Pancreatic β Cells Inhibit Glucagon Secretion from α Cells: An In Vitro Demonstration of α–β Cell Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Buffers
2.2. Culture of α-TC1-6 and MIN-6 Cells
2.3. Experimental Set-Up
2.4. Glucagon Secretion and Insulin Secretion
2.5. Morphology and Immunocytochemistry
2.6. Quantitative RT-PCR
2.6.1. RNA Isolation
2.6.2. Reverse Transcription Reaction
2.6.3. Preamplification
2.7. Viability of α-TC1-6 Cells
2.8. Data and Statistical Analyses
3. Results
3.1. GSIS of Mono- and Co-Cultured MIN-6 and α-TC1-6 Cells
3.2. Glucagon Secretion of Mono- and Co-Cultured MIN-6 and α-TC1-6 Cells
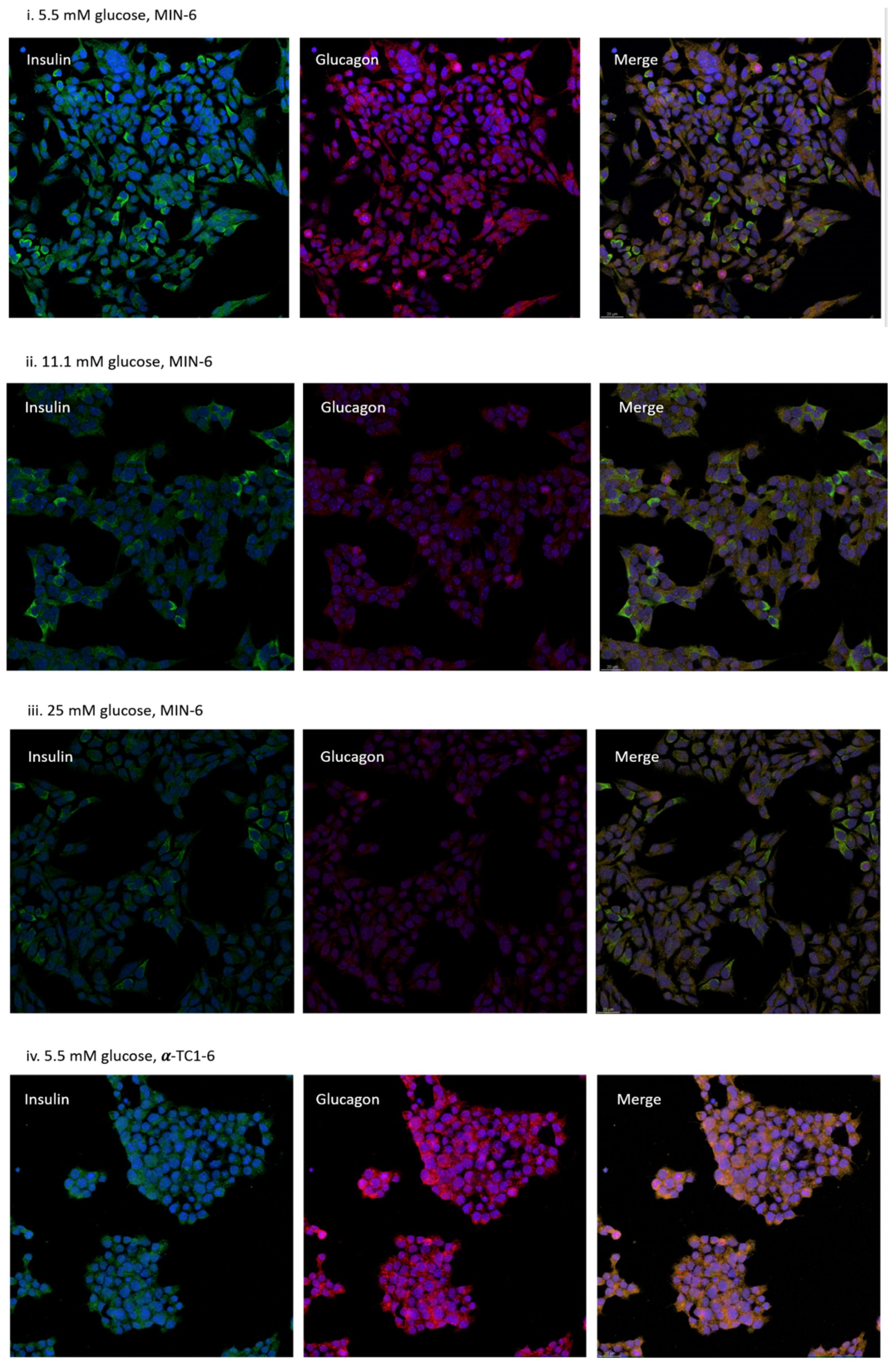
3.3. Distribution of MIN-6 and α-TC1-6 Cells in Co-Cultured System
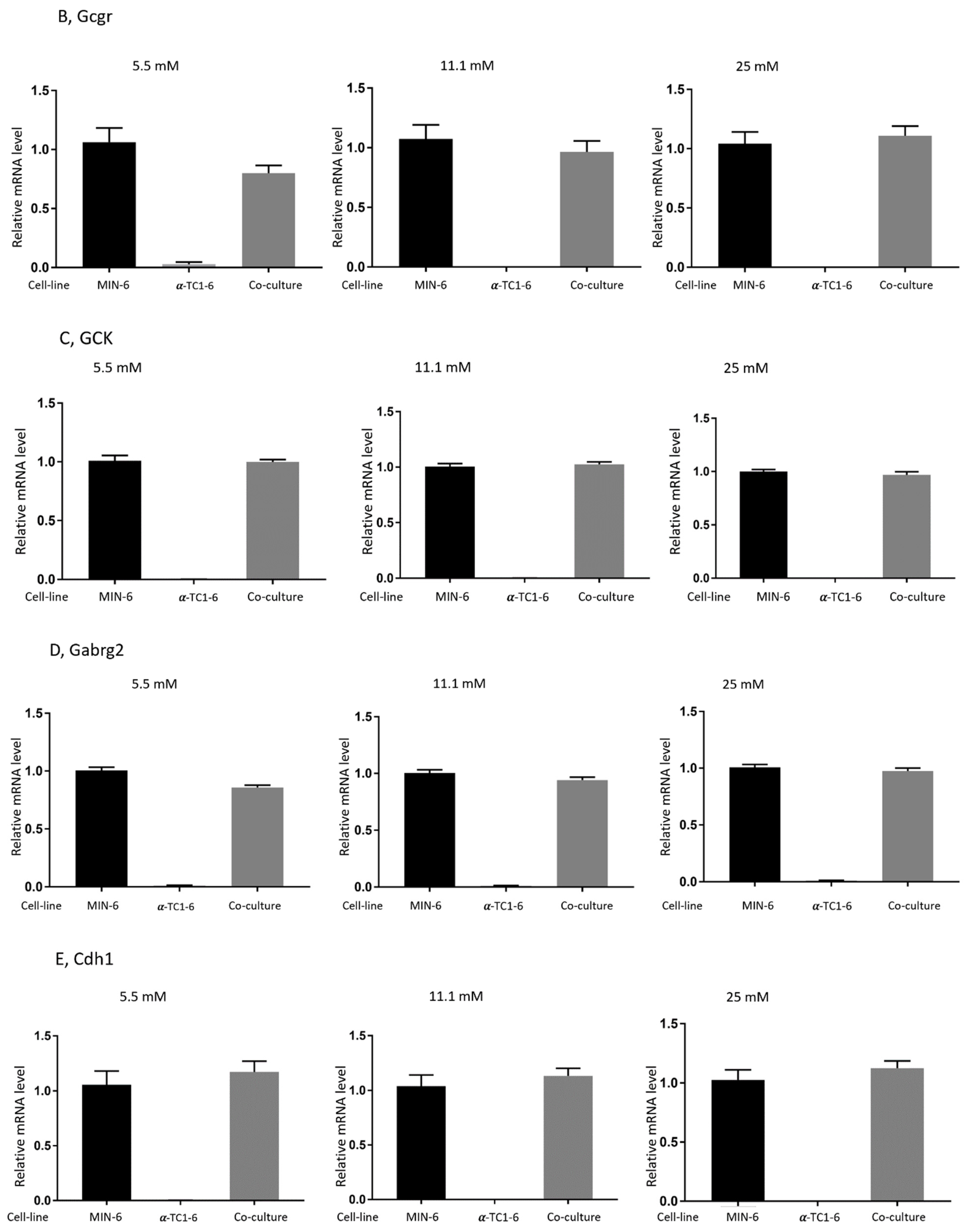
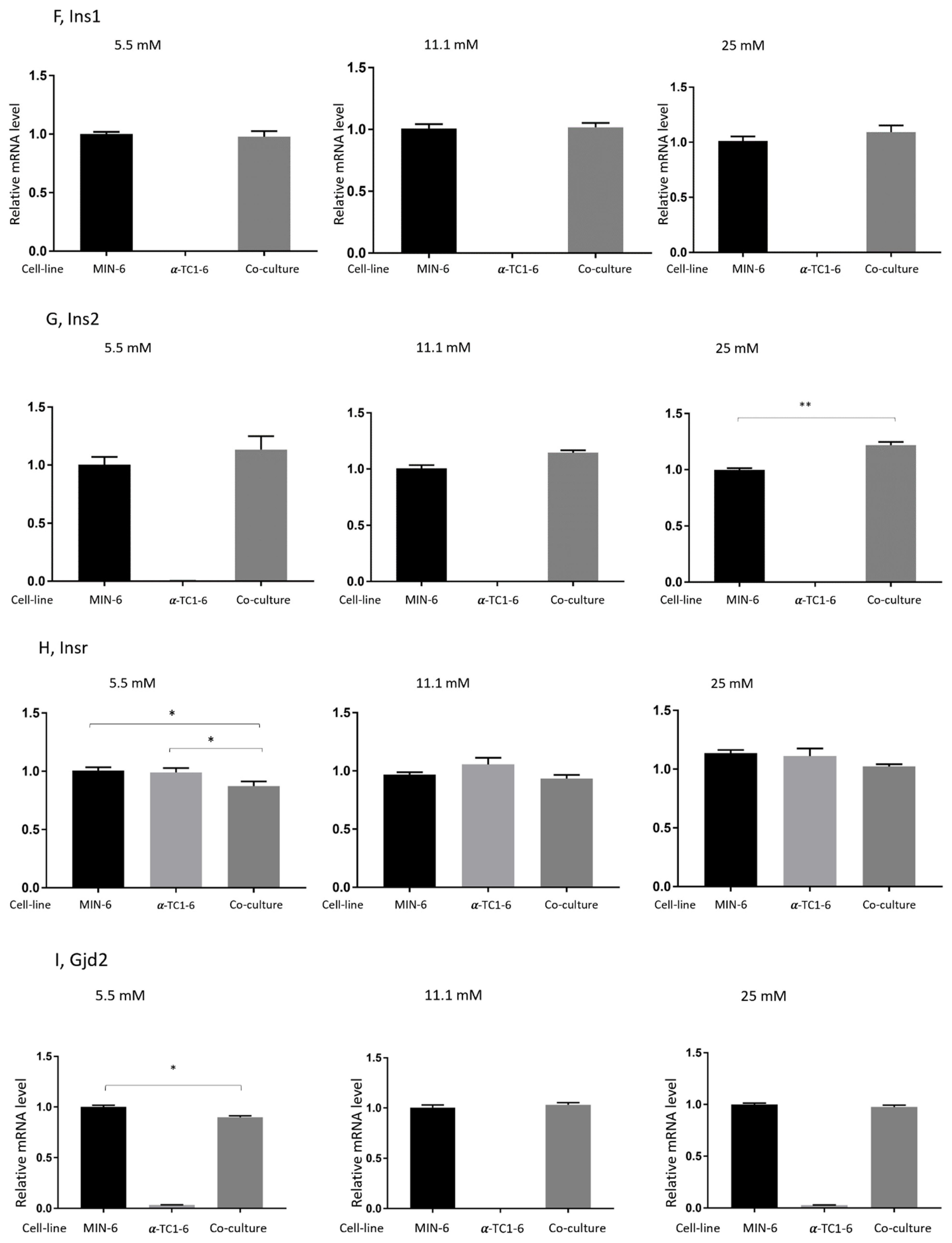
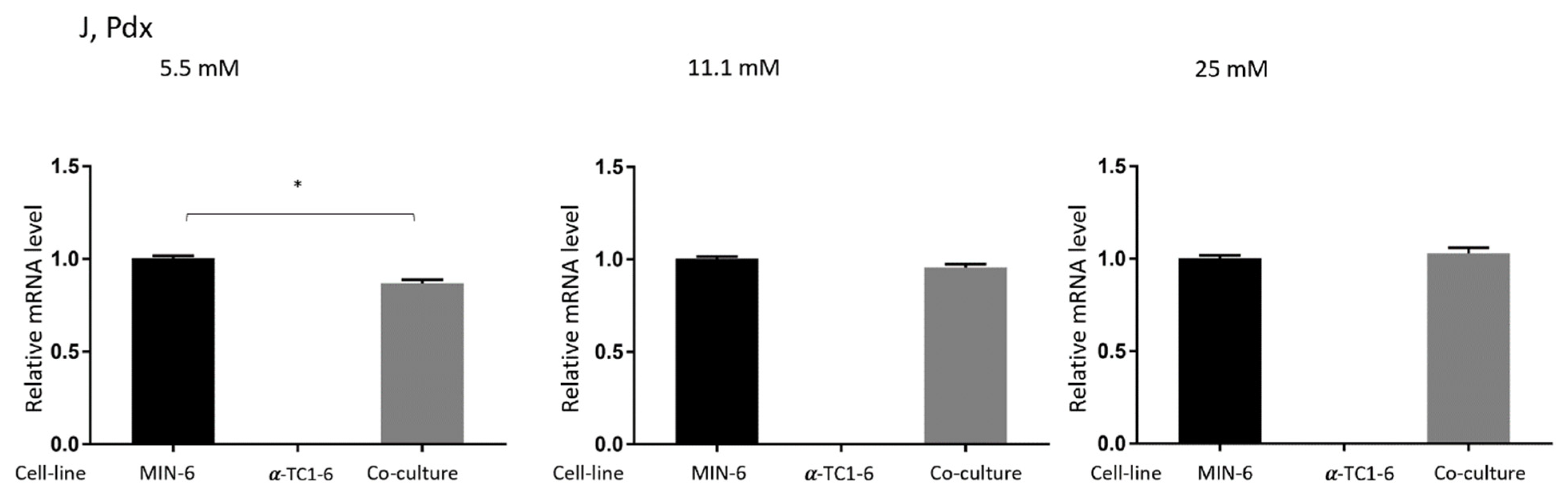
3.4. Effects on Gene Expression in Mono- or Co-Cultured MIN-6 and α-TC1-6 Cells
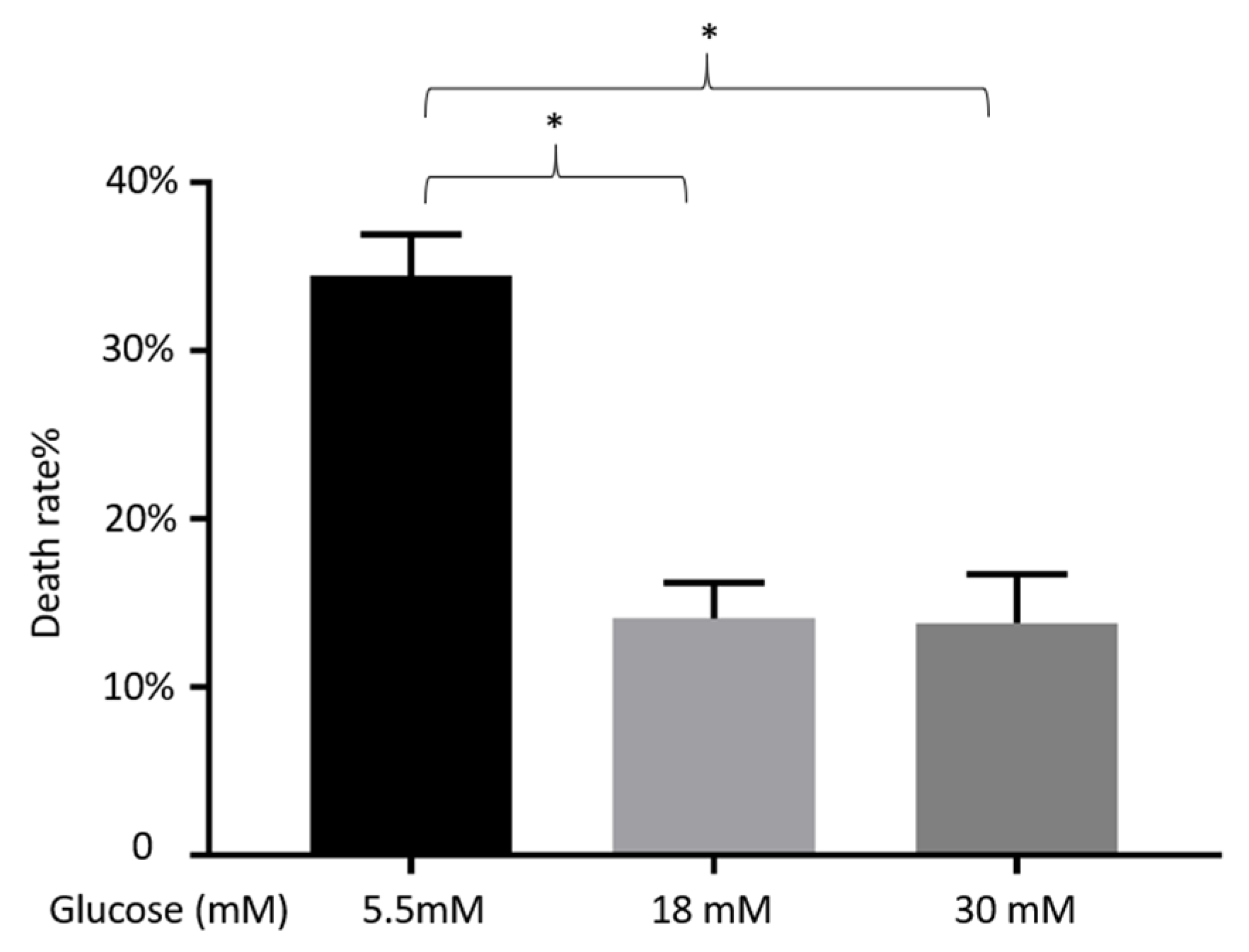
3.5. Impact of Different Concentrations of Glucose on the Viability of α-TC1-6 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabrera, O.; Berman, D.M.; Norma, S.K.; Kenyon, C.R.; Berggren, P.-O. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. USA 2006, 103, 2334–2339. [Google Scholar] [CrossRef] [PubMed]
- Donald, J.S.; Abraham, K.; Kevin, M.; Manami, H. Pancreatic islet plasticity: Interspecies comparison of islet architecture and composition. Islets 2010, 2, 135–145. [Google Scholar]
- Elayat, A.A.; el-Naggar, M.M.; Tahir, M. An immunocytochemical and morphometric study of the rat pancreatic islets. J. Anat. 1995, 186 Pt 3, 629–637. [Google Scholar]
- Orci, L.; Unger, R.H. Functional subdivision of islets of Langerhans and possible role of D cells. Lancet 1975, 2, 1243–1244. [Google Scholar] [CrossRef]
- Samols, E.; Bonner-Weir, S.; Weir, G.C. Intra-islet insulin-glucagon-somatostatin relationships. Clin. Endocrinol. Metab. 1986, 15, 33–58. [Google Scholar] [CrossRef]
- Da Silva Xavier, G. The Cells of the Islets of Langerhans. J. Clin. Med. 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Bavamian, S.; Klee, A.P.; Britan, C.P.; Caille, J.D.; Cancela, A.C.; Meda, P. Islet-cell-to-cell communication as basis for normal insulin secretion. Diabetes Obes. Metab. 2007, 9 (Suppl. 2), 118–132. [Google Scholar] [CrossRef]
- Aamodt, K.I.; Powers, A.C. Signals in the pancreatic islet microenvironment influence beta-cell proliferation. Diabetes Obes. Metab. 2017, 19 (Suppl. 1), 124–136. [Google Scholar] [CrossRef] [PubMed]
- Otter, S.; Lammert, E. Exciting Times for Pancreatic Islets: Glutamate Signaling in Endocrine Cells. Trends Endocrinol. Metab. 2016, 27, 177–188. [Google Scholar] [CrossRef]
- Bonaventura, M.M.; Catalano, A.P.; Chamson-Reig, E.; Arany, D.; Hill, B.; Bettler, F.; Saravia, C.; Libertun, C. GABAB receptors and glucose homeostasis: Evaluation in GABAB receptor knockout mice. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E157–E167. [Google Scholar] [CrossRef]
- Di Cairano, E.S.; Stefania, M.; Paola, M.; Vellea, F.S.; Michela, C.; Alberto, D.; Franco, F.; Carla, P. Neurotransmitters and Neuropeptides: New Players in the Control of Islet of Langerhans’ Cell Mass and Function. J. Cell Physiol. 2016, 231, 756–767. [Google Scholar] [CrossRef]
- Rorsman, P.; Per-Olof, B.; Krister, B.; Hans, E.; Hanns, M.; Claes-Göran, Ö.; Paul, A.S. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 1989, 341, 233–236. [Google Scholar] [CrossRef]
- Braun, M.; Reshma, R.; Martin, B.; Anne, C.; Jonathan, N.W.; Paul, R.J.; Patrik, R. Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes 2010, 59, 1694–1701. [Google Scholar] [CrossRef]
- Street, C.N.; Jonathan, R.T.; James, S.; Sharleen, I.; Ray, V.R.; Edmond, A.R.; James, G.; Tatsuya, K.; Jose, A.; Toshiaki, T.; et al. Islet graft assessment in the Edmonton Protocol: Implications for predicting long-term clinical outcome. Diabetes 2004, 53, 3107–3114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakuraba, H.; Mizukami, H.; Yagihashi, N.; Wada, R.; Hanyu, C.; Yagihashi, S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 2002, 45, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Orci, L.; Danielle, B.; Mariella, R.; Yolande, S.; Francine, M. Pancreatic polypeptide and glucagon: Non-random distribution in pancreatic islets. Life Sci. 1976, 19, 1811–1815. [Google Scholar] [CrossRef]
- Rahier, J.; Goebbels, R.M.; Henquin, J.C. Cellular composition of the human diabetic pancreas. Diabetologia 1983, 24, 366–371. [Google Scholar] [CrossRef]
- Geary, N. Postprandial Suppression of Glucagon Secretion: A Puzzlement. Diabetes 2017, 66, 1123–1125. [Google Scholar] [CrossRef]
- Brereton, H.; Melanie, J.C.; Shantam, J.P.; Peter, M.J. Islet alpha-cells do not influence insulin secretion from beta-cells through cell-cell contact. Endocrine 2007, 31, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; McClenaghan, N.H.; Flatt, P.R. Role of islet structure and cellular interactions in the control of insulin secretion. Islets 2011, 3, 41–47. [Google Scholar] [CrossRef]
- Hamaguchi, K.; Naoko, U.; Ryosaburo, T.; Hironobu, Y.; Toshiie, S. Cellular interaction between mouse pancreatic alpha-cell and beta-cell lines: Possible contact-dependent inhibition of insulin secretion. Exp. Biol. Med. 2003, 228, 1227–1233. [Google Scholar] [CrossRef]
- Kelly, C.; Hong, G.P.; Jane, T.M.; Peter, R.F.; Neville, H.M. The role of glucagon- and somatostatin-secreting cells in the regulation of insulin release and beta-cell function in heterotypic pseudoislets. Diabetes Metab. Res. Rev. 2010, 26, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Brock, B.; Gregersen, S.; Kristensen, K.; Thomsen, L.; Buschard, K.; Kofod, K. The insulinotropic effect of endothelin-1 is mediated by glucagon release from the islet alpha cells. Diabetologia 1999, 42, 1302–1307. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Traub, S.; Meier, D.T.; Schulze, F.; Dror, E.; Nordmann, T.M.; Goetz, N.; Koch, N.; Dalmas, E.; Stawiski, M.; Makshana, V.; et al. Pancreatic alpha Cell-Derived Glucagon-Related Peptides Are Required for beta Cell Adaptation and Glucose Homeostasis. Cell Rep 2017, 18, 3192–3203. [Google Scholar] [CrossRef]
- Bosco, D.; Orci, L.; Meda, P. Homologous but not heterologous contact increases the insulin secretion of individual pancreatic B-cells. Exp. Cell Res. 1989, 184, 72–80. [Google Scholar] [CrossRef]
- Rodriguez-Diaz, R.; Molano, R.D.; Weitz, J.R.; Abdulreda, M.H.; Berman, D.M.; Leibiger, B.; Leibiger, I.B.; Kenyon, N.S.; Ricordi, C.; Pileggi, A.; et al. Paracrine Interactions within the Pancreatic Islet Determine the Glycemic Set Point. Cell Metab. 2018, 27, 549–558.e4. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, A.E.; Svendsen, B.; Lam, B.Y.H.; Yeo, G.S.H.; Holst, J.J.; Reimann, F.; Gribble, F.M. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia 2016, 59, 2156–2165. [Google Scholar] [CrossRef]
- Huypens, P.; Ling, Z.; Pipeleers, D.; Schuit, F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 2000, 43, 1012–1019. [Google Scholar] [CrossRef]
- Ravier, M.A.; Rutter, G.A. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes 2005, 54, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, D.; Kurpad, A.J.; Hu, J.; Liew, C.W.; Shih, J.L.; Ford, E.L.; Herrera, P.L.; Polonsky, K.S.; McGuinness, O.P.; Kulkarni, R.N. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009, 9, 350–361. [Google Scholar] [CrossRef]
- Svendsen, B.; Larsen, O.; Gabe, M.B.N.; Christiansen, C.B.; Rosenkilde, M.M.; Drucker, D.J.; Holst, J.J. Insulin Secretion Depends on Intra-islet Glucagon Signaling. Cell Rep. 2018, 25, 1127–1134.e2. [Google Scholar] [CrossRef]
- Schuit, F.C.; Pipeleers, D.G. Regulation of adenosine 32′,5′-monophosphate levels in the pancreatic B cell. Endocrinology 1985, 117, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, C.J.; Havel, P.J.; Taborsky, G.J., Jr.; Klaff, L.J. Intra-islet insulin permits glucose to directly suppress pancreatic A cell function. J. Clin. Investig. 1991, 88, 767–773. [Google Scholar] [CrossRef]
- Starke, A.; Imamura, T.; Unger, R.H. Relationship of glucagon suppression by insulin and somatostatin to the ambient glucose concentration. J. Clin. Investig. 1987, 79, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Franklin, I.; Gromada, J.; Gjinovci, A.; Theander, S.; Wollheim, C.B. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 2005, 54, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Olsen, H.L.; Theander, S.; Bokvist, K.; Buschard, K.; Wollheim, C.B.; Gromada, J. Glucose stimulates glucagon release in single rat alpha-cells by mechanisms that mirror the stimulus-secretion coupling in beta-cells. Endocrinology 2005, 146, 4861–4870. [Google Scholar] [CrossRef]
- Vergari, E.; Knudsen, J.G.; Ramracheya, R.; Salehi, A.; Zhang, Q.; Adam, J.; Asterholm, I.W.; Benrick, A.; Briant, L.J.B.; Chibalina, M.V.; et al. Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion. Nat. Commun. 2019, 10, 139. [Google Scholar] [CrossRef]
- Wendt, A.; Birnir, B.; Buschard, K.; Gromada, J.; Salehi, A.; Sewing, S.; Rorsman, P.; Braun, M. Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring beta-cells. Diabetes 2004, 53, 1038–1045. [Google Scholar] [CrossRef]
- Ben-Othman, N.; Vieira, A.; Courtney, M.; Record, F.; Gjernes, E.; Avolio, F.; Hadzic, B.; Druelle, N.; Napolitano, T.; Navarro-Sanz, S.; et al. Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-like Cell Neogenesis. Cell 2017, 168, 73–85.e11. [Google Scholar] [CrossRef]
- Bailey, S.J.; Ravier, M.A.; Rutter, G.A. Glucose-dependent regulation of gamma-aminobutyric acid (GABA A) receptor expression in mouse pancreatic islet alpha-cells. Diabetes 2007, 56, 320–327. [Google Scholar] [CrossRef]
- Xu, E.; Kumar, M.; Zhang, Y.; Ju, W.; Obata, T.; Zhang, N.; Liu, S.; Wendt, A.; Deng, S.; Ebina, Y.; et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006, 3, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, A.S.; Stillings, S.N.; Hover, B.; Martin, D.M.; Matschinsky, F.M. Glucose modulation of amino acid-induced glucagon and insulin release in the isolated perfused rat pancreas. J. Clin. Investig. 1974, 54, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Vieira, E.; Gylfe, E. Paradoxical stimulation of glucagon secretion by high glucose concentrations. Diabetes 2006, 55, 2318–2323. [Google Scholar] [CrossRef]
- Vieira, E.; Salehi, A.; Gylfe, E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia 2007, 50, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.N.; Ramracheya, R.; Zhang, Q.; Johnson, P.R.; Braun, M.; Rorsman, P. Regulation of glucagon secretion by glucose: Paracrine, intrinsic or both? Diabetes Obes. Metab. 2011, 13 (Suppl. 1), 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, E.J.; Jung, H.S.; Han, N.; Kim, Y.J.; Kim, T.K.; Kim, T.N.; Kwon, M.J.; Lee, S.H.; Park, J.H.; et al. Co-Culture of α TC-6 Cells and β TC-1 Cells: Morphology and Function. Endocrinol. Metab. 2015, 30, 92–97. [Google Scholar] [CrossRef]
- Matta, S.G.; Wobken, J.D.; Williams, F.G.; Bauer, G.E. Pancreatic islet cell reaggregation systems: Efficiency of cell reassociation and endocrine cell topography of rat islet-like aggregates. Pancreas 1994, 9, 439–449. [Google Scholar] [CrossRef]
- Kojima, N. In vitro reconstitution of pancreatic islets. Organogenesis 2014, 10, 225–230. [Google Scholar] [CrossRef]
- Singh, B.; Khattab, F.; Chae, H.; Desmet, L.; Herrera, P.L.; Gilon, P. KATP channel blockers control glucagon secretion by distinct mechanisms: A direct stimulation of α-cells involving a [Ca2+]c rise and an indirect inhibition mediated by somatostatin. Mol. Metab. 2021. [Google Scholar] [CrossRef] [PubMed]





| Glucose Levels | Cell Types | Cell Numbers/Well |
|---|---|---|
| 5.5, 11.1, or 25 mM | MIN6 cells | 2.625 × 105 MIN6 cells |
| α-TC1-6 cells | 0.875 × 105 α-TC1-6 | |
| Co-culture | 2.625 × 105 MIN6 cells + 0.875 × 105 α-TC1-6 |
| Gene Symbol | TaqMan Assay ID | Name |
|---|---|---|
| Ins1 | Mm01259683_g1 | Insulin I |
| Ins2 | Mm00731595_gH | Insulin II |
| Pdx 1 | Mm00435565_m1 | Pancreatic and duodenal homeobox 1 |
| Akt1 | Mm01331626_m1 | Thymoma viral proto-oncogene 1 |
| GCK | Mm00439129_m1 | Glucokinase |
| PLCxd3 | Mm01307828_m1 | Phosphatidylinositol-specific phospholipase C, X domain containing 3 |
| Sirt 1 | Mm01168521_m1 | Sirtuin 1 |
| Beta2/Neurod1 | Mm01946604_s1 | Neurogenic differentiation 1 |
| Lcn2 | Mm01324470_m1 | Lipocalin 2 |
| Insr | Mm01211875_m1 | Insulin receptor |
| Gcg | Mm00801712_m1 | Glucagon |
| Gcgr | Mm00433546_m1 | Glucagon receptor |
| PCSK2 | Mm00500981_m1 | Pyruvate dehydrogenase kinase, isoenzyme 1 |
| Gabrg2 | Mm00433489_m1 | Proprotein convertase subtilisin/kexin type 2 |
| Gjd2 | Mm00439121_m1 | Gamma-aminobutyric acid (GABA) A receptor |
| Gja1 | Mm00439105_m1 | Connexin36 |
| Cdh1 | Mm01247357_m1 | Connexin43, gap junction protein |
| N-CAM | Mm00493049_m1 | E-cad, Ecad, L-CAM |
| Slc2a2 | Mm00446229_m1 | L1 cell adhesion molecule |
| Slc39a5 | Mm00511105_m1 | Solute carrier family 2 (facilitated glucose transporter), member |
| Solute carrier family 39 (metal ion transporter) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, W.; Anker, C.C.B.; Christiansen, C.B.; Moede, T.; Berggren, P.-O.; Hermansen, K.; Gregersen, S.; Jeppesen, P.B. Pancreatic β Cells Inhibit Glucagon Secretion from α Cells: An In Vitro Demonstration of α–β Cell Interaction. Nutrients 2021, 13, 2281. https://doi.org/10.3390/nu13072281
Gu W, Anker CCB, Christiansen CB, Moede T, Berggren P-O, Hermansen K, Gregersen S, Jeppesen PB. Pancreatic β Cells Inhibit Glucagon Secretion from α Cells: An In Vitro Demonstration of α–β Cell Interaction. Nutrients. 2021; 13(7):2281. https://doi.org/10.3390/nu13072281
Chicago/Turabian StyleGu, Wenqian, Camilla Christine Bundgaard Anker, Christine Bodelund Christiansen, Tilo Moede, Per-Olof Berggren, Kjeld Hermansen, Søren Gregersen, and Per Bendix Jeppesen. 2021. "Pancreatic β Cells Inhibit Glucagon Secretion from α Cells: An In Vitro Demonstration of α–β Cell Interaction" Nutrients 13, no. 7: 2281. https://doi.org/10.3390/nu13072281
APA StyleGu, W., Anker, C. C. B., Christiansen, C. B., Moede, T., Berggren, P.-O., Hermansen, K., Gregersen, S., & Jeppesen, P. B. (2021). Pancreatic β Cells Inhibit Glucagon Secretion from α Cells: An In Vitro Demonstration of α–β Cell Interaction. Nutrients, 13(7), 2281. https://doi.org/10.3390/nu13072281






