2014, 27, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.A.; Verlengia, R.; Crisp, A.H.; Novais, P.F.S.; Rasera-Junior, I.; de Oliveira, M.R.M. Micronutrient supplementation in gastric bypass surgery: Prospective study on inflammation and iron metabolism in premenopausal women. Nutr. Hosp. 2017, 34, 369–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, A.R.; Lobato, C.B.; Pereira, S.S.; Guimarães, M.; Faria, S.; Nora, M.; Monteiro, M.P. Insights from the Impact of Meal Composition on Glucose Profile Towards Post-bariatric Hypoglycemia Management. Obes. Surg. 2019, 30, 249–255. [Google Scholar] [CrossRef]
- McLean, K.L.; Moore, C.E.; Miketinas, D.C.; Champagne, C.M. Comparison of dietary habits and plans for dietary changes in black and white women seeking bariatric surgery. Surg. Obes. Relat. Dis. 2018, 14, 106–111. [Google Scholar] [CrossRef]
- Melendez-Araújo, M.S.; Arruda, S.L.D.M.; Kelly, E.D.O.; De Carvalho, K.M.B. Preoperative Nutritional Interventions in Morbid Obesity: Impact on Body Weight, Energy Intake, and Eating Quality. Obes. Surg. 2012, 22, 1848–1854. [Google Scholar] [CrossRef]
- Melo, T.L.; Froeder, L.; Baia, L.D.C.; Heilberg, I.P. Bone turnover after bariatric surgery. Arch. Endocrinol. Metab. 2017, 61, 332–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercachita, T.; Santos, Z.; Limao, J.; Carolino, E.; Mendes, L. Anthropometric Evaluation and Micronutrients Intake in Patients Submitted to Laparoscopic Roux-en-Y Gastric Bypass with a Postoperative Period of ≥1 Year. Obes. Surg. 2013, 24, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.D.; Norris, A.; Fernandez, A. Changes in Nutrients and Food Groups Intake Following Laparoscopic Roux-en-Y Gastric Bypass (RYGB). Obes. Surg. 2014, 24, 1926–1932. [Google Scholar] [CrossRef] [Green Version]
- Mischler, R.A.; Armah, S.M.; Wright, B.N.; Mattar, S.G.; Rosen, A.D.; Gletsu-Miller, N. Influence of diet and supplements on iron status after gastric bypass surgery. Surg. Obes. Relat. Dis. 2016, 12, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Moizé, V.; Deulofeu, R.; Torres, F.; De Osaba, J.M.; Vidal, J. Nutritional Intake and Prevalence of Nutritional Deficiencies Prior to Surgery in a Spanish Morbidly Obese Population. Obes. Surg. 2011, 21, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Moizé, V.; Andreu, A.; Flores, L.; Torres, F.; Ibarzabal, A.; Delgado, S.; Lacy, A.; Rodriguez, L.; Vidal, J. Long-Term Dietary Intake and Nutritional Deficiencies following Sleeve Gastrectomy or Roux-En-Y Gastric Bypass in a Mediterranean Population. J. Acad. Nutr. Diet. 2013, 113, 400–410. [Google Scholar] [CrossRef]
- Netto, B.D.M.; Earthman, C.P.; Farias, G.; Masquio, D.C.L.; Clemente, A.P.G.; Peixoto, P.; Bettini, S.C.; von Der Heyde, M.E.; Dâmaso, A.R. Eating patterns and food choice as determinant of weight loss and improvement of metabolic profile after RYGB. Nutrition 2017, 33, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.E.; Sherman, V. Effectiveness of B Vitamin Supplementation Following Bariatric Surgery: Rapid Increases of Serum Vitamin B12. Obes. Surg. 2014, 25, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, C.F.; Lima, T.P.; Donadelli, S.P.; Salgado, W.; Marchini, J.S.; Nonino, C.B. New look at nutritional care for obese patient candidates for bariatric surgery. Surg. Obes. Relat. Dis. 2013, 9, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, C.F.; Oliveira, B.; Barbin, R.; Marchini, J.S.; Junior, W.S.; Nonino, C.B. Red meat intolerance in patients submitted to gastric bypass: A 4-year follow-up study. Surg. Obes. Relat. Dis. 2015, 11, 842–846. [Google Scholar] [CrossRef]
- Nicoletti, C.F.; Kimura, B.M.; Oliveira, B.; De Pinhel, M.A.S.; Salgado, W.; Marchini, J.S.; Nonino, C.B. Role ofUCP2polymorphisms on dietary intake of obese patients who underwent bariatric surgery. Clin. Obes. 2016, 6, 354–358. [Google Scholar] [CrossRef]
- Nicoletti, C.F.; Esteves, G.P.; Genario, R.; Santo, M.A.; de Cleva, R.; Gualano, B.; Roschel, H. Nutritional Inadequacies Among Post-bariatric Patients During COVID-19 Quarantine in Sao Paulo, Brazil. Obes. Surg. 2021, 31, 2330–2334. [Google Scholar] [CrossRef] [PubMed]
- Nonino, C.B.; Oliveira, B.; Chaves, R.C.P.; Silva, L.T.P.E.; Pinhel, M.A.D.S.; Ferreira, F.D.C.; Rocha, G.D.C.; Donadelli, S.P.; Marchini, J.S.; Salgado-Junior, W.; et al. Is there any change in phenotypic characteristics comparing 5 to 10 years of follow-up in obese patients undergoing roux-en-y gastric bypass? Arq. Bras. Cir. Dig. 2019, 32, e1453. [Google Scholar] [CrossRef] [PubMed]
- Nosso, G.; Lupoli, R.; Saldalamacchia, G.; Griffo, E.; Cotugno, M.; Costabile, G.; Riccardi, G.; Capaldo, B. Diabetes remission after bariatric surgery is characterized by high glycemic variability and high oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 949–955. [Google Scholar] [CrossRef]
- Novais, P.F.S.; Rasera, I.; Leite, C.V.D.S.; Marin, F.A.; de Oliveira, M.R.M. Food intake in women two years or more after bariatric surgery meets adequate intake requirements. Nutr. Res. 2012, 32, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Olbers, T.; Björkman, S.; Lindroos, A.; Maleckas, A.; Lönn, L.L.; Sjöström, L.; Lönroth, H. Body Composition, Dietary Intake, and Energy Expenditure After Laparoscopic Roux-en-Y Gastric Bypass and Laparoscopic Vertical Banded Gastroplasty. Ann. Surg. 2006, 244, 715–722. [Google Scholar] [CrossRef]
- Ortega, J.; Ortega-Evangelio, G.; Cassinello, N.; Sebastiá, V. What Are Obese Patients Able to Eat after Roux-en-Y Gastric Bypass? Obes. Facts 2012, 5, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Papalazarou, A.; Yannakoulia, M.; Kavouras, S.; Komesidou, V.; Dimitriadis, G.; Papakonstantinou, A.; Sidossis, L.S. Lifestyle Intervention Favorably Affects Weight Loss and Maintenance Following Obesity Surgery. Obesity 2010, 18, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.L.; Juvanhol, L.L.; Bressan, J. Increase in Protein Intake After 3 Months of RYGB Is an Independent Predictor for the Remission of Obesity in the First Year of Surgery. Obes. Surg. 2019, 29, 3780–3785. [Google Scholar] [CrossRef]
- Quesada, K.R.; Novais, P.F.S.; Detregiachi, C.R.P.; Barbalho, S.M.; Rasera, I.; Oliveira, M.R.M. Comparative Analysis of Approaches for Assessing Energy Intake Underreporting by Female Bariatric Surgery Candidates. J. Am. Coll. Nutr. 2014, 33, 155–162. [Google Scholar] [CrossRef]
- Raatz, S.K.; Johnson, L.K.; Caliquary, A.; King, W.C.; Kalarchian, M.A.; Devlin, M.J.; Marcus, M.D.; Mitchell, J.E. Reported nutrient intake over 7 years after Roux-en-Y gastric bypass in the Longitudinal Assessment of Bariatric Surgery-3 (LABS-3) psychosocial study. Surg. Obes. Relat. Dis. 2020, 16, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.E.R.; Oparina, E.; Plourde, H.; Andersen, R.E. Energy Intake and Food Habits between Weight Maintainers and Regainers, Five Years after Roux-en-Y Gastric Bypass. Can. J. Diet. Pract. Res. 2016, 77, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, T.; Vidal, J.; de Hollanda, A.; Scheer, F.; Garaulet, M.; Izquierdo-Pulido, M. Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clin. Nutr. 2016, 35, 1308–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Tovar, J.; Bozhychko, M.; Del-Campo, J.M.; Boix, E.; Zubiaga, L.; Muñoz, J.L.; Llavero, C. Changes in Frequency Intake of Foods in Patients Undergoing Sleeve Gastrectomy and Following a Strict Dietary Control. Obes. Surg. 2017, 28, 1659–1664. [Google Scholar] [CrossRef]
- Sánchez, A.; Rojas, P.; Basfi-Fer, K.; Carrasco, F.; Inostroza, J.; Codoceo, J.; Valencia, A.; Papapietro, K.; Csendes, A.; Ruz, M. Micronutrient Deficiencies in Morbidly Obese Women Prior to Bariatric Surgery. Obes. Surg. 2016, 26, 361–368. [Google Scholar] [CrossRef]
- Sarwer, D.B.; Moore, R.H.; Spitzer, J.C.; Wadden, T.A.; Raper, S.E.; Williams, N.N. A pilot study investigating the efficacy of postoperative dietary counseling to improve outcomes after bariatric surgery. Surg. Obes. Relat. Dis. 2012, 8, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Sarwer, D.B.; Wadden, T.A.; Moore, R.H.; Baker, A.W.; Gibbons, L.M.; Raper, S.E.; Williams, N.N. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg. Obes. Relat. Dis. 2008, 4, 640–646. [Google Scholar] [CrossRef] [Green Version]
- Schoemacher, L.A.H.M.; Boerboom, A.B.; Thijsselink, M.M.R.; Aarts, E.O. The Relationship Between Energy Intake and Weight Loss in Bariatric Patients. Obes. Surg. 2019, 29, 3874–3881. [Google Scholar] [CrossRef]
- Seki, Y.; Pantanakul, S.; Kasama, K.; Kikkawa, E.; Nakazato, T.; Porciuncula, J.P. Impact of metabolic surgery on health-related quality of life and quality of alimentation. Surg. Obes. Relat. Dis. 2019, 15, 488–496. [Google Scholar] [CrossRef]
- Shah, M.; Adams-Huet, B.; Rao, S.; Snell, P.; Quittner, C.; Garg, A. The effect of dietary counseling on nutrient intakes in gastric banding surgery patients. J. Investig. Med. 2013, 61, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Shai, I.; Henkin, Y.; Weitzman, S.; Levi, I. Long-term Dietary Changes after Vertical Banded Gastroplasty: Is the Trade-off Favorable? Obes. Surg. 2002, 12, 805–811. [Google Scholar] [CrossRef]
- Soares, F.L.; De Sousa, L.B.; Corradi-Perini, C.; Da Cruz, M.R.R.; Nunes, M.G.J.; Branco-Filho, A.J. Food Quality in the Late Postoperative Period of Bariatric Surgery: An Evaluation Using the Bariatric Food Pyramid. Obes. Surg. 2014, 24, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Solga, S.; Alkhuraishe, A.R.; Clark, J.M.; Torbenson, M.; Greenwald, A.; Diehl, A.M.; Magnuson, T. Dietary Composition and Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2004, 49, 1578–1583. [Google Scholar] [CrossRef]
- Søvik, T.T.; Karlsson, J.; Aasheim, E.T.; Fagerland, M.W.; Björkman, S.; Engström, M.; Kristinsson, J.; Olbers, T.; Mala, T. Gastrointestinal function and eating behavior after gastric bypass and duodenal switch. Surg. Obes. Relat. Dis. 2013, 9, 641–647. [Google Scholar] [CrossRef]
- Rossi, R.G.D.T.; Dos Santos, M.T.A.; De Souza, F.I.S.; Aquino, R.D.C.D.; Sarni, R.O.S. Nutrient Intake of Women 3 Years After Roux-en-Y Gastric Bypass Surgery. Obes. Surg. 2012, 22, 1548–1553. [Google Scholar] [CrossRef]
- Trostler, N.; Mann, A.; Zilberbush, N.; Avinoach, E.; Charuzi, I. Weight Loss and Food Intake 18 Months following Vertical Banded Gastroplasty or Gastric Bypass for Severe Obesity. Obes. Surg. 1995, 5, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, J.; Ernst, B.; Wilms, B.; Thurnheer, M.; Schultes, B. Roux-en Y Gastric Bypass Surgery Reduces Hedonic Hunger and Improves Dietary Habits in Severely Obese Subjects. Obes. Surg. 2013, 23, 50–55. [Google Scholar] [CrossRef]
- Verger, E.O.; Aron-Wisnewsky, J.; Dao, M.C.; Kayser, B.D.; Oppert, J.-M.; Bouillot, J.-L.; Torcivia, A.; Clément, K. Micronutrient and Protein Deficiencies After Gastric Bypass and Sleeve Gastrectomy: A 1-year Follow-up. Obes. Surg. 2016, 26, 785–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, F.T.; Faria, S.L.C.M.; Dutra, E.S.; Ito, M.K.; Reis, C.; Da Costa, T.H.M.; de Carvalho, K.M.B. Perception of Hunger/Satiety and Nutrient Intake in Women Who Regain Weight in the Postoperative Period After Bariatric Surgery. Obes. Surg. 2019, 29, 958–963. [Google Scholar] [CrossRef]
- Vieira, R.A.L.; Filho, L.V.R.; Burgos, M.G.P.D.A. Consumo alimentar e sua associação com estado nutricional, atividade física e fatores sociodemográficos de candidatos à cirurgia bariátrica. Rev. Colégio Bras. Cir. 2019, 46, e20192382. [Google Scholar] [CrossRef] [PubMed]
- Vinolas, H.; Barnetche, T.; Ferrandi, G.; Monsaingeon-Henry, M.; Pupier, E.; Collet, D.; Gronnier, C.; Gatta-Cherifi, B. Oral Hydration, Food Intake, and Nutritional Status Before and After Bariatric Surgery. Obes. Surg. 2019, 29, 2896–2903. [Google Scholar] [CrossRef]
- Wardé-Kamar, J.; Rogers, M.; Flancbaum, L.; Laferrere, B. Calorie Intake and Meal Patterns up to 4 Years after Roux-en-Y Gastric Bypass Surgery. Obes. Surg. 2004, 14, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Werling, M.; Olbers, T.; Fändriks, L.; Bueter, M.; Lönroth, H.; Stenlöf, K.; Le Roux, C.W. Increased Postprandial Energy Expenditure May Explain Superior Long Term Weight Loss after Roux-en-Y Gastric Bypass Compared to Vertical Banded Gastroplasty. PLoS ONE 2013, 8, e60280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, E.; Utech, M.; Stehle, P.; Büsing, M.; Stoffel-Wagner, B.; Ellinger, S. Preoperative micronutrient status in morbidly obese patients before undergoing bariatric surgery: Results of a cross-sectional study. Surg. Obes. Relat. Dis. 2015, 11, 1157–1163. [Google Scholar] [CrossRef]
- Zaparolli, M.R.; Da-Cruz, M.R.R.; Frehner, C.; Branco-Filho, A.J.; Schieferdecker, M.E.M.; Campos, A.C.L.; Taconeli, C.A.; Parreira, G. Food intake evaluation during the first year of postoperative of patients with type 2 diabetes mellitus or glycemic alteration submitted to roux-en-y gastric bypass. Arq. Bras. Cir. Dig. 2018, 31, e1367. [Google Scholar] [CrossRef]
- Ziadlou, M.; Hosseini-Esfahani, F.; Khosravi, H.M.; Hosseinpanah, F.; Barzin, M.; Khalaj, A.; Valizadeh, M. Dietary macro- and micro-nutrients intake adequacy at 6th and 12th month post-bariatric surgery. BMC Surg. 2020, 20, 232. [Google Scholar] [CrossRef]
- Yang, Y.J.; Kim, M.K.; Hwang, S.H.; Ahn, Y.; Shim, J.E.; Kim, D.H. Relative validities of 3-day food records and the food frequency questionnaire. Nutr. Res. Pr. 2010, 4, 142–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touvier, M.; Kesse-Guyot, E.; Méjean, C.; Pollet, C.; Malon, A.; Castetbon, K.; Hercberg, S. Comparison between an interactive web-based self-administered 24 h dietary record and an interview by a dietitian for large-scale epidemiological studies. Br. J. Nutr. 2011, 105, 1055–1064. [Google Scholar] [CrossRef]
- Alcantara, I.; Haardörfer, R.; Gazmararian, J.A.; Hartman, T.J.; Greene, B.; Kegler, M.C. Relative validation of fruit and vegetable intake and fat intake among overweight and obese African-American women. Public Health Nutr. 2014, 18, 1932–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, L.F.; Tomten, H.; Haggarty, P.; Løvø, A.; Hustvedt, B.-E. Validation of energy intake estimated from a food frequency questionnaire: A doubly labelled water study. Eur. J. Clin. Nutr. 2003, 57, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroder, H.; Covas, M.; Marrugat, J.; Vila-Domènech, J.S.; Pena, A.; Alcántara, M.; Masiá, R. Use of a three-day estimated food record, a 72-hour recall and a food-frequency questionnaire for dietary assessment in a Mediterranean Spanish population. Clin. Nutr. 2001, 20, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Salvo, V.L.M.A.; Gimeno, S.G.A. Reprodutibilidade e validade do questionário de freqüência de consumo de alimentos. Rev. Saúde Públ. 2002, 36, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Andersen, L.F.; Solvoll, K.; Johansson, L.R.K.; Salminen, I.; Aro, A.; Drevon, C.A. Evaluation of a Food Frequency Questionnaire with Weighed Records, Fatty Acids, and Alpha-Tocopherol in Adipose Tissue and Serum. Am. J. Epidemiol. 1999, 150, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Block, G.; Woods, M.; Potosky, A.; Clifford, C. Validation of a self-administered diet history questionnaire using multiple diet records. J. Clin. Epidemiol. 1990, 43, 1327–1335. [Google Scholar] [CrossRef]
- Lindroos, A.K.; Lissner, L.; Sjöström, L. Validity and reproducibility of a self-administered dietary questionnaire in obese and non-obese subjects. Eur. J. Clin. Nutr. 1993, 47, 461–481. [Google Scholar]
- Lissner, L. Measuring food intake in studies of obesity. Public Health Nutr. 2002, 5, 889–892. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Study | Year | Country | PMID | Population | Dietary Assessment | Surgery Type | Objective | Study Design | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age ± SD | Sex | BMI ± SD | Tool | Validation | |||||||
| Al Assal et al. [16] | 2020 | Brazil | 31973130 | 25 | 45.8 ± 7.9 | 100% women | 46.4 ± 5.5 | Food record (7 days) | None | RYGB | Assess the gut microbiota profile before and after RYGB and the correlation with food intake and postoperative type 2 diabetes remission. | Prospective |
| Al-Ozairi et al. [17] | 2019 | Kuwait | 30756296 | 50 | 38.8 ± 9.1 | 84% women | 29.2 ± 6.2 | Photo-assisted diet capture method | Yes | SG | Evaluate the use of digital food photography in comparison to conventional methods among patients after sleeve gastrectomy. | Cross-sectional |
| Amundsen et al. [18] | 2017 | Norway | 27914028 | 49 | 46 | 82% women | 44.1 | FFQ | Yes | GB | Compare GB patients experiencing suboptimal weight loss or significant weigh regain with successful controls, regarding postoperative food intake, eating behavior, physical activity, and psychometrics. | Case-control |
| Andersen and Larsen [19] | 1989 | Denmark | 2556911 | 18 | 35 | 89% women | N/D | Food record (7 days) | None | Gastroplasty | Evaluate diet compliance and nutritional safety. | Longitudinal |
| Anderson et al. [20] | 2007 | United-states | 17557983 | 84 | AA: 41 ± 10; white: 43 ± 10 | 76% women | AA: 55 ± 10; white: 53 ± 11 | Food record (N/D) | None | RYGB | Compare weight loss between AA and white severely obese patients after RYGB and examined differences in dietary intake and cardiovascular risk factors before and after weight loss. | Retrospective |
| Andreu et al. [21] | 2010 | Spain | 20820937 | 101 | 43.2 | 75% women | 47.7 | Food record (3 days) | None | RYGB (66%) or SG (34%) | Examine the accomplishment of the recommended protein intake, and the influence of protein intake on free fat mass and protein status following bariatric surgery. | Longitudinal |
| Anthone et al. [22] | 2003 | United States | 14530733 | 701 | 42.3 ± 10.4 | 78% women | 52.3 ± 9.6 | Questionnaire | None | DS | Determine the safety and efficacy of the duodenal switch procedure as surgical treatment of morbid obesity. | Prospective |
| Aron-Wisnewsky et al. [23] | 2016 | France | 26891123 | 22 | GBP: 40.5; AGB 40.5 | 100% women | GBP 46.3; AGB 42.8 | 3 × 24-h dietary recall | Yes | GBP or AGB | Analyze the effect of food restriction on nutritional parameters in the short-term (≤3 months) period after bariatric surgery in morbid obesity. | Prospective |
| Barnadas et al. [24] | 2021 | Spain | 33435751 | 41 | 49.7 ± 10 | 75% women | 44.3 ± 6.2 | Food record (7 days) | None | SG | Study the alterations of the circadian rhythmicity due to morbid obesity and the recovery of the circadian pattern after weight loss in a cohort of patients who underwent sleeve gastrectomy. | Prospective |
| Bavaresco et al. [25] | 2010 | Brazil | 18931884 | 48 | 41.9 | 85% women | 51.9 | 24-h dietary recall | None | RYGB | Assess the metabolic and nutritional profile of grade III obese patients for a period of 12 months after bariatric surgery. | Longitudinal |
| Benaiges et al. [26] | 2019 | Spain | 31288988 | 60 | 43.1 ± 7.9 | 71.7% women | 44.1 ± 4.9 | FFQ | Yes | RYGB (43%) | Evaluate dietary modifications during the preoperative and postoperative periods of bariatric surgery. | Observational, prospective |
| Bobbioni-Harsch et al. [27] | 2002 | Switzerland | 12032656 | 50 | 38.4 | 100% women | 45.2 | Food record (3 days) | Yes | RYGB | Evaluate whether or not the individual differences in the substrates content of the diet had any impact on body weight loss and, consequently, could contribute to its variability. | Longitudinal |
| Brolin et al. [28] | 1994 | United-states | 7986146 | 138 | VBG: 39 ± 9; RYGB: 38 ± 10 | 85% women | VBG: 42 ± 4; RYGB: 43 ± 4 | Dietary history + 24-h dietary recall | Yes | VBG (30) or RYGB (108) | Determine whether assessment of preoperative eating patterns and food preferences can be used to predict weight loss outcome after surgery. | Prospective longitudinal |
| Buzga et al. [29] | 2014 | Czech Republic | 25561993 | 37 | 43.5 | 78% women | 43 | Questionnaire | None | SG | Assess changes in dietary habits in obese patients 6 and 12 months after SG, compare changes in hormonal levels and dietary habits after this procedure. | Longitudinal |
| Carrasco et al. [30] | 2007 | Chile | 17658019 | 38 | 36.3 | 89% women | 44 | FFQ + Food record (3 days) | None | RYGB | Detect metabolic or behavioral parameters that could predict the reduction in weight, the loss in body fat and the improvement in cardiovascular risk factors. | Longitudinal |
| Carrasco et al. [31] | 2012 | Chile | 22305536 | 50 | 37.6 ± 10.2 | 100% women | 43.8 ± 4.8 | Food record (3 days) | None | GBP | Evaluate the relation between weight loss and food intake and between weight loss and changes in serum ghrelin concentrations 1 y after GBP with resection of the bypassed stomach and without resection. | Prospective |
| Carvalho et al. [32] | 2020 | Brazil | 32728839 | 122 | 33 | 77% women | N/D | 2 × 24-h dietary recall | Yes | RYGB or SG | Evaluate the association between social jet lag, a measure of circadian misalignment, and anthropometric, metabolic and food intake outcomes 6 months after bariatric surgery. | Longitudinal, observational |
| Casagrande et al. [33] | 2010 | Brazil | 20411350 | 33 | 35.9 | 100% women | 43.2 | FFQ + 24-h dietary recall | Yes | RYGB | Evaluate bone metabolism/mineral density and nutritional profile in morbidly obese women before surgery. | Prospective longitudinal |
| Chou et al. [34] | 2017 | Taiwan | 28589529 | 40 | 33.5 ± 9.7 | 75% women | 37.9 ± 6.6 | FFQ + 24-h dietary recall | Yes | SG | Investigate long-term dietary intake and weight status after SG. | Retrospective |
| Coluzzi et al. [35] | 2016 | Italy | 26744284 | 30 | 35 | 73% women | 43.9 | 24-h dietary recall | None | SG | Evaluate the quantitative reduction and qualitative changes in food intake post surgery and analyzed the association between weight loss and changes in eating behavior. | Prospective longitudinal |
| Cooper et al. [36] | 1999 | Australia | 10340816 | 26 | 23–59 | 96% women | 31.6–52.7 | Food record (4 days) | None | MLVG | Perform detailed dietary analyses together with anthropometric, haematological and food intolerance assessment of a group of subjects undergoing MLVG, who received preoperative dietary education and regular postoperative followup, with some dietary advice over the subsequent year. | Longitudinal |
| Correia Horvath et al. [37] | 2014 | Brazil | 24528344 | 77 | 44.5 | 65% women | 48.8 | 24-h dietary recall | None | N/D | Assess food consumption by severely obese patients and describe their main nutritional deficiencies on the basis of dietary reference intake. | Cross-sectional |
| Coupaye et al. [38] | 2014 | France | 24122661 | 86 | SG: 45 ± 11; RYGB: 44 ± 9 | 72% women | SG = 48.5 ± 9.6; RYGB = 48.6 ± 7.8 | Food record (4 days) + interview | None | RYGB or SG | Compare nutritional status after SG and RYGB in subjects matched for postoperative weight | Prospective |
| Custodio et al. [39] | 2012 | Brazil | 23165553 | 22 | 37.9 ± 9.1 | 100% women | 44.3 ± 5.4 | Food record (3 days) | None | RYGB | Evaluate the influence of changes in food intake on body composition and some hematologic and biochemical variables in the period of eight weeks after RYGB. | Prospective |
| Dagan et al. [40] | 2016 | Israel | 26797718 | 100 | 41.9 | 60% women | 42.3 | FFQ | None | SG | Evaluate and compare between genders dietary intake and micronutrient deficiencies among 100 candidates for surgery. | Cross-sectional |
| Dagan et al. [41] | 2017 | Israel | 28303504 | 77 | 43.1 | 57% women | 42.1 | Food record (3 days) | None | SG | Evaluate adherence to dietary and lifestyle recommendations and its relation to weight post surgery. | Prospective |
| Davies et al. [42] | 2020 | New Zealand | 32447634 | 44 | RYGB: 48.5 ± 5.5 SG: 47.7 ± 6.9 | 52% women | RYGB: 38.2 ± 5.7; SG: 40.0 ± 5.9 | Food record (5 days) | None | RYGB or SG | Identify whether there were surgery-specific changes in gut microbiota among obese people with Type 2 diabetes randomised to either SG or RYGB and whether there were common taxa and gut microbiota functional capacity changes among those who achieved T2D remission, irrespective of surgery type. | Prospective |
| da Silva et al. [43] | 2014 | Brazil | 25518027 | 10 | 46.5 ± 6.6 | 100% women | 45.7 ± 4.1 | Food record (7 days) | N/D | RYGB | Compare the Virtual Nutri Plus®® and Dietpro 5i®® software systems in assessing nutrient intake in obese patients with type 2 diabetes mellitus who underwent a RYGB. | Prospective |
| da Silva et al. [44] | 2016 | Brazil | 27544005 | 80 | 46 | 88.8% women | 49.8 ± 9.3 | 2 × 24-h dietary recall | None | RYGB | Investigate factors associated with weight regain long after RYGB. | Retrospective |
| Dias et al. [45] | 2006 | Brazil | 16680324 | 40 | 42.5 ± 10.8 | 100% women | 51.9 ± 11.8 | 24-h dietary recall | None | RYGB | Systematically document nutrient intake at 3-month intervals, during the first 12 months after uncomplicated RYGB. | Prospective |
| Duffey et al. [46] | 2007 | United States | 18289566 | 45 | 47.0 ± 10.5 | 71% women | 49.5 ± 9.1 | Food record (1 day) | None | N/D | Evaluate baseline risk factors for stone formation in a group of morbidly obese patients presenting for gastric bypass surgery and the changes that may occur after bariatric surgery. | Cross-sectional |
| El Labban et al. [47] | 2015 | Lebanon | 25982803 | 60 | RYGB: 39.6 ± 11.3; SG: 33.0 ± 12.3 | 60% women | RYGB: 42.7 ± 5.2; SG: 41.2 ± 4.1 | FFQ + 3 × 24-h dietary recall | Yes | RYGB or SG | Compare dietary intake, food preferences, and gastro-intestinal symptoms in subjects with extreme obesity after RYGB and SG. | Cross-sectional |
| Ernst et al. [48] | 2009 | Germany | 19034589 | 121 | RYGB: 40.2 ± 1.5; GB: 44.0 ± 1.2 | 79% women | RYGB: 46.5 ± 0.7; GB: 44.6 ± 0.5 | FFQ | None | GBP (48) or GB (73) + 45 obese controls | Assesse dietary habits in patients who have underwent a bariatric surgery and compare their data with those of an obese as well as a nonobese control group. | Cross-sectional |
| Faria et al. [49] | 2014 | Brazil | 25409965 | 60 | N/D | 87% women | N/D | 3 × 24-h dietary recall | None | RYGB | Compare weight loss, consumption of macronutrients and the frequency of vomiting among patients who underwent RYGB with and without the placement of a constriction ring around the pouch. | Retrospective |
| Farias et al. [50] | 2020 | Brazil | 32200267 | 32 | 40.1 ± 10.1 | 94% women | 43.9 ± 1.1 | FFQ | Yes | RYGB | Analyze the contribution of unprocessed, processed, and ultra-processed foods 2 years after RYGB. | Prospective |
| Federico et al. [51] | 2016 | Italy | 27107092 | 28 | 26–63 | 71% women | Women: 48.6 ± 8.1; Men: 54.3 ± 18.5 | Food record (7 days) | None | BI | Evaluate the dietary intake, the nutritional status, as well as plasma levels of a number of gastrointestinal peptides that regulate food intake and fecal microbiota in severely obese patients and healthy non-obese control subjects and evaluate whether bariatric surgery affected gastrointestinal peptides plasma levels and fecal microbiota. | Prospective longitudinal |
| Forbes et al. [52] | 2016 | United-states | 26328533 | 18 | 36.6 ± 2.3 | 100% women | 44.0 ± 1.0 | Food record (3 days) | None | RYGB (13) or AGB (5) | Describe compositional changes in plasma phospholipids during 6 months following bariatric surgery procedures. | Longitudinal |
| Freeth et al. [53] | 2012 | United-states | 22714824 | 15 | 18–80 (min-max) | N/D | N/D | Food record (3 days) | None | RYGB (6) or GB (9) | Comprehensively analyze selenium intake before and after bariatric surgery while simultaneous looking at the serum selenium level and functional measurement of selenium. | Prospective longitudinal |
| Freeman et al. [54] | 2013 | Australia | 24743015 | 130 | Control: 47; AGB 46; RYGB 58; SG 50 | 68% women | Control 43.2; AGB 45.5; RYGB 42.4; SG 43.2 | Questionnaire + 24-h dietary recall | None | AGB, SG or RYGB | Assess food tolerance and diet quality in AGB, SG and RYGBP patients 2–4 years post-surgery, comparing findings with an obese control group. | Prospective, cross-sectional |
| Furet et al. [55] | 2010 | France | 20876719 | 30 | nDb: 42 ± 2; Db: 49 ± 5 | 90% women | nDb: 48.3 ± 1.6; Db: 45.4 ± 3.5 | Questionnaire | None | RYGB | Examine the association between gut microbiota changes and a range of body composition, metabolic, and inflammatory markers. | Prospective longitudinal |
| Furtado et al. [56] | 2018 | Brazil | 30307293 | 105 | Succes group 43.3 ± 11.4; Failure group 43.4 ± 10.7 | 84% women | SG 48.8 ± 8.4; Failure group 49.9 ± 6.6 | 24-h dietary recall + food record (3 days) + FFQ | None | RYGB | Analyse wheter feeding behavior, evaluated by caloric intake, dietary preferences and tolerance, can be considered as a determinant factor for weight loss in obese patients submitted to RYGB. | Cross-sectional |
| Gesquiere et al. [57] | 2017 | Belgium | 27591033 | 54 | 48 | 61% women | 40.4 | Food record (2 days) | None | RYGB | Study dietary and supplement intake of micronutrients before and after RYGB and examine the association between the total micronutrient intakes and status markers. | Prospective longitudinal |
| Gimenes et al. [58] | 2017 | Brazil | 28102495 | 25 | 35.7 | 100% women | 50.1 ± 6.5 | Food record (1 day) | None | RYGB | Evaluate nutritional and biochemical indicators of women who became pregnant after RYGB. | Retrospective |
| Giusti et al. [59] | 2015 | Switzerland | 26675775 | 16 | 39.4 ± 2.4 | 100% women | 44.1 ± 1.6 | Food record (7 days) | None | RYGB | Evaluate energy and macronutrient intakes, body composition, and the basal metabolic rate in obese female patients during the initial 3 y after an RYGB. | Observational |
| Gobato et al. [60] | 2014 | Brazil | 25264334 | 36 | 37.7 | 75% women | 44.2 | 24-h dietary recall | None | RYGB | Evaluate the nutritional status of minerals and vitamins and the food consumption in patients before and after RYGB. Evaluate the overall effect of RYGB by monitoring additional risk factors of related chronic diseases. | Prospective longitudinal |
| Gobato et al. [61] | 2018 | Brazil | 30306500 | 75 | 38 ± 10 | 89% women | 43.94 ± 5.89 | Food record (3 days) | None | RYGB | Evaluate the food intolerance after banded RYGB, correlating the data of food ingestion. | Observational, prospective |
| Golpaie et al. [62] | 2011 | Iran | 22266100 | 30 | 32.5 | 70% women | 44.1 ± 4.9 | Food record (3 days) | None | AGB (15) or (16) TGVP | Investigate the possible short-term effects of surgery on vaspin and other metabolic variables relevant to insulin sensitivity and evaluate the possible relationship between dietary intake and serum vaspin. | Longitudinal |
| Golzarand et al. [63] | 2018 | Iran | 30251098 | 43 | N/D | N/D | RYGB: 45.9 ± 4.6 SG: 39.5 ± 4.2 | Food record (3 days) | Yes | RYGB or SG | Compare the changes in body composition, dietary intake, and substrate oxidation 6 months post-sugery in obese patients who underwent RYGB and SG. | Prospective |
| Jastrzębska-Mierzyńska et al. [64] | 2012 | Poland | 23256020 | 27 | Women: 40.4 ± 13.9; Men: 39.6 ±12.7 | 68% women | W: 45.9 ± 6.8; M: 48.1 ± 7.7 | 24-h dietary recall | None | N/A | Assess dietary habits, nutritional status and biochemical parameters of blood in patients being prepared for different bariatric procedures. | Cross-sectional |
| Johnson et al. [65] | 2013 | Norway | 23110916 | 72 | 42.6 ± 11 | 69% women | 46.2 ± 5.9 | FFQ | Yes | RYGB | Compare changes in the dietary patterns of morbidly obese patients undergoing either laparoscopic gastric bypass surgery or a comprehensive lifestyle intervention program. | Interventional (clinical trial) |
| Kanerva et al. [66] | 2017 | Sweden | 28756049 | 1695 | 47.3 ± 5.9 | 69.8% women | 42.5 ± 4.5 | Questionnaire | Yes | LAGB OR VBG OR RYGB | Explore whether pre-surgical sociodemographic and lifestyle characteristics, together with the type of surgery, could predict 10-year changes in dietary intake following bariatric surgery. | Prospective, matched, non-randomized, surgical intervention trial |
| Kops et al. [67] | 2017 | Brazil | 28760427 | 120 | N/D | Adherent: 74.4% women; Non adherent 81.8% women | Adherent: 45.8 ± 6.8 Non adherents: 49.1 ± 8.1 | 24-h dietary recall (3×) | Yes | N/D | Evaluate the possible association between the Thr54 allele and anthropometric and lipid profile of severely obese indivieuals, taking into account the dietary intake of these participants. | Cross-sectional |
| Kruseman et al. [68] | 2010 | Switzerland | 20338278 | 141 | 40 | 93% women | 46 | Food record (4 days) | None | GBP | Document weight and body composition changes among patients after bariatric surgery and to assess whether dietary, behavior, or psychological factors were associated with long-term weight outcome. | Retrospective longitudinal |
| Laurenius et al. [69] | 2013 | Sweden | 23299713 | 43 | 43 ± 10 | 72% women | 44.3 ± 4.9 | Questionnaire | Yes | RYGB | Test the hypothesis that dietary energy density decreases after RYGB. | Longitudinal |
| Leite Faria et al. [70] | 2009 | Brazil | 18830780 | 75 | 36.8 ± 10.7 | 80% women | 43 ± 5.5 | Food record (4 days) | None | RYGB | Assess postoperative eating patterns, relating them to weight loss. | Cross-sectional |
| Le Roux et al. [71] | 2011 | Sweden | 21734019 | 16 | N/D | 69% women | N/D | Questionnaire | Yes | RYGB or VBG | Investigate how RYGB affects intake of and preference for high-fat food in an experimental (rat) study and within a trial setting (human). | Prospective |
| Ledoux et al. [72] | 2017 | France | 27943093 | 78 | 43 | 81% women | 44 | Food record (4 days) | None | RYGB, SG or AGB | Explore whether self-reported preoperative changes in dietary habits and physical activity during a multidisciplinary preparation were predictive of postoperative weight loss. | Interventional |
| Magno et al. [73] | 2014 | Brazil | 25409962 | 30 | W: 48.4 ± 12.9; M: 49.8 ± 8.1 | 73% women | 50.8 ± 14.5 | 24-h dietary recall | None | N/D | Evaluate the nutritional profile of the patients included into a multidisciplinary program for the treatment of severe obesity and bariatric presurgery. | Retrospective |
| Marin et al. [74] | 2017 | Brazil | 28421792 | 45 | 20–45 | 100% women | Group 1: 47.8; Groupe 2: 41.5 | Food record (3 days) | None | RYGB | Assess the effect of two micronutrient supplementation schemes on inflammation and iron metabolism in premenopausal women who had undergone RYGB surgery. | Prospective |
| Marques et Al. [75] | 2020 | Portugal | 31435901 | 17 | Symptomatic: 46.4 ± 1.7 Control: 42.1 ± 3.4 | 94% women | Symptomatic: 39.4 ± 1.8; Control 42.4 ± 1.2 | Food and symptom diary (FSD) | None | RYGB | Evaluate the influence of meal nutritional composition on interstitial fluid glucose profiles and symptom profile after RYGB. | Cross-sectional |
| McLean et al. [76] | 2018 | United States | 29100900 | 200 | 46.3 ± 8.5 | 100% women | 48.9 ± 5.8 | FFQ | None | RYGB, SG or LAGB | Identify usual dietary habits of black and white women seeking bariatric surgery and examine potential differences between these ethnic groups; to describe participants’plans to change dietary behaviors after surgery. | Cross-sectional |
| Melendez-Araùjo et al. [77] | 2012 | Brazil | 23054569 | 32 | 39 ± 10.6 | N/D | 41.9 ± 5.2 | 24-h dietary recall | None | RYGB | Evaluate the impact of intensive and standard nutritional interventions on body weight, energy intake, and eating quality. | Retrospective |
| Melo et al. [78] | 2017 | Brazil | 28724055 | 61 | 47.1 ± 9.9 | 84% women | 31.5 ± 6.0 | 3 × 24-h dietary recall | None | RYGB or BPDS | Evaluate parameters of bone and mineral metabolism after bariatric surgery. | Sectional, retrospective |
| Mercachita et al. [79] | 2014 | Portugal | 23955522 | 60 | 41.9 ± 12.2 | 65% women | 42.3 ± 6.7 | 24-h dietary recall | None | RYGB | Quantify the intake of micronutrients in patients that were submitted to RYGB, determine the micronutrients deficiencies, and verify if the recommended vitamin and mineral supplementation intake would prevent theses deficiencies. | Retrospective longitudinal |
| Miller et al. [80] | 2014 | United States | 24748474 | 17 | 47.3 ± 2.2 | 94% women | 53.6 ± 1.7 | Food record (4 days) | None | RYGB | Examine changes in macro- and micronutrients, food groups, and selected foods during 12-months of follow-up in post RYGB individuals. | Prospective |
| Mischler et al. [81] | 2015 | United states | 26806728 | 36 | 45 | 97% women | 32 | Food record (3 days) | None | RYGB | Explore the impact of dietary and supplemental sources of iron and absorptive factors on iron status. | Cross-sectional |
| Moizé et al. [82] | 2011 | Spain | 21298509 | 231 | 45.6 ± 9.9 | 72.3% women | 48.2 ± 7.8 | Food record (4 days) + 24-h dietary recall | None | N/D | Evaluate the dietetic intake and the prevalence of nutritional deficiencies in obese patients who are candidates for bariatric surgery. | Cross-sectional |
| Moizé et al. [83] | 2013 | Spain | 23438491 | 355 | SG = 46.4 ± 11.6; RYGB = 45.2 ± 10.6 | 75% women | SG = 51.6 ± 6.7; RYGB = 47.4 ± 6.0 | Food record (3 days) + 24-h dietary recall | None | RYGB or SG | Prospectively compare dietary changes and nutritional deficiencies in grade 3 obese patients 5 years after SG and RYGB. | Longitudinal, prospective, observational |
| Molin Netto et al. [84] | 2017 | Brazil | 27474230 | 41 | 39.4 ± 10.9 | 95% women | 44.6 ± 6.3 | FFQ | Yes | RYGB | Evaluate the early post-RYGB changes in the quality of eating patterns and their relationship to weight loss and metabolic parameters. | Longitudinal |
| Moore et al. [85] | 2015 | United-states | 25270794 | 22 | 41± 12 | 100% women | 46.7 ± 8 | 24-h dietary recall | None | RYGB (11) or SG (11) | Determine the response to 3 months of thiamin, B12, and folate supplementations. | Prospective observational |
| Nicoletti et al. [86] | 2013 | Brazil | 21978750 | 80 | 45 ± 11 | 81% women | 54 ± 8 | 24-h dietary recall | None | RYGB | Characterize the eating, anthropometric, and biochemical profile of obese candidates for bariatric surgery at a university hospital and assess their preoperative risk of nutritional deficiency. | Retrospective |
| Nicoletti et al. [87] | 2015 | Brazil | 25851774 | 72 | 42 ± 9 | 86% women | 53 ± 8 | 24-h dietary recall | None | RYGB | Evaluate the influence of red meat intolerance on the dietary pattern, biochemical indicators, and clinical symptoms after Roux-en-Y gastric bypass. | Retrospective |
| Nicoletti et al. [88] | 2016 | Brazil | 27256164 | 150 | 47.2 ± 10.5 | 80% women | 51.3 ± 7.3 | 24-h dietary recall | None | RYGB | Investigate the contribution of UCP2 gene variants on energy and macronutrients intake in a population after bariatric surgery. | Retrospective |
| Nicoletti et al. [89] | 2020 | Brazil | 33231819 | 65 | 47.2 ± 11.4 | 86% women | 35.5 ± 6.8 | 3 × 24-h dietary recall | None | RYGB or VBG | Investigate dietary habits and food intake during COVID-19 quarantine among patients who recently underwent bariatric surgery. | Cross-sectional |
| Nonino et al. [90] | 2019 | Brazil | 31644673 | 441 | 44 ± 10 | 82.7% women | 50.5 ± 8.0 | 24-h dietary recall | N/D | RYGB | Investigate nutritional status in 10 years follow-up. | Longitudinal retrospective |
| Nosso et al. [91] | 2017 | Italy | 28969883 | 22 | 50 ± 9 | 54.5% women | 31 ± 6 | Food record (7 days) | None | RYGB (11) or SG (11) | Evaluate glycemic variability and oxidative stress in patients who achieved type 2 diabetes remission after bariatric surgery. | Cross-sectional |
| Novais et al. [92] | 2012 | Brazil | 22652372 | 141 | 44 ± 9 | 100% women | 45.9 ± 16.4 | 2 × 24-h dietary recall | Yes | RYGB | Assess the adequacy of food intake in women two or more years after bariatric surgery according to the excess weight lost. | Cross-sectional |
| Olbers et al. [93] | 2006 | Sweden | 17060764 | 75 | GB: 37.4 ± 0.4 VGB: 37.4 ± 0.5 | 50% women | GB: 42.3 ± 4.5; VBG: 42.6 ± 4.2 | Questionnaire | Yes | RYGB(36) or VBG(39) | Evaluate the effect of dietary intake of on body composition and energy expenditure after sugery. | Prospective longitudinal |
| Ortega et al. [94] | 2012 | Spain | 22722236 | 107 | 41.8 ± 9.8 | 79% women | 50.7 ± 11.8 | Food record (3 days) | None | RYGB | Analyze the likelihood of patients undergoing RYGB to recover a normal daily food intake, and the possible influence of dietary and exercise habits on long-term weight loss. | Cross-sectional |
| Papalazarou et al. [95] | 2010 | Greece | 19834466 | 30 | Usual care: 33.4 ± 2. Lifestyle intervention: 32.7 ± 1.6 | 100% women | Usual Care: 49.8 ± 1.6. Lifestyle intervention:48.5 ± 2. | 24-h dietary recall | None | VBG | Evaluate the 3 year effects of a lifestyle intervention on weight loss and maintenance, dietary, and physical activity habits and eating behavior of patients following VBG. | Cross-sectional |
| Pinto et al. [96] | 2019 | Brazil | 31376133 | 51 | 39.34 ± 9.38 | 68.7% women | 43.0 ± 5.7 | 24-h dietary recall | None | RYGB | Evaluate changes in dietary intake and predictive factors of obesity remission in the first 12 months after RYGB. | Observational, prospective |
| Quesada et al. [97] | 2014 | Brazil | 24724773 | 100 | 33.3 ± 6.08 | 100% women | 45.75 ± 6.05 | 24-h dietary recall | Yes | Gastroplasty | Test 6 variations in the Goldberg equation to evaluate underreporting among obese women on a bariatric surgery waiting list. | Cross-sectional |
| Raatz [98] | 2020 | United States | 32418771 | 72 | 44.1 ± 11.7 | 81% women | 47.3 ± 6.9 | 2 × 24-h dietary recall | None | RYGB | Evaluate the reported macro- and micronutrient intake of adults who underwent RYGB over 7 years after surgery. | Longitudinal |
| Reid et al. [99] | 2016 | Canada | 27744735 | 27 | 53.2 ± 8.3 | 89% women | 33.8 ± 8.1 | Food record (3 days) | Yes | RYGB | Compare the differences in dietary intake (caloric and macronutrient) between individuals who have maintained weight loss (maintainers) to those who have regained their lost weight (regainers) on average 12 years after RYGB and examine behaviours/habits between weight regainers and maintainers. | Retrospective |
| Ruiz-Lozano et al. [100] | 2016 | Spain | 26948400 | 270 | 52 ± 11 | 82% women | 46.5 ± 6 | Food record (4 days) | None | RYGB (203) or SG (67) | Evaluate if food timing is associated with the weight loss effectiveness following bariatric surgery. | Observational |
| Ruiz-Tovar et al. [101] | 2017 | Spain | 29250751 | 93 | 45.7 ± 10.8 | 78% women | 46.4 ± 7.9 | FFQ | None | SG | Evaluate the changes in the frequency intake of different foods in patients undergoing sleeve gastrectomy and following a strict dietary control. | Prospective, observational |
| Sanchez et al. [102] | 2016 | Chile | 26108638 | 103 | 36 ± 9.6 | 100% women | 43.1 ±5.3 | FFQ | None | RYGB or SG | Evaluate dietary intake and nutritional status of various micronutrients in morbidly obese women prior to bariatric surgery. | Cross-sectional |
| Sarwer et al. [103] | 2012 | United-states | 22551576 | 84 | 42 ± 9.9 | 63% women | 51.6 ± 9.2 | FFQ | Yes | RYGB (62) or AGB (16) | Evaluate the impact of dietary counselling on weight loss, dietary intake and eating behaviour after surgery. | Interventional |
| Sarwer et al. [104] | 2008 | United States | 18586571 | 200 | 43.2 ± 9.8 | 82% women | 52.1 ± 9.3 | FFQ | Yes | RYGB | Investigate the relationship between preoperative eating behavior, postoperative dietary adherence and weight loss following gastric bypass surgery. | Prospective |
| Schoemacher et al. [105] | 2019 | the Netherlands | 31313238 | 135 | 46.5 ± 9.5 | 83.7% women | 44.6 ± 6.7 | Food record (2 days) | None | RYGB or SG | Explore the relationship between total energy intake and % total body weight loss over a period of 4 years post-surgery. | Longitudinal, observational |
| Seki et al. [106] | 2019 | Japan | 30711445 | 46 | 64.5 ± 8.1 | 47% women | 31.7 ± 2.2 | 24-h dietary recall | None | DBP | Investigate the impact of metabolic surgery for diabetic patients with body mass index < 35 kg/m2 on health-related quality of life, food tolerance, and food satisfaction in a single institution. | Retrospective |
| Shah et al. [107] | 2013 | United States | 24113734 | 23 | 49.3 ± 10.5 | 91% women | 41.1 ± 6.2 | Food record (3 days) | None | GB | Examine whether dietary counseling improves micronutrient and macronutrient intakes in GB surgery patients. | Prospective |
| Shai et al. [108] | 2002 | Israel | 12568186 | 75 | 34.4 ± 9.4 | 81% women | 41.4 ± 6.0 | FFQ | None | VBG | Evaluate the long-term nutritional changes that occur in VBG patients compared with their nutrition before surgery. | Retrospective |
| Soares et al. [109] | 2014 | Brazil | 24500225 | 172 | 42.4 ± 9.0 | 92.5% women | 46.9 ± 6.0 | FFQ | None | RYGB | Evaluate the life habits and diet quality of patients who have undergone bariatric surgery (who have been recovering for at least 6 months) based on the specific food pyramid. | Retrospective |
| Solga et al. [110] | 2004 | United-states | 15573908 | 70 | 44 ± 9 | 89% women | 55 (median) | 24-h dietary recall | None | RYGB | Determine whether overall calorie intake and diet composition are associated with the severity of NAFLD histopathology. | Retrospective |
| Sovik et al. [111] | 2013 | Norway/Sweden | 22951078 | 60 | GB: 35.2 ± 7 DS: 36.1 ± 5.26 | 70% women | GB: 54.8 ± 3.24 DS: 55.2 ± 3.49 | Food record (4 days) | None | BPD (29) or RYGB (31) | Evaluate the gastrointestinal side effects, caloric intake, and changes in obesity-specific quality of life 2 years after surgery. | Prospective longitudinal |
| Torres et al. [112] | 2012 | Brazil | 22688468 | 44 | 45.4 ± 9.5 | 100% women | 31.3 ± 4.8 | Food record (4 days) | None | RYGB | Evaluate the nutrient intake of women who had undergone RYGB surgery. | Cross-sectional |
| Trostler et al. [113] | 1995 | Israel | 10733792 | 55 | RYGB: M: 41± 4/W: 32 ± 4 VBG: M: 32 ± 3/W: 37 ± 2 | 73% women | RYGB: 43 ± 4/W:43 ± 6 VGB: M:45± 7/W: 42± 8 | FFQ + 24-h dietary recall | None | RYGB (19) or VBG (36) | Compare 2 surgeries with a low energy diet and dietary counseling. Compare the food intake pattern and nutritional composition of the food consumed over time. | Longitudinal |
| Ullrich et al. [114] | 2013 | Switzerland | 22941334 | 44 | N/D | N/D | 47.3 ± 1.1 | FFQ | None | RYGB | Investigate changes in the hedonic hunger and dietary habits after RYGB surgery | Longitudinal |
| Verger et al. [115] | 2016 | France | 26205215 | 52 | RYGB: 43.5; SG:41.0 | 67% women | RYGB: 45.5; SG:43.2 | 24-h dietary recall | Yes | RYGB (22) or SG (30) | Analyze food restriction effects on the nutritional adequacy of the diet, on macro- and micronutrient intake evolution, as well as their consequences in terms of bioclinical evolution and micronutrient serum level post surgery. | Retrospective |
| Vieira et al. [116] | 2019 | Brazil | 30565102 | 40 | stable weight 38± 7; weight regain 42 ± 11 | 100% women | SW 41.7 ± 6.5; WR 41.3 ± 3.5 | 24-h dietary recall + 2 food record (1 day) | None | RYGB | Investigate the perception of hunger and satiety and its association with nutrient intake in women who regain weight in the postoperative period after bariatric surgery. | Cross-sectional |
| Vieira et al. [117] | 2020 | Brazil | 32022115 | 60 | 38.8 ± 9.6 | 78% women | 47.3 ± 6.9 | FFQ | None | N/D | Evaluate the association of food consumption with nutritional status, physical activity and sociodemographic factors in the bariatric surgery period preoperative | Cross-sectional |
| Vinolas et al. [118] | 2019 | France | 31102207 | 57 | RYGB: 42.9 ± 11 SG: 45.2 ± 9.2 | N/D | RYGB: 46.8 ± 6.9 SG: 44.1 ± 9.4 | Food record (7 days) | None | RYGB or SG | Evaluate nutritional status, micro- and macronutrient intake, and oral hydration in patients before and regularly during 1 year after RYGB and SG. | Retrospective |
| Wardé-Kamar et al. [119] | 2004 | United States | 15479596 | 73 | 46 ± 11 | 93% women | 54 ± 12 | 24-h dietary recall | None | RYGB | Investigate self-reported food intake, diet composition and meal patterns, in relation to long-term weight loss outcomes after RYGB. | Retrospective, longitudinal |
| Werling et al. [120] | 2013 | Sweden | 23573244 | 14 | GB: 59.7; VBG: 50.2 | 100% women | GB: 30.8; VBG: 35.0 | Questionnaire | Yes | VGB or GB | Investigate alterations in postprandial EE after gastric bypass and VBG in humans. | Cross-sectional |
| Wolf et al. [121] | 2015 | Germany | 25980331 | 43 | 44 ± 12 | 63% women | 52.6 ± 10.5 | Food record (3 days) | Yes | N/D | Assess the status of micronutrients in morbidly obese patients seeking bariatric surgery and to correlate extra-cellular nutrient levels with the corresponding nutrient intake. | Cross-sectional |
| Zaparolli et al. [122] | 2018 | Brazil | 29972395 | 106 | 48 (20–64y) | 90.5% women | 39.6 (32.8–67.8) | 24-h dietary recall | None | RYGB | Analyze food intake evolution during the first postoperative year of Roux-en-y gastric bypass in patients with type 2 diabetes or glycemic alteration. | Retrospective, longitudinal, observational |
| Ziadlou et al. [123] | 2020 | Iran | 33046020 | 58 | 37 ± 8 | 71% women | 44 ± 6 | 3 × 24-h dietary recall | None | RYGB or SG | Assess the adequacy of dietary nutrient intakes at 6th and 12th month after bariatric surgery. | Longitudinal |
| Author | Surgery Type | Reference Method | Validation | Conclusions About Validity | ||
|---|---|---|---|---|---|---|
| Bariatric Population | Pre-and/or Post-Surgery | Directly in the Study | ||||
| Food records (FR) | ||||||
| Bobbioni-Harsch et al. [27] | RYGB | Indirect calorimetry (resting energy expenditure; glucose, lipid and protein oxidation) | Yes | Pre-surgery | Yes | The degree of mis-report averages −17% of the evaluated energy requirements, in pre-surgery conditions; it represents a reasonable degree of inaccuracy [27]. |
| Golzarand et al. [63] | RYGB or SG | Indirect calorimetry (resting metabolic rate, glucose, lipid and protein oxidation) | Yes | Pre- & post-surgery | Yes | In accordance with dietary intake reduction, protein and carbohydrate oxidation significantly decreased in both procedures post-surgery, while fat oxidation increased, but was not significant. |
| Reid et al. [99] | RYGB | 9-days food record (energy, macro and micronutrients) | No | No | Relative validity of 3-days FR appears to be acceptable as dietary assessment tool [124]. | |
| Wolf et al. [121] | N/A | Correlation with vitamin A, D, E and C plasmatic values | Yes | Pre-surgery | Yes | No correlations were found between serum/plasma concentrations and nutritional intake nor associations between low concentrations and inadequate intakes. |
| 24-h dietary recall (24HR) | ||||||
| Aron-Wisnewsky et al. [23] | RYGB or AGB | 24HR conducted by a dietitian (food consumption, energy and macro- and micronutrient intakes) | No | No | Agreement between the two methods was high, although it may have been overestimated because the two assessments were consecutives to one another. The tool may be highly advantageous for large population-based surveys [125]. | |
| Carvalho et al. [32] | RYGB or SG | Compared to Behavioral Risk Factor Surveillance System’s Fruit and Vegetable Consumption Module and the National Cancer Institute’s Percentage Energy from Fat Screener. | No | No | Validity of brief dietary intake measures may vary by demographic characteristics of the sample. Additional measurement work may be needed to accurately measure dietary intake in obese African-American women [126]. | |
| Kops et al. [67] | N/D | 24-h urine sample (urinary urea to assess protein intake) | Yes | Pre-surgery | Yes | The 24HR was accepted as appropriate. Only 37.4% of patients gave an accurate record; another 37.4% underrreported, and 25.2% overreported. |
| Novais et al. [92] | RYGB | 3-days FR (energy and nutrients) | Yes | Post-surgery | Yes | The agreement between the two methods (r = 0.91 to 0.98) evidenced low variability of the meals consumed by the group. |
| Quesada et al. [97] | GP | Indirect calorimetry (resting metabolic rate, energy requirement) | Yes | Pre-surgery | Yes | Comparing the results obtained for the modified Goldberg equations in this study, there was considerable variation in the proportion of underreporting (55% to 97%). |
| Verger et al. [115] | RYGB or SG | Indirect calorimetry (basal metabolic rate) | Yes | Pre- & post-surgery | Yes | Values revealed that patients from both groups underreported their caloric intake by 8% pre-surgery. |
| Food frequency questionnaire (FFQ) | ||||||
| Amundsen et al. [18] | GB | Doubly labelled water (total energy expenditure) | No | No | The data showed that there was substantial variability in the accuracy of the FFQ at the individual level. Furthermore, the results showed that the questionnaire was more accurate for groups than individuals [127]. | |
| Benaiges et al. [26] | RYGB (43%) | 3-day FR (dietary intakes) | No | No | A reasonable relative validity of the FFQ and 3-day FR for estimating nutrient intake was found [128]. | |
| Chou et al. [34] | SG | 24HR (energy and macronutrients intakes) | Yes | Post-surgery | Yes | The energy intake according to the dietary questionnaire was 1230 kcal/day 5 years after LSG, and the 24HR method reported a daily energy intake of approximately 1083 kcal/day. |
| Farias et al. [50] | RYGB | 3x 24HR (energy and macronutrients intakes) | No | No | Food consumption reports of overweight individuals tend to be underestimated. Despite its limitations, FFQ could be used in epidemiological studies to assess the regular food consumption of overweight individuals [129]. | |
| Johnson et al. [65] | RYGB | 14-day FR (energy from fat and sugar) and correlation of fatty acids and Alpha-tocopherol in adipose tissue with serum | No | No | On average, 39% of the men were classified in the same quartile with the two methods, and 3% in the opposite quartile. Very-long chain n-3 fatty acids in adipose tissue and total serum lipids reflect the dietary intake of very-long-chain n-3 fatty acids to the same degree. No associations were observed between intake of alpha-tocopherol and concentration in adipose tissue and serum [130]. | |
| Molin Netto et al. [84] | RYGB | 3 × 24HR (energy and macronutrients intakes) | No | No | Idem Farias et al. 2020 [129]. | |
| Sarwer et al. 2012 [103] | RYGB or AGB | 4 and 7-day FR (energy and macronutrients intakes) | No | No | Correlations between questionnaire and FR for percent of energy from fat were 0.67 and 0.65 respectively in the two groups; most correlations were similar to those achievable by a single 4-day FR [131]. | |
| Sarwer et al. 2008 [104] | RYGB | 4 and 7-day FR (energy and macronutrients intakes) | No | No | Idem Sarwer et al. 2012 [131]. | |
| Questionnaires | ||||||
| Kanerva et al. [66] | LAGB or VBG or RYGB | 4-day FR, 24-h energy expenditure and nitrogen excretion (nutrient intake, basal matabolic rate) | No | No | People with obesity reported energy and protein intakes 35% higher with the questionnaire compared with FR and nitrogen excretion [132]. | |
| Laurenius et al. [69] | RYGB | 4-day FR, 24-h energy expenditure and nitrogen excretion (nutrient intake, basal matabolic rate) | No | No | Idem Kaverna et al. 2017 [132]. | |
| Le Roux et al. [71] | RYGB or VBG | 4-day FR, 24-h energy expenditure and nitrogen excretion (nutrient intake, basal matabolic rate) | No | No | Idem Kaverna et al. 2017 [132]. | |
| Olbers et al. [93] | RYGB or VBG | 4-day FR, 24-h energy expenditure and nitrogen excretion (nutrient intake, basal matabolic rate) | No | No | Idem Kaverna et al. 2017 [132]. | |
| Werling et al. [120] | GB or VBG | 4-day FR, 24-h energy expenditure and nitrogen excretion (nutrient intake, basal matabolic rate) | No | No | Idem Kaverna et al. 2017 [132]. | |
| Other dietary assessment methods | ||||||
| Al-Ozairi et al. [17] | SG | 24HR by a dietitian (energy, macronutrients, fiber, total fat, saturated fat, mono- polyinsaturated fat, cholesterol and sodium) | Yes | Post-surgery | Yes | After SG, patients reported higher total energy intake and energy intake from carbohydrates compared to estimations using photographs. Digital photography appears reliable and accurate in adults in measuring energy intake in a cafeteria setting. |
| Mixed methods | ||||||
| Brolin et al. [28] | VBG or RYGB | 1 week FFQ (energy, protein, carbohydrate and fat intake) | Yes | Pre- & post-surgery | Yes | Multiple tools were used to obtain a mean of energy intake and macronutrients. |
| Casagrande et al. [33] | RYGB | FFQ + 24HR (total energy, macro and micronutrients) | Yes | Pre-surgery | Yes | The FFQ underestimated total energy value intake as compared with the 24HR. Protein and lipid intakes were lower if evaluated by the FFQ as compared to the 24HR. Calcium intake was higher when evaluated by the FFQ as compared with the 24HR. |
| El Labban [47] | RYGB or SG | N/D | No | No | N/D | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legault, M.; Leblanc, V.; Marchand, G.B.; Iceta, S.; Drolet-Labelle, V.; Lemieux, S.; Lamarche, B.; Michaud, A. Evaluation of Dietary Assessment Tools Used in Bariatric Population. Nutrients 2021, 13, 2250. https://doi.org/10.3390/nu13072250
Legault M, Leblanc V, Marchand GB, Iceta S, Drolet-Labelle V, Lemieux S, Lamarche B, Michaud A. Evaluation of Dietary Assessment Tools Used in Bariatric Population. Nutrients. 2021; 13(7):2250. https://doi.org/10.3390/nu13072250
Chicago/Turabian StyleLegault, Marianne, Vicky Leblanc, Geneviève B. Marchand, Sylvain Iceta, Virginie Drolet-Labelle, Simone Lemieux, Benoît Lamarche, and Andréanne Michaud. 2021. "Evaluation of Dietary Assessment Tools Used in Bariatric Population" Nutrients 13, no. 7: 2250. https://doi.org/10.3390/nu13072250
APA StyleLegault, M., Leblanc, V., Marchand, G. B., Iceta, S., Drolet-Labelle, V., Lemieux, S., Lamarche, B., & Michaud, A. (2021). Evaluation of Dietary Assessment Tools Used in Bariatric Population. Nutrients, 13(7), 2250. https://doi.org/10.3390/nu13072250








