Vitamin D, Its Role in Recovery after Muscular Damage Following Exercise
Abstract
:1. Introduction
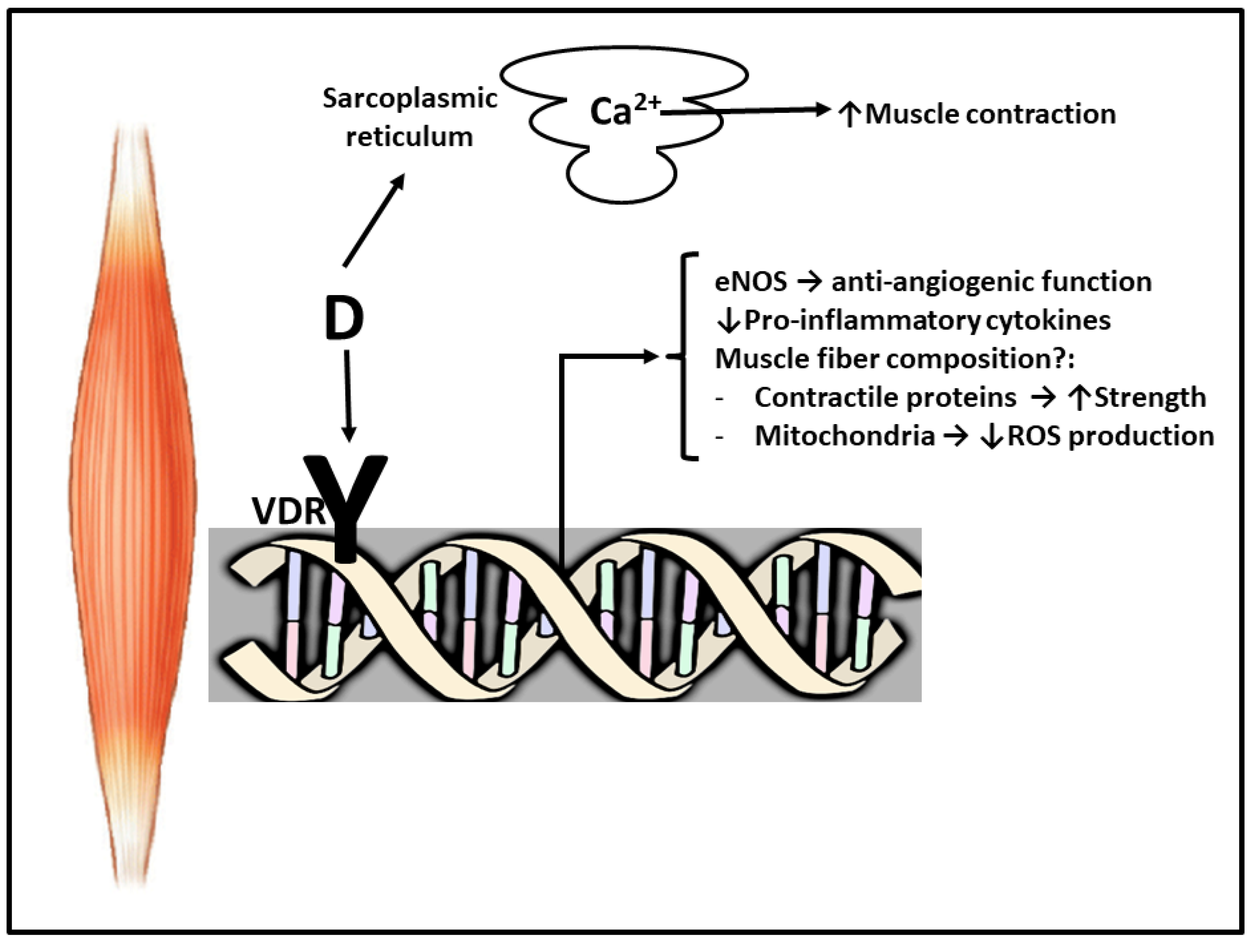
2. Vitamin D and Muscle
3. Vitamin D and Exercise
4. Exercise and Inflammation
5. Why Vitamin D Supplements Improve Muscular Damage Recovery?
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morton, J.P.; Iqbal, Z.; Drust, B.; Burgess, D.; Close, G.L.; Brukner, P.D. Seasonal Variation in Vitamin D Status in Professional Soccer Players of the English Premier League. Appl. Physiol. Nutr. Metab. 2012, 37, 798–802. [Google Scholar] [CrossRef] [Green Version]
- Rawson, E.S.; Miles, M.P.; Larson-Meyer, D.E. Dietary Supplements for Health, Adaptation, and Recovery in Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 188–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Hurst, P.R.; Beck, K.L. Vitamin D and Skeletal Muscle Function in Athletes. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 539–545. [Google Scholar] [CrossRef]
- Garay, E.; Jankowski, P.; Lizano, P.; Marczak, S.; Maehr, H.; Adorini, L.; Uskokovic, M.R.; Studzinski, G.P. Calcitriol Derivatives with Two Different Side-Chains at C-20. Part 4: Further Chain Modifications that Alter VDR-Dependent Monocytic Differentiation Potency in Human Leukemia Cells. Bioorg. Med. Chem. 2007, 15, 4444–4455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, W.; Khan, N.; Glueck, C.J.; Pandey, S.; Wang, P.; Goldenberg, N.; Uppal, M.; Khanal, S. Low Serum 25 (OH) Vitamin D Levels (<32 ng/mL) Are Associated with Reversible Myositis-Myalgia in Statin-Treated Patients. Transl. Res. 2009, 153, 11–16. [Google Scholar] [CrossRef]
- Singh, P. Treatment of Vitamin D Deficiency and Comorbidities: A Review. J. Assoc. Physicians India 2018, 66, 75–82. [Google Scholar]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the Immune System. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and Innate and Adaptive Immunity. In Vitamins & Hormones; Elsevier: Barcelona, Spain, 2011; Volume 86, pp. 23–62. [Google Scholar]
- Xu, H.; Soruri, A.; Gieseler, R.K.; Peters, J.H. 1,25-Dihydroxyvitamin D3 Exerts Opposing Effects to IL-4 on MHC Class-II Antigen Expression, Accessory Activity, and Phagocytosis of Human Monocytes. Scand. J. Immunol. 1993, 38, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Larsen-Meyer, D.E.; Willis, K.S. Vitamin D and Athletes. Curr. Sports Med. Rep. 2010, 9, 220–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willis, K.S.; Smith, D.T.; Broughton, K.S.; Larson-Meyer, D.E. Vitamin D Status and Biomarkers of Inflammation in Runners. J. Sports Med. 2012, 3, 35–42. [Google Scholar]
- Campbell, P.M.F.; Allain, T.J. Muscle Strength and Vitamin D in Older People. Gerontology 2006, 52, 335–338. [Google Scholar] [CrossRef]
- Ceglia, L. Vitamin D and Skeletal Muscle Tissue and Function. Mol. Asp. Med. 2008, 29, 407–414. [Google Scholar] [CrossRef]
- Dinarello, C.A. Anti-Cytokine Therapeutics and Infections. Vaccine 2003, 21 (Suppl. S2), S24–S34. [Google Scholar] [CrossRef]
- Prineas, J.W.; Mason, A.S.; Henson, R.A. Myopathy in Metabolic Bone Disease. Br. Med. J. 1965, 1, 1034–1036. [Google Scholar] [CrossRef] [Green Version]
- Dalakas, M.C. Immunotherapy of Myositis: Issues, Concerns and Future Prospects. Nat. Rev. Rheumatol. 2010, 6, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ceglia, L.; Harris, S.S. Vitamin D and Its Role in Skeletal Muscle. Calcif. Tissue Int. 2013, 92, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Irani, P.F. Electromyography in Nutritional Osteomalacic Myopathy. J. Neurol. Neurosurg. Psychiatry 1976, 10, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Barker, T.; Henriksen, V.T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Schneider, E.D.; Dixon, B.M.; Weaver, L.K. Higher Serum 25-Hydroxyvitamin D Concentrations Associate with a Faster Recovery of Skeletal Muscle Strength after Muscular Injury. Nutrients 2013, 5, 1253–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratos, I.; Li, Z.; Herlyn, P.; Rotter, R.; Behrendt, A.K.; Mittlmeier, T.; Vollmar, B. Vitamin D Increases Cellular Turnover and Functionally Restores the Skeletal Muscle after Crush Injury in Rats. Am. J. Pathol. 2013, 182, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Mikkelsen, U.R.; Raastad, T.; Peake, J.M. Leucocytes, Cytokines and Satellite Cells: What Role Do They Play in Muscle Damage and Regeneration Following Eccentric Exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar] [PubMed]
- Warren, G.L.; Lowe, D.A.; Armstrong, R.B. Measurement Tools Used in the Study of Eccentric Contraction-Induced Injury. Sports Med. 1999, 27, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Park, H.; Cho, S.; Lee, M. Vitamin D3 Supplementation Modulates Inflammatory Responses from the Muscle Damage Induced by High-Intensity Exercise in SD Rats. Cytokine 2013, 63, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Pojednic, R.M.; Ceglia, L.; Lichtenstein, A.; Dawson-Hughes, B.; Fielding, R. Vitamin D Receptor Protein Is Associated with Interleukin-6 in Human Skeletal Muscle. Endocrine 2015, 49, 512–520. [Google Scholar] [CrossRef]
- Sørensen, O.H.; Lund, B.; Saltin, B.; Lund, B.; Andersen, R.B.; Hjorth, L.; Melsen, F.; Mosekilde, L. Myopathy in Bone Loss of Ageing: Improvement by Treatment with 1 Alpha-Hydroxycholecalciferol and Calcium. Clin. Sci. 1979, 56, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Srikuea, R.; Zhang, X.; Park-Sarge, O.K.; Esser, K.A. VDR and CYP27B1 Are Expressed in C2C12 Cells and Regenerating Skeletal Muscle: Potential Role in Suppression of Myoblast Proliferation. Am. J. Physiol. Cell Physiol. 2012, 303, C396–C405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foo, L.H.; Zhang, Q.; Zhu, K.; Ma, G.; Hu, X.; Greenfield, H.; Fraser, D.R. Low Vitamin D Status Has an Adverse Influence on Bone Mass, Bone Turnover, and Muscle Strength in Chinese Adolescent Girls. J. Nutr. 2009, 139, 1002–1007. [Google Scholar] [CrossRef] [Green Version]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on Skeletal and Extraskeletal Health and the Need for Supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [Green Version]
- Brech, G.C.; Ciolac, E.G.; Peterson, M.D.; Greve, J.M. Serum 25-Hydroxyvitamin D Levels Are Associated with Functional Capacity but Not with Postural Balance in Osteoporotic Postmenopausal Women. Clinics (Sao Paulo) 2017, 72, 11–16. [Google Scholar] [CrossRef]
- Barker, T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Henriksen, V.T.; Dixon, B.M.; Schneider, E.D.; Dern, A.; Weaver, L.K. Different Doses of Supplemental Vitamin D Maintain Interleukin-5 without Altering Skeletal Muscle Strength: A Randomized, Double-Blind, Placebocontrolled Study in Vitamin D Sufficient Adults. Nutr. Metab. 2012, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Barker, T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Dixon, B.M.; Schneider, E.D.; Henriksen, V.T.; Weaver, L.K. Circulating Pro-Inflammatory Cytokines Are Elevated and Peak Power Output Correlates with 25-Hydroxyvitamin D in Vitamin D Insufficient Adults. Eur. J. Appl. Physiol. 2013, 113, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Schleithoff, S.S.; Zittermann, A.; Tenderich, G.; Berthold, H.K.; Stehle, P.; Koerfer, R. Vitamin D Supplementation Improves Cytokine Profiles in Patients with Congestive Heart Failure: A Double-Blind, Randomized, Placebo-Controlled Trial. Am. J. Clin. Nutr. 2006, 83, 754–759. [Google Scholar] [CrossRef]
- Barker, T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Dixon, B.M.; Schneider, E.D.; Henriksen, V.T.; Weaver, L.K. Vitamin D Sufficiency Associates with An Increase in Anti-Inflammatory Cytokines after Intense Exercise in Humans. Cytokine 2014, 65, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Shuler, F.D.; Wingate, M.K.; Moore, G.H.; Giangarra, C. Sports Health Benefits of Vitamin D. Sports Health 2012, 4, 496–501. [Google Scholar] [CrossRef] [Green Version]
- Visser, M.; Deeg, D.J.H.; Lips, P. Low Vitamin D and High Parathyroid Hormone Levels as Determinants of Loss of Muscle Strength and Muscle Mass (Sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef]
- Close, G.L.; Hamilton, D.L.; Philp, A.; Burke, L.M.; Morton, J.P. New Strategies in Sport Nutrition to Increase Exercise Performance. Free Radic. Biol. Med. 2016, 98, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Wyon, M.A.; Koutedakis, Y.; Wolman, R.; Nevill, A.M.; Allen, N. The Influence of Winter Vitamin D Supplementation on Muscle Function and Injury Occurrence in Elite Ballet Dancers: A Controlled Study. J. Sci. Med. Sport 2014, 17, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Leckey, J.; Patterson, M.; Bradley, W.; Owens, D.J.; Fraser, W.D.; Morton, J.P. The Effects of Vitamin D(3) Supplementation on Serum Total 25 [OH] D Concentration and Physical Performance: A Randomised Dose-Response Study. Br. J. Sports Med. 2013, 47, 692–696. [Google Scholar] [CrossRef]
- Carswell, A.T.; Oliver, S.J.; Wentz, L.M.; Kashi, D.S.; Roberts, R.; Tang, J.C.; Izard, R.M.; Jackson, S.; Allan, D.; Rhodes, L.E.; et al. Influence of Vitamin D Supplementation by Sunlight or Oral D3 on Exercise Performance. Med. Sci. Sports Exerc. 2018, 50, 2555–2564. [Google Scholar] [CrossRef] [Green Version]
- Koundourakis, N.E.; Androulakis, N.E.; Malliaraki, N.; Margioris, A.N. Vitamin D and Exercise Performance in Professional Soccer Players. PLoS ONE 2014, 9, e101659. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Calleja-González, J.; Urdampilleta, A.; León-Guereño, P.; Córdova, A.; Caballero-García, A.; Fernandez-Lázaro, D. Effects of Vitamin D Supplementation on Haematological Values and Muscle Recovery in Elite Male Traditional Rowers. Nutrients 2018, 10, 1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, B.; Whiteley, R.; Farooq, A.; Chalabi, H. Vitamin D Concentration in 342 Professional Football Players and Association with Lower Limb Isokinetic Function. J. Sci. Med. Sport 2014, 17, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Agergaard, J.; Trøstrup, J.; Uth, J.; Iversen, J.V.; Boesen, A.; Andersen, J.L.; Schjerling, P.; Langberg, H. Does Vitamin-D Intake during Resistance Training Improve the Skeletal Muscle Hypertrophic and Strength Response in Young and Elderly Men?—A Randomized Controlled Trial. Nutr. Metab. (Lond) 2015, 12, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Rocha, A.L.; Pinto, A.P.; Kohama, E.B.; Pauli, J.R.; de Moura, L.P.; Cintra, D.E.; Ropelle, E.R.; da Silva, A.D.S. The Proinflammatory Effects of Chronic Excessive Exercise. Cytokine 2019, 119, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Córdova, A.; Álvarez de Mon, M. Inmunidad en el Deporte; Gymnos: Madrid, Spain, 2001. [Google Scholar]
- Regueiro, J.; López, C.; González, S.; Martínez, E. Inmunología. Biología y Patología del Sistema Inmunitario; Editorial Medica Panamericana: Madrid, Spain, 2010. [Google Scholar]
- Peake, J.M.; Della Gatta, P.; Suzuki, K.; Nieman, D.C. Cytokine Expression and Secretion by Skeletal Muscle Cells: Regulatory Mechanisms and Exercise Effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar]
- Feghali, C.A.; Wright, T.M. Cytokines in Acute and Chronic Inflammation. Front. Biosci. 1997, 2, 12–26. [Google Scholar]
- Dinarello, C.A. Proinflammatory Cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef]
- Allen, J.; Sun, Y.; Woods, J.A. Exercise and the Regulation of Inflammatory Responses. Prog. Mol. Biol. Transl. Sci. 2015, 135, 337–354. [Google Scholar]
- Moldoveanu, A.I.; Shephard, R.J.; Shek, P.N. The Cytokine Response to Physical Activity and Training. Sports Med. 2001, 31, 115–144. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The Anti-Inflammatory Effects of Exercise: Mechanisms and Implications for the Prevention and Treatment of Disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Córdova, A.; Martin, J.F.; Reyes, E.; Alvarez-Mon, M. Protection against Muscle Damage in Competitive Sports Players: The Effect of the Immunomodulator AM3. J. Sports Sci. 2004, 22, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Córdova, A.; Monserrat, J.; Villa, G.; Reyes, E.; Soto, M.A. Effects of AM3 (Inmunoferon) on Increased Serum Concentrations of Interleukin-6 and Tumour Necrosis Factor Receptors I and II in Cyclists. J. Sports Sci. 2006, 24, 565–573. [Google Scholar] [CrossRef]
- Córdova, A.; Sureda, A.; Pons, A.; Alvarez-Mon, M. Modulation of TNF-α, TNF-α Receptors and IL-6 after Treatment with AM3 in Professional Cyclists. J. Sports Med. Phys. Fitness 2015, 55, 345–351. [Google Scholar]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Seco-Calvo, J.; Córdova-Martínez, A.; Caballero-García, A.; Fernandez-Lazaro, C.I. Modulation of Exercise-Induced Muscle Damage, Inflammation, and Oxidative Markers by Curcumin Supplementation in a Physically Active Population: A Systematic Review. Nutrients 2020, 12, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrera-Quintanar, L.; Funes, L.; Herranz-López, M.; Martínez-Peinado, P.; Pascual-García, S.; Sempere, J.M.; Boix-Castejón, M.; Córdova, A.; Pons, A.; Micol, V.; et al. Antioxidant Supplementation Modulates Neutrophil Inflammatory Response to Exercise-Induced Stress. Antioxidants 2020, 9, 1242. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Ferrer, M.D.; Tauler, P.; Maestre, I.; Aguiló, A.; Córdova, A.; Tur, J.A.; Roche, E.; Pons, A. Intense Physical Activity Enhances Neutrophil Antioxidant Gene Expression. Immunocytochemistry Evidence for Catalase Secretion. Free Rad. Res. 2007, 41, 874–883. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Li, J.J. The Role of NF-KappaB in the Regulation of Cell Stress Responses. Int. Immunopharmacol. 2002, 2, 1509–1520. [Google Scholar] [CrossRef]
- Baumann, H.; Gauldie, J. The Acute Phase Response. Immunol. Today 1994, 15, 74–80. [Google Scholar] [CrossRef]
- Kammuler, M.E. Recombinant Human Interleukin-6: Safety Issues of a Pleiotropic Growth Factor. Toxicology 1995, 105, 91–107. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the Immune System: Regulation, Integration, and Adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Totsuka, M.; Sato, K.; Sugawara, K. Systemic Inflammatory Response to Exhaustive Exercise. Cytokine Kinetics. Exerc. Immunol. Rev. 2002, 8, 6–48. [Google Scholar] [PubMed]
- Billiau, A. Interferon Gamma: Biology and Role in Pathogenesis. Adv. Immunol. 1996, 62, 61–130. [Google Scholar] [PubMed]
- Viti, A.; Muscettola, M.; Paulesu, L.; Bocci, V.; Almi, A. Effect of Exercise on Plasma Interferon Levels. J. Appl. Physiol. 1985, 59, 426–428. [Google Scholar] [CrossRef]
- Ruggiero, V.; Tavernier, J.; Fiers, W.; Baglioni, C. Induction of Synthesis of Tumor Necrosis Factor Alpha by Interferon Gamma. J. Immunol. 1986, 136, 2445–2450. [Google Scholar] [PubMed]
- Lowenstein, C.J.; Snyder, S.H. Nitric Oxide, a Novel Biologic Messenger. Cell 1992, 70, 705–707. [Google Scholar] [CrossRef]
- Kanda, K.; Sugama, K.; Hayashida, H.; Sakuma, J.; Kawakami, Y.; Miura, S.; Yoshioka, H.; Mori, Y.; Suzuki, K. Eccentric Exercise-Induced Delayed-Onset Muscle Soreness and Changes in Markers of Muscle Damage and Inflammation. Exerc. Immunol. Rev. 2013, 19, 72–85. [Google Scholar]
- Tidball, J.G. Inflammatory Processes in Muscle Injury and Repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R345–R353. [Google Scholar] [CrossRef] [Green Version]
- Toumi, H.; F’guyer, S.; Best, T.M. The Role of Neutrophils in Injury and Repair Following Muscle Stretch. J. Anat. 2006, 208, 459–470. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Ulker, P.; Meiselman, H.J. Nitric Oxide, Erythrocytes and Exercise. Clin. Hemorheol. Microcirc. 2011, 49, 175–181. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Thompson, H.S. Antioxidants: What Role Do They Play in Physical Activity and Health? Am. J. Clin. Nutr. 2000, 72 (Suppl. S2), 637S–646S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceglia, L.; Chiu, G.R.; Harris, S.S.; Araujo, A.B. Serum 25-Hydroxyvitamin D Concentration and Physical Function in Adult Men. Clin. Endocrinol. 2011, 74, 370–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimaldi, A.S.; Parker, B.A.; Capizzi, J.A.; Clarkson, P.M.; Pescatello, L.S.; White, C.M.; Thompson, P.D. 25(OH) Vitamin D Is Associated with Greater Muscle Strength in Healthy Men and Women. Med. Sci. Sports Exerc. 2013, 45, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marantes, I.; Achenbach, S.J.; Atkinson, E.J.; Khosla, S.; Melton, L.J., III; Amin, S. Is Vitamin D a Determinant of Muscle Mass and Strength? J. Bone Miner. Res. 2011, 26, 2860–2871. [Google Scholar] [CrossRef]
- Houston, D.K.; Tooze, J.A.; Neiberg, R.H.; Hausman, D.B.; Johnson, M.A.; Cauley, J.A.; Bauer, D.C.; Cawthon, P.M.; Shea, M.K.; Schwartz, G.G.; et al. Health ABC Study. 25-Hydroxyvitamin D Status and Change in Physical Performance and Strength in Older Adults: The Health, Aging, and Body Composition Study. Am. J. Epidemiol. 2012, 176, 1025–1034. [Google Scholar] [CrossRef]
- Bischoff, H.A.; Stahelin, H.B.; Urscheler, N.; Ehrsam, R.; Vonthein, R.; Perrig-Chiello, P.; Tyndall, A.; Theiler, R. Muscle Strength in the Elderly: Its Relation to Vitamin D Metabolites. Arch. Phys. Med. Rehab. 1999, 80, 54–58. [Google Scholar] [CrossRef]
- Sohl, E.; van Schoor, N.M.; de Jongh, R.T.; Visser, M.; Deeg, D.J.; Lips, P. Vitamin D Status Is Associated with Functional Limitations and Functional Decline in Older Individuals. J. Clin. Endocrinol. Metab. 2013, 98, E1483–E1490. [Google Scholar] [CrossRef]
- Cannell, J.J.; Hollis, B.W.; Sorenson, M.B.; Taft, T.N.; Anderson, J.J. Athletic Performance and Vitamin D. Med. Sci. Sports Exerc. 2009, 41, 1102–1110. [Google Scholar] [CrossRef]
- De la Puente Yagüe, M.; Collado, L.; Ciudad-Cabañas, M.J.; Cuadrado-Cenzual, M.A. Role of Vitamin D in Athletes and Their Performance: Current Concepts and New Trends. Nutrients 2020, 12, 579. [Google Scholar] [CrossRef] [Green Version]
- Steinacker, J.M.; Lormes, W.; Reissnecker, S.; Liu, Y. New Aspects of the Hormone and Cytokine Response to Training. Eur. J. Appl. Physiol. 2004, 91, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Hennigar, S.R.; McClung, J.P.; Pasiakos, S.M. Nutritional Interventions and the IL-6 Response to Exercise. FASEB J. 2017, 31, 3719–3728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandenburg, V.M.; Vervloet, M.G.; Marx, N. The Role of Vitamin D in Cardiovascular Disease: From Present Evidence to Future Perspectives. Atherosclerosis 2012, 225, 253–263. [Google Scholar] [CrossRef]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanikarla-Marie, P.; Jain, S.K. 1,25(OH)2D3 Inhibits Oxidative Stress and Monocyte Adhesion by Mediating the Upregulation of GCLC and GSH in Endothelial Cells Treated with Acetoacetate (ketosis). J. Steroid Biochem. Mol. Biol. 2016, 159, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrukhova, O.; Slavic, S.; Zeitz, U.; Riesen, S.C.; Heppelmann, M.S.; Ambrisko, T.D.; Markovic, M.; Kuebler, W.M.; Erben, R.G. Vitamin D Is a Regulator of Endothelial Nitric Oxide Synthase and Arterial Stiffness in Mice. Mol. Endocrinol. 2014, 28, 53–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischoff-Ferrari, H.A. Relevance of Vitamin D in Muscle Health. Rev. Endocr. Metab. Disord. 2012, 13, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The Roles of Vitamin D in Skeletal Muscle: Form, Function, and Metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, B. Vitamin D and Athletic Performance: The Potential Role of Muscle. Asian J. Sports Med. 2011, 2, 211–219. [Google Scholar] [CrossRef] [Green Version]
- DeLuca, H.F. Overview of General Physiologic Features and Functions of Vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [Green Version]
- Haussler, M.R.; Haussler, C.A.; Bartik, L.; Whitfield, G.K.; Hsieh, J.C.; Slater, S.; Jurutka, P.W. Vitamin D Receptor: Molecular Signaling and Actions of Nutritional Ligands in Disease Prevention. Nutr. Rev. 2008, 66 (Suppl. S2), S98–S112. [Google Scholar] [CrossRef]
- Li, J.; Mihalcioiu, M.; Li, L.; Zakikhani, M.; Camirand, A.; Kremer, R. Vitamin D Prevents Lipid Accumulation in Murine Muscle through Regulation of Pparγ and Perilipin-2 Expression. J. Steroid Biochem. Mol. Biol. 2018, 177, 116–124. [Google Scholar] [CrossRef]
- Hamilton, B. Vitamin D and Human Skeletal Muscle. Scand. J. Med. Sci. Sports 2010, 20, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Bruyere, O. The Effects of Vitamin D on Skeletal Muscle Strength, Muscle Mass, and Muscle Power: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, M.; Saaristo, P.; Salonoja, M.; Vaapio, S.; Vahlberg, T.; Lamberg-Allardt, C.; Kivela, S.L. Vitamin D Status and Physical Function in Older Finnish People: A One-Year Follow-Up Study. Arch. Gerontol. Geriatr. 2015, 61, 419–424. [Google Scholar] [CrossRef]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.D.; Wang, X.; Lanza, I.R.; Schaible, N.S.; Kumar, R. 1alpha,25-Dihydroxyvitamin D3 Regulates Mitochondrial Oxygen Consumption and Dynamics in Human Skeletal Muscle Cells. J. Biol. Chem. 2016, 291, 1514–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of Vitamin D on Skeletal Muscle Function: Oxidative Stress, Energy Metabolism and Anabolic State. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogan, D.; Pritchett, K. Vitamin D and the Athlete: Risks, Recommendations, and Benefits. Nutrients 2013, 5, 1856–1868. [Google Scholar] [CrossRef] [Green Version]
- Carlberg, C.; Haq, A. The Concept of the Personal Vitamin D Response Index. J. Steroid Biochem. Mol. Biol. 2018, 175, 12–17. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero-García, A.; Córdova-Martínez, A.; Vicente-Salar, N.; Roche, E.; Pérez-Valdecantos, D. Vitamin D, Its Role in Recovery after Muscular Damage Following Exercise. Nutrients 2021, 13, 2336. https://doi.org/10.3390/nu13072336
Caballero-García A, Córdova-Martínez A, Vicente-Salar N, Roche E, Pérez-Valdecantos D. Vitamin D, Its Role in Recovery after Muscular Damage Following Exercise. Nutrients. 2021; 13(7):2336. https://doi.org/10.3390/nu13072336
Chicago/Turabian StyleCaballero-García, Alberto, Alfredo Córdova-Martínez, Néstor Vicente-Salar, Enrique Roche, and Daniel Pérez-Valdecantos. 2021. "Vitamin D, Its Role in Recovery after Muscular Damage Following Exercise" Nutrients 13, no. 7: 2336. https://doi.org/10.3390/nu13072336





