Vitamin Intake and Loss of Muscle Mass in Older People with Type 2 Diabetes: A Prospective Study of the KAMOGAWA-DM Cohort
Abstract
:1. Introduction
2. Method
2.1. Study Design
2.2. Setting
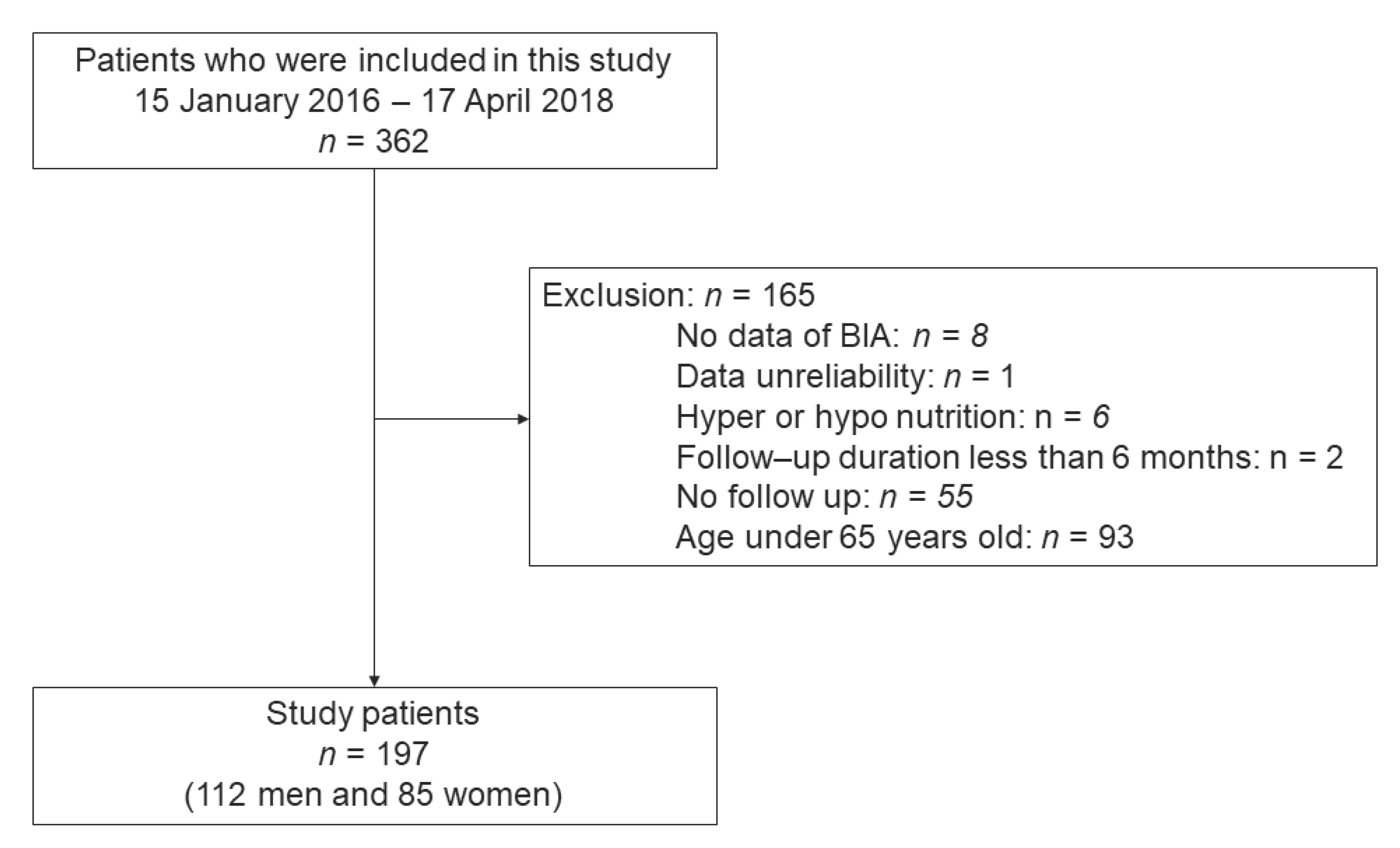
2.3. Study Participants
2.4. Ethics
2.5. Variables
2.6. Definition of Loss of Muscle Mass
2.7. Statistical Method
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradley, D.; Hsueh, W. Type 2 Diabetes in the Elderly: Challenges in a Unique Patient Population. J. Geriatr. Med. Gerontol. 2016, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Umegaki, H. Sarcopenia and frailty in older patients with diabetes mellitus. Geriatr. Gerontol. Int. 2016, 16, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Malmstrom, T.K.; Rodriguez-Mañas, L.; Sinclair, A.J. Frailty, sarcopenia and diabetes. J. Am. Med. Dir. Assoc. 2014, 15, 853–859. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 2 (Suppl. 2), S157–S163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamura, T.; Miki, A.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Osaka, T.; Hamaguchi, M.; Yamazaki, M.; Fukui, M. Shortage of energy intake rather than protein intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort. J. Diabetes 2019, 11, 477–483. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kaji, A.; Sakai, R.; Hamaguchi, M.; Okada, H.; Ushigome, E.; Asano, M.; Yamazaki, M.; Fukui, M. Sarcopenia is associated with blood pressure variability in older patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort study. Geriatr. Gerontol. Int. 2018, 18, 1345–1349. [Google Scholar] [CrossRef]
- Lai, S.; Muscaritoli, M.; Andreozzi, P.; Sgreccia, A.; De Leo, S.; Mazzaferro, S.; Mitterhofer, A.P.; Pasquali, M.; Protopapa, P.; Spagnoli, A.; et al. Sarcopenia and cardiovascular risk indices in patients with chronic kidney disease on conservative and replacement therapy. Nutrition 2019, 62, 108–114. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S. Is sarcopenia associated with an increased risk of all-cause mortality and functional disability? Exp. Gerontol. 2017, 96, 100–103. [Google Scholar] [CrossRef]
- Landi, F.; Cruz-Jentoft, A.J.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia and mortality risk in frail older persons aged 80 years and older: Results from ilSIRENTE study. Age Ageing 2013, 42, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, Y.; Kaji, A.; Sakai, R.; Takahashi, F.; Kawano, R.; Hamaguchi, M.; Fukui, M. Effect of Exercise Habit on Skeletal Muscle Mass Varies with Protein Intake in Elderly Patients with Type 2 Diabetes: A Retrospective Cohort Study. Nutrients 2020, 12, 3220. [Google Scholar] [CrossRef]
- Kawano, R.; Takahashi, F.; Hashimoto, Y.; Okamura, T.; Miki, A.; Kaji, A.; Sakai, R.; Kitagawa, N.; Senmaru, T.; Majima, S.; et al. Short energy intake is associated with muscle mass loss in older patients with type 2 diabetes: A prospective study of the KAMOGAWA-DM cohort. Clin. Nutr. 2021, 40, 1613–1620. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [Green Version]
- Santiago, E.; Roriz, A.; Ramos, L.B.; Ferreira, A.; Oliveira, C.C.; Gomes-Neto, M. Comparison of calorie and nutrient intake among elderly with and without sarcopenia: A systematic review and meta-analysis. Nutr. Rev. 2021, nuaa145. [Google Scholar] [CrossRef]
- Balboa-Castillo, T.; Struijk, E.A.; Lopez-Garcia, E.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillon, P. Low vitamin intake is associated with risk of frailty in older adults. Age Ageing 2018, 47, 872–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stöckle, U.; Ochs, G.; de Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A.K. Crucial Role of Vitamin D in the Musculoskeletal System. Nutrients 2016, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Hirose, Y.; Onishi, T.; Miura, S.; Hatazawa, Y.; Kamei, Y. Vitamin D Attenuates FOXO1-Target Atrophy Gene Expression in C2C12 Muscle Cells. J. Nutr. Sci. Vitaminol. (Tokyo) 2018, 64, 229–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lappe, J.M.; Binkley, N. Vitamin D and Sarcopenia/Falls. J. Clin. Densitom. 2015, 18, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Cesari, M.; Ferrucci, L.; Cherubini, A.; Maggio, D.; Bartali, B.; Johnson, M.A.; Schwartz, G.G.; Kritchevsky, S.B. Association between vitamin D status and physical performance: The InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Bartali, B.; Frongillo, E.A.; Bandinelli, S.; Lauretani, F.; Semba, R.D.; Fried, L.P.; Ferrucci, L. Low nutrient intake is an essential component of frailty in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 589–593. [Google Scholar] [CrossRef]
- Kositsawat, J.; Kuo, C.L.; Barry, L.C.; Melzer, D.; Bandinelli, S.; Ferrucci, L.; Wu, R.; Kuchel, G.A. Interaction between Vitamin D and Interleukin 6 on Slow Gait Speed: 6-Year Follow-up Data of Older Adults from InCHIANTI. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1161–1166. [Google Scholar] [CrossRef]
- Verlaan, S.; Aspray, T.J.; Bauer, J.M.; Cederholm, T.; Hemsworth, J.; Hill, T.R.; McPhee, J.S.; Piasecki, M.; Seal, C.; Sieber, C.C.; et al. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: A case-control study. Clin. Nutr. 2017, 36, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Villamor, E.; Fawzi, W.W. Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin. Microbiol. Rev. 2005, 18, 446–464. [Google Scholar] [CrossRef] [Green Version]
- Qian, B.; Shen, S.; Zhang, J.; Jing, P. Effects of Vitamin B6 Deficiency on the Composition and Functional Potential of T Cell Populations. J. Immunol. Res. 2017, 2017, 2197975. [Google Scholar] [CrossRef] [Green Version]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Aĭdarkhanov, B.B.; Lokshina, E.A.; Lenskaia, E.G. Molekuliarnye aspekty makhanizma antiokislitel’noĭ aktivnost’ vitamina E: Osobennosti deĭstviia alpha- i gamma-tokoferolov [Molecular aspects of the mechanism of anti-oxidant activity of vitamin E: Features of the action of alpha- and gamma-tocopherol]. Vopr. Med. Khim. 1989, 35, 2–9. [Google Scholar]
- Gori, A.M.; Sofi, F.; Corsi, A.M.; Gazzini, A.; Sestini, I.; Lauretani, F.; Bandinelli, S.; Gensini, G.F.; Ferrucci, L.; Abbate, R. Predictors of vitamin B6 and folate concentrations in older persons: The InCHIANTI study. Clin. Chem. 2006, 52, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Ble, A.; Cherubini, A.; Volpato, S.; Bartali, B.; Walston, J.D.; Windham, B.G.; Bandinelli, S.; Lauretani, F.; Guralnik, J.M.; Ferrucci, L. Lower plasma vitamin E levels are associated with the frailty syndrome: The InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Manzetti, S.; Zhang, J.; van der Spoel, D. Thiamin function, metabolism, uptake, and transport. Biochemistry 2014, 53, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Tomar, S.K.; Singh, A.K.; Mandal, S.; Arora, S. Riboflavin and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2017, 57, 3650–3660. [Google Scholar] [CrossRef] [PubMed]
- Sakai, R.; Hashimoto, Y.; Ushigome, E.; Miki, A.; Okamura, T.; Matsugasumi, M.; Fukuda, T.; Majima, S.; Matsumoto, S.; Senmaru, T.; et al. Late-night-dinner is associated with poor glycemic control in people with type 2 diabetes: The KAMOGAWA-DM cohort study. Endocr. J. 2018, 65, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Kaji, A.; Hashimoto, Y.; Sakai, R.; Okada, H.; Hamaguchi, M.; Ushigome, E.; Majima, S.; Yamazaki, M.; Fukui, M. Frequent Usage of Convenience Stores is Associated with Low Diet Quality. Nutrients 2019, 11, 1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, K.; Sasaki, S.; Takahashi, Y.; Okubo, H.; Hosoi, Y.; Horiguchi, H.; Oguma, E.; Kayama, F. Dietary glycemic index and load in relation to metabolic risk factors in Japanese female farmers with traditional dietary habits. Am. J. Clin. Nutr. 2006, 83, 1161–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shinkai, S.; Murayama, H.; Mori, S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatr. Gerontol. Int. 2015, 15, 1013–1022. [Google Scholar] [CrossRef]
- Lemmens, H.J.; Brodsky, J.B.; Bernstein, D.P. Estimating ideal body weight—A new formula. Obes. Surg. 2005, 15, 1082–1083. [Google Scholar] [CrossRef]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, Y.; Tanaka, M.; Miki, A.; Kobayashi, Y.; Wada, S.; Kuwahata, M.; Kido, Y.; Yamazaki, M.; Fukui, M. Intake of Carbohydrate to Fiber Ratio Is a Useful Marker for Metabolic Syndrome in Patients with Type 2 Diabetes: A Cross-Sectional Study. Ann. Nutr. Metab. 2018, 72, 329–335. [Google Scholar] [CrossRef]
- Muscariello, E.; Nasti, G.; Siervo, M.; Di Maro, M.; Lapi, D.; D’Addio, G.; Colantuoni, A. Dietary protein intake in sarcopenic obese older women. Clin. Interv. Aging 2016, 11, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, K.E.; von Allmen, M.T.; Devries, M.C.; Phillips, S.M. Muscle Disuse as a Pivotal Problem in Sarcopenia-related Muscle Loss and Dysfunction. J. Frailty Aging 2016, 5, 33–41. [Google Scholar]
- Sasaki, T.; Sugawara, M.; Fukuda, M. Sodium-glucose cotransporter 2 inhibitor-induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: The Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J. Diabetes Investig. 2019, 10, 108–117. [Google Scholar] [CrossRef]
- Perna, S.; Guido, D.; Bologna, C.; Solerte, S.B.; Guerriero, F.; Isu, A.; Rondanelli, M. Liraglutide and obesity in elderly: Efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin. Exp. Res. 2016, 28, 1251–1257. [Google Scholar] [CrossRef]
- von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Hajj, C.; Fares, S.; Chardigny, J.M.; Boirie, Y.; Walrand, S. Vitamin D supplementation and muscle strength in pre-sarcopenic elderly Lebanese people: A randomized controlled trial. Arch. Osteoporos. 2018, 14, 4. [Google Scholar] [CrossRef]
- Nasri, H.; Ardalan, M.R. Association of serum vitamin D level with age in individuals with normal renal function. J. Nephropharmacol. 2012, 1, 7–9. [Google Scholar]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, M.; Deeg, D.J.; Lips, P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.Y.; Lee, J.Y.; Kim, D.H. Low 25-hydroxyvitamin D levels and the risk of frailty syndrome: A systematic review and dose-response meta-analysis. BMC Geriatr. 2018, 18, 206. [Google Scholar] [CrossRef]
- Bischoff, H.A.; Borchers, M.; Gudat, F.; Duermueller, U.; Theiler, R.; Stähelin, H.B.; Dick, W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem. J. 2001, 33, 19–24. [Google Scholar] [CrossRef]
- Chen, S.; Villalta, S.A.; Agrawal, D.K. FOXO1 Mediates Vitamin D Deficiency-Induced Insulin Resistance in Skeletal Muscle. J. Bone Miner. Res. 2016, 31, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Nazarian, S.; St Peter, J.V.; Boston, R.C.; Jones, S.A.; Mariash, C.N. Vitamin D3 supplementation improves insulin sensitivity in subjects with impaired fasting glucose. Transl. Res. 2011, 158, 276–281. [Google Scholar] [CrossRef] [Green Version]
- Parildar, H.; Cigerli, O.; Unal, D.A.; Gulmez, O.; Demirag, N.G. The impact of Vitamin D Replacement on Glucose Metabolism. Pak. J. Med. Sci. 2013, 29, 1311–1314. [Google Scholar] [CrossRef]
- Naharci, I.; Bozoglu, E.; Kocak, N.; Doganci, S.; Doruk, H.; Serdar, M. Effect of vitamin D on insulin sensitivity in elderly patients with impaired fasting glucose. Geriatr. Gerontol. Int. 2012, 12, 454–460. [Google Scholar] [CrossRef]
- von Hurst, P.R.; Stonehouse, W.; Coad, J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—A randomised, placebo-controlled trial. Br. J. Nutr. 2010, 103, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittas, A.G.; Harris, S.S.; Stark, P.C.; Dawson-Hughes, B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2007, 30, 980–986. [Google Scholar] [CrossRef] [Green Version]
- Garcia, L.A.; King, K.K.; Ferrini, M.G.; Norris, K.C.; Artaza, J.N. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 2011, 152, 2976–2986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.X.; Liu, X.D.; Li, K.; Liu, W.Z.; Ren, Y.S.; Zhang, J.X. Different dietary energy intake affects skeletal muscle development through an Akt-dependent pathway in Dorper × Small Thin-Tailed crossbred ewe lambs. Domest. Anim. Endocrinol. 2016, 57, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.X.; Liu, X.D.; Zhang, J.X.; Li, H.Q. Effect of different dietary energy on collagen accumulation in skeletal muscle of ram lambs. J. Anim. Sci. 2015, 93, 4200–4210. [Google Scholar] [CrossRef]
- Uh, S.T.; Koo, S.M.; Kim, Y.K.; Kim, K.U.; Park, S.W.; Jang, A.S.; Kim, D.J.; Kim, Y.H.; Park, C.S. Inhibition of vitamin d receptor translocation by cigarette smoking extracts. Tuberc. Respir. Dis. 2012, 73, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Xu, W.G.; Luo, Y.; Han, F.F.; Yao, X.H.; Yang, T.Y.; Zhang, Y.; Pi, W.F.; Guo, X.J. Cigarette smoke-induced skeletal muscle atrophy is associated with up-regulation of USP-19 via p38 and ERK MAPKs. J. Cell. Biochem. 2011, 112, 2307–2316. [Google Scholar] [CrossRef]
- Kuo, H.K.; Liao, K.C.; Leveille, S.G.; Bean, J.F.; Yen, C.J.; Chen, J.H.; Yu, Y.H.; Tai, T.Y. Relationship of homocysteine levels to quadriceps strength, gait speed, and late-life disability in older adults. J. Gerontol. A. Biol. Sci. Med. Sci. 2007, 62, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Kado, D.M.; Bucur, A.; Selhub, J.; Rowe, J.W.; Seeman, T. Homocysteine levels and decline in physical function: MacArthur Studies of Successful Aging. Am. J. Med. 2002, 113, 537–542. [Google Scholar] [CrossRef]
- Koehler, K.M.; Romero, L.J.; Stauber, P.M.; Pareo-Tubbeh, S.L.; Liang, H.C.; Baumgartner, R.N.; Garry, P.J.; Allen, R.H.; Stabler, S.P. Vitamin supplementation and other variables affecting serum homocysteine and methylmalonic acid concentrations in elderly men and women. J. Am. Coll. Nutr. 1996, 15, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D.; Martin, J.J. Homocysteine. Int. J. Biochem. Cell. Biol. 2000, 32, 385–389. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef]
- Sudevan, S.; Takiura, M.; Kubota, Y.; Higashitani, N.; Cooke, M.; Ellwood, R.A.; Etheridge, T.; Szewczyk, N.J.; Higashitani, A. Mitochondrial dysfunction causes Ca2+ overload and ECM degradation-mediated muscle damage in C. elegans. FASEB J. 2019, 33, 9540–9550. [Google Scholar] [CrossRef]
- Kashiba, M.; Oka, J.; Ichikawa, R.; Kasahara, E.; Inayama, T.; Kageyama, A.; Kageyama, H.; Osaka, T.; Umegaki, K.; Matsumoto, A.; et al. Impaired ascorbic acid metabolism in streptozotocin-induced diabetic rats. Free Radic. Biol. Med. 2002, 33, 1221–1230. [Google Scholar] [CrossRef]
- Gopaul, N.K.; Manraj, M.D.; Hébé, A.; Lee Kwai Yan, S.; Johnston, A.; Carrier, M.J.; Anggård, E.E. Oxidative stress could precede endothelial dysfunction and insulin resistance in Indian Mauritians with impaired glucose metabolism. Diabetologia 2001, 44, 706–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| All n= 197 | Loss of Muscle Mass (−) n = 104 | Loss of Muscle Mass (+) n = 93 | p | |
|---|---|---|---|---|
| Age (years) | 72.3 (5.2) | 72.3 (5.1) | 72.3 (5.3) | 0.983 |
| Sex (men/women) | 112/85 | 54/50 | 58/35 | 0.182 |
| Duration of diabetes (years) | 16.1 (10.1) | 15.5 (9.6) | 16.8 (10.6) | 0.381 |
| Family history of diabetes (−/+) | 115/82 | 62/42 | 53/40 | 0.819 |
| BMI (kg/m2) | 23.7 (3.9) | 23.6 (3.2) | 23.9 (4.5) | 0.529 |
| Appendicular muscle mass at baseline (kg) | 19.6 (4.3) | 17.3 (4.0) | 18.2 (3.6) | 0.103 |
| Appendicular muscle mass at follow-up (kg) | 17.5 (3.9) | 17.6 (4.1) | 17.4 (3.7) | 0.700 |
| SMI at baseline (kg/m2) | 6.9 (0.9) | 6.7 (1.0) | 7.0 (0.9) | 0.048 |
| SMI at follow-up (kg/m2) | 6.8 (1.0) | 6.8 (1.0) | 6.7 (0.9) | 0.212 |
| SBP (mmHg) | 134.0 (18.9) | 133.8 (19.1) | 134.3 (18.7) | 0.873 |
| DBP (mmHg) | 77.1 (10.5) | 77.0 (11.2) | 77.3 (9.7) | 0.830 |
| Insulin (−/+) | 144/53 | 77/27 | 67/26 | 0.877 |
| Sodium glucose cotransporter-2 inhibitor (−/+) | 172/25 | 91/13 | 81/12 | 1.000 |
| Glucagon-like peptide-1 agonist (−/+) | 174/23 | 88/16 | 86/7 | 0.136 |
| Biguanides (−/+) | 132/65 | 65/39 | 67/26 | 0.204 |
| Steroids usage (−/+) | 192/5 | 103/1 | 89/4 | 0.301 |
| Habit of smoking (−/+) | 169/28 | 90/14 | 79/14 | 0.908 |
| Habit of exercise (−/+) | 85/112 | 41/63 | 44/49 | 0.331 |
| Triglycerides (mmol/L) | 1.4 (0.9) | 1.4 (0.9) | 1.5 (1.0) | 0.755 |
| HDL cholesterol (mmol/L) | 1.6 (0.4) | 1.6 (0.4) | 1.6 (0.4) | 0.820 |
| Blood glucose levels (mmol/L) | 8.2 (2.8) | 8.2 (2.6) | 8.2 (3.1) | 0.866 |
| HbA1c (mmol/mol) | 55.3 (10.7) | 54.9 (10.7) | 55.8 (10.7) | 0.533 |
| HbA1c (%) | 7.2 (1.0) | 7.2 (1.0) | 7.3 (1.0) | 0.533 |
| Creatinine (umol/L) | 74.8 (26.1) | 71.7 (23.9) | 78.4 (28.0) | 0.070 |
| eGFR (mL/min/1.73 m2) | 65.6 (17.5) | 67.0 (17.0) | 64.1 (18.0) | 0.241 |
| Uric acid (mmol/L) | 310.4 (71.3) | 307.2 (66.7) | 313.0 (76.2) | 0.513 |
| All n = 197 | Loss of Muscle Mass (−) n = 81 | Loss of Muscle Mass (+) n = 93 | p | |
|---|---|---|---|---|
| Total energy (kcal/day) | 1746.4 (596.3) | 1818.8 (596.1) | 1665.4 (589.2) | 0.071 |
| Energy (kcal/IBW/day) | 30.9 (10.0) | 32.5 (10.1) | 29.2 (9.7) | 0.021 |
| Total protein (g/day) | 75.3 (30.3) | 79.5 (29.0) | 70.6 (31.3) | 0.040 |
| Protein (g/BW/day) | 1.3 (0.6) | 1.4 (0.5) | 1.2 (0.6) | 0.079 |
| Protein per energy (%) | 17.3 (3.6) | 17.8 (4.1) | 16.8 (3.0) | 0.058 |
| Animal protein (g/day) | 47.2 (23.8) | 50.7 (23.1) | 43.2 (24.1) | 0.026 |
| Animal protein (g/BW/day) | 0.8 (0.4) | 0.9 (0.4) | 0.7 (0.5) | 0.045 |
| Vegetable protein (g/day) | 28.1 (9.7) | 28.7 (9.7) | 27.4 (9.8) | 0.348 |
| Vegetable protein (g/BW/day) | 0.5 (0.2) | 0.5 (0.2) | 0.5 (0.2) | 0.285 |
| Animal/vegetable protein ratio | 1.8 (1.0) | 1.9 (1.2) | 1.6 (0.6) | 0.017 |
| Total fat (g/day) | 55.6 (22.1) | 58.2 (21.0) | 52.8 (23.1) | 0.088 |
| Fat (g/IBW/day) | 1.0 (0.4) | 1.1 (0.4) | 0.9 (0.4) | 0.034 |
| Fat per energy (%) | 28.9 (6.5) | 29.3 (6.8) | 28.4 (6.1) | 0.300 |
| Total carbohydrate (g/day) | 218.1 (82.2) | 225.3 (86.0) | 210.1 (77.3) | 0.197 |
| Carbohydrate (g/IBW/day) | 3.9 (1.4) | 4.0 (1.5) | 3.7 (1.2) | 0.089 |
| Carbohydrate per energy (%) | 50.2 (9.1) | 49.3 (9.7) | 51.2 (8.3) | 0.138 |
| Dietary fiber (g/day) | 12.5 (5.3) | 12.9 (5.4) | 12.1 (5.3) | 0.308 |
| Carbohydrate/fiber ratio | 18.9 (7.1) | 18.8 (6.8) | 19.1 (7.4) | 0.752 |
| Alcohol consumption (g/day) | 7.5 (17.4) | 8.1 (19.3) | 6.9 (15.1) | 0.631 |
| Vitamin A (µg RAE/day) | 807.6 (612.0) | 824.7 (596.1) | 788.4 (632.0) | 0.679 |
| Vitamin E (mg/day) | 7.9 (3.1) | 8.2 (3.0) | 7.6 (3.2) | 0.130 |
| Vitamin B1 (mg/day) | 0.8 (0.3) | 0.8 (0.3) | 0.8 (0.3) | 0.031 |
| Vitamin B2 (mg/day) | 1.5 (0.6) | 1.5 (0.5) | 1.4 (0.6) | 0.175 |
| Vitamin B6 (mg/day) | 1.4 (0.6) | 1.4 (0.5) | 1.3 (0.6) | 0.075 |
| Vitamin B12 (µg/day) | 12.4 (8.0) | 13.4 (7.5) | 11.2 (8.3) | 0.049 |
| Vitamin C (mg/day) | 126.2 (62.5) | 129.7 (66.2) | 122.4 (58.3) | 0.412 |
| Vitamin D (µg/day) | 19.2 (12.9) | 21.6 (13.0) | 16.5 (12.2) | 0.004 |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Vitamin A (µg RAE/day) | 1.00 (1.00–1.00) | 0.679 | 1.00 (1.00–1.00) | 0.637 | 1.00 (1.00–1.00) | 0.471 |
| Vitamin E (mg/day) | 0.93 (0.85–1.02) | 0.132 | 0.96 (0.80–1.14) | 0.616 | 0.95 (0.79–1.13) | 0.556 |
| Vitamin B1 (mg/day) | 0.36 (0.14–0.93) | 0.034 | 0.15 (0.02–1.28) | 0.083 | 0.13 (0.01–1.23) | 0.075 |
| Vitamin B2 (mg/day) | 0.70 (0.42–1.17) | 0.176 | 0.79 (0.25–2.51) | 0.694 | 0.77 (0.23–2.53) | 0.664 |
| Vitamin B6 (mg/day) | 0.63 (0.38–1.05) | 0.078 | 0.42 (0.11–1.57) | 0.197 | 0.43 (0.11–1.72) | 0.233 |
| Vitamin B12 (µg/day) | 0.96 (0.93–1.00) | 0.054 | 0.92 (0.84–1.00) | 0.038 | 0.93 (0.85–1.01) | 0.090 |
| Vitamin C (mg/day) | 1.00 (0.99–1.00) | 0.411 | 1.00 (1.00–1.01) | 0.996 | 1.00 (0.99–1.01) | 0.953 |
| Vitamin D (µg/day) | 0.97 (0.94–0.99) | 0.006 | 0.91 (0.87–0.96) | <0.001 | 0.93 (0.88–0.97) | 0.003 |
| Cut-Off Point; Rate of SMI Reduction ≥ 0.5% | ||||||
| Model 1 | Model 2 | Model 3 | ||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Vitamin A (µgRAE/day) | 1.00 (1.00–1.00) | 0.770 | 1.00 (1.00–1.00) | 0.667 | 1.00 (1.00–1.00) | 0.684 |
| Vitamin E (mg/day) | 0.94 (0.85–1.03) | 0.166 | 0.94 (0.79–1.13) | 0.517 | 0.94 (0.78–1.14) | 0.535 |
| Vitamin B1 (mg/day) | 0.48 (0.19–1.20) | 0.117 | 0.29 (0.04–2.33) | 0.243 | 0.32 (0.04–2.79) | 0.303 |
| Vitamin B2 (mg/day) | 0.72 (0.43–1.21) | 0.214 | 0.53 (0.16–1.69) | 0.283 | 0.56 (0.17–1.91) | 0.355 |
| Vitamin B6 (mg/day) | 0.71 (0.43–1.16) | 0.170 | 0.57 (0.16–2.09) | 0.399 | 0.68 (0.18–2.59) | 0.570 |
| Vitamin B12 (µg/day) | 0.97 (0.93–1.01) | 0.090 | 0.93 (0.86–1.01) | 0.101 | 0.94 (0.86–1.02) | 0.145 |
| Vitamin C (mg/day) | 1.00 (1.00–1.00) | 0.901 | 1.00 (1.00–1.01) | 0.388 | 1.00 (1.00–1.01) | 0.327 |
| Vitamin D (µg/day) | 0.97 (0.95–1.00) | 0.021 | 0.94 (0.89–0.98) | 0.007 | 0.94 (0.89–0.99) | 0.020 |
| Cut-Off Point; Rate of SMI Reduction ≥ 2.0% | ||||||
| Model 1 | Model 2 | Model 3 | ||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Vitamin A (µgRAE/day) | 1.00 (1.00–1.00 | 0.563 | 1.00 (1.00–1.00) | 0.883 | 1.00 (1.00–1.00) | 0.727 |
| Vitamin E (mg/day) | 0.95 (0.87–1.05) | 0.335 | 0.96 (0.80–1.14) | 0.635 | 0.94 (0.78–1.12) | 0.485 |
| Vitamin B1 (mg/day) | 0.56 (0.21–1.47) | 0.237 | 0.40 (0.05–3.47) | 0.406 | 0.32 (0.03–3.00) | 0.320 |
| Vitamin B2 (mg/day) | 0.75 (0.44–1.28) | 0.291 | 0.60 (0.18–1.98) | 0.399 | 0.61 (0.18–2.10) | 0.435 |
| Vitamin B6 (mg/day) | 0.83 (0.50–1.38) | 0.474 | 1.05 (0.28–3.95) | 0.946 | 1.11 (0.28–4.37) | 0.887 |
| Vitamin B12 (µg/day) | 0.97 (0.94–1.01) | 0.188 | 0.92 (0.85–1.00) | 0.063 | 0.93 (0.85–1.02) | 0.120 |
| Vitamin C (mg/day) | 1.00 (1.00–1.00) | 0.927 | 1.00 (1.00–1.01) | 0.703 | 1.00 (0.99–1.01) | 0.869 |
| Vitamin D (µg/day) | 0.98 (0.95–1.00) | 0.054 | 0.92 (0.88–0.97) | 0.002 | 0.93 (0.88–0.98) | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Kawate, Y.; Okamura, T.; Kondo, Y.; Fukuda, T.; Kitagawa, N.; Okada, H.; et al. Vitamin Intake and Loss of Muscle Mass in Older People with Type 2 Diabetes: A Prospective Study of the KAMOGAWA-DM Cohort. Nutrients 2021, 13, 2335. https://doi.org/10.3390/nu13072335
Takahashi F, Hashimoto Y, Kaji A, Sakai R, Kawate Y, Okamura T, Kondo Y, Fukuda T, Kitagawa N, Okada H, et al. Vitamin Intake and Loss of Muscle Mass in Older People with Type 2 Diabetes: A Prospective Study of the KAMOGAWA-DM Cohort. Nutrients. 2021; 13(7):2335. https://doi.org/10.3390/nu13072335
Chicago/Turabian StyleTakahashi, Fuyuko, Yoshitaka Hashimoto, Ayumi Kaji, Ryosuke Sakai, Yuka Kawate, Takuro Okamura, Yuriko Kondo, Takuya Fukuda, Noriyuki Kitagawa, Hiroshi Okada, and et al. 2021. "Vitamin Intake and Loss of Muscle Mass in Older People with Type 2 Diabetes: A Prospective Study of the KAMOGAWA-DM Cohort" Nutrients 13, no. 7: 2335. https://doi.org/10.3390/nu13072335
APA StyleTakahashi, F., Hashimoto, Y., Kaji, A., Sakai, R., Kawate, Y., Okamura, T., Kondo, Y., Fukuda, T., Kitagawa, N., Okada, H., Nakanishi, N., Majima, S., Senmaru, T., Ushigome, E., Hamaguchi, M., Asano, M., Yamazaki, M., & Fukui, M. (2021). Vitamin Intake and Loss of Muscle Mass in Older People with Type 2 Diabetes: A Prospective Study of the KAMOGAWA-DM Cohort. Nutrients, 13(7), 2335. https://doi.org/10.3390/nu13072335










