Iron-Containing Oral Contraceptives and Their Effect on Hemoglobin and Biomarkers of Iron Status: A Narrative Review
Abstract
:1. Introduction
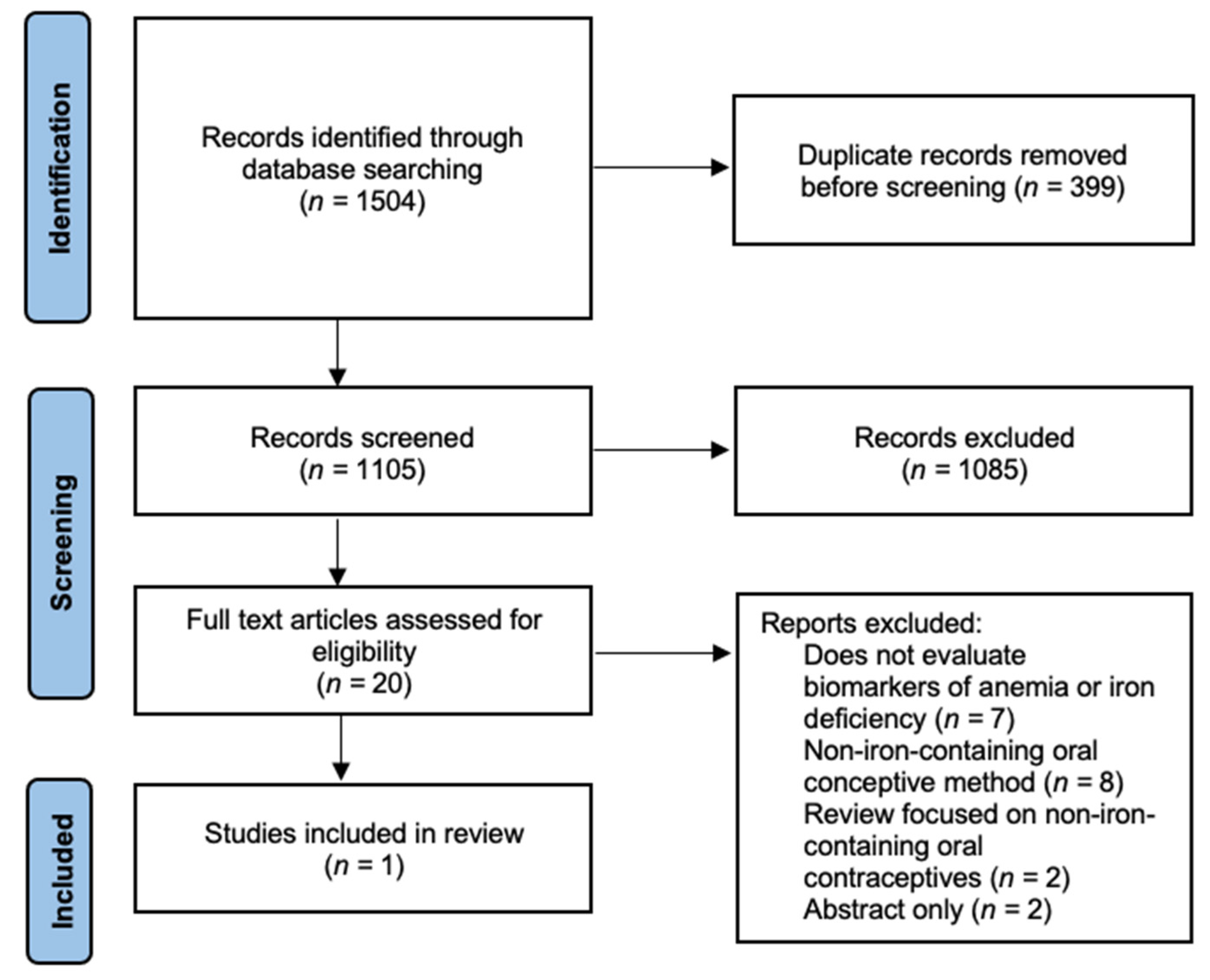
2. Materials and Methods
2.1. Search Methods
2.2. Selection Criteria and Eligibility
3. Results
3.1. Iron-Containing Oral Contraceptives
3.2. Trials Evaluating the Effect of Iron-Containing Oral Contraceptives on Biomarkers of Anemia and Iron Status
3.3. Attitudes towards Inclusion of Iron in Oral Contraceptives
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cleland, J.; Conde-Agudelo, A.; Peterson, H.; Ross, J.; Tsui, A. Family Planning 2: Contraception and health. Lancet 2012, 380, 149–156. [Google Scholar] [CrossRef]
- United Nations. Contraceptive Use by Method 2019: Data Booklet; United Nations: Geneva, Switzerland, 2019. [Google Scholar]
- Britton, L.E.; Alspaugh, A.; Greene, M.Z.; McLemore, M.R. CE: An evidence-based update on contraception. Am. J. Nurs. 2020, 120, 22–33. [Google Scholar] [CrossRef]
- Urrutia, R.P.; Polis, C.B. Fertility awareness based methods for pregnancy prevention. BMJ 2019, 366. [Google Scholar] [CrossRef]
- United Nations Goal 3|Department of Economic and Social Affairs. Available online: https://sdgs.un.org/goals/goal3 (accessed on 4 June 2021).
- Frye, C.A. An overview of oral contraceptives: Mechanism of action and clinical use. Neurology 2006, 66, 29–36. [Google Scholar] [CrossRef]
- Dhont, M. Non-contraceptive benefits of oral contraceptives. Open Access J. Contracept. 2011, 2, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [Green Version]
- Sriprasert, I.; Pakrashi, T.; Kimble, T.; Archer, D.F. Heavy menstrual bleeding diagnosis and medical management. Contracept. Reprod. Med. 2017, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mansour, D.; Hofmann, A.; Gemzell-Danielsson, K. A review of clinical guidelines on the management of iron deficiency and iron-deficiency anemia in women with heavy menstrual bleeding. Adv. Ther. 2021, 38, 201–225. [Google Scholar] [CrossRef]
- Lethaby, A.; Wise, M.R.; Weterings, M.A.J.; Rodriguez, M.B.; Brown, J. Combined hormonal contraceptives for heavy menstrual bleeding. Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef]
- Yeasmin, T.; Haque, M.S.; Yeasmin, S.; Amin, M.R. Iron status in women using oral contraceptives. Bangladesh J. Physiol. Pharmacol. 2014, 26, 25–29. [Google Scholar] [CrossRef]
- Hasanat, F.; Chakroborty, P.; Hasanat, A.; Sharmin, S.; Mannan, M.; Nargis, S. Status of serum iron and copper in women taking oral contraceptive. Bangladesh J. Med. Biochem. 2018, 10, 5–9. [Google Scholar] [CrossRef]
- Milman, N.; Clausen, J.; Byg, K.E. Iron status in 268 Danish women aged 18–30 years: Influence of menstruation, contraceptive method, and iron supplementation. Ann. Hematol. 1998, 77, 13–19. [Google Scholar] [CrossRef]
- Milman, N.; Rosdahl, N.; Lyhne, N.; Jørgensen, T.; Graudal, N. Iron status in Danish women aged 35–65 years: Relation to menstruation and method of contraception. Acta Obstet. Gynecol. Scand. 1993, 72, 601–605. [Google Scholar] [CrossRef]
- Wilson, S.M.C.; Bivins, B.N.; Russell, K.A.; Bailey, L.B. Oral contraceptive use: Impact on folate, vitamin B 6, and vitamin B 12 status. Nutr. Rev. 2011, 69, 572–583. [Google Scholar] [CrossRef]
- Lynch, S.; Pfeiffer, C.M.; Georgieff, M.K.; Brittenham, G.; Fairweather-Tait, S.; Hurrell, R.F.; McArdle, H.J.; Raiten, D.J. Biomarkers of nutrition for development (BOND)- Iron review. J. Nutr. 2018, 148, 1001S–1067S. [Google Scholar] [CrossRef] [Green Version]
- Camaschella, C. Review Series: Iron metabolism and its disorders—Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- World Health Organization. Worldwide Prevalence of Anaemia 1993–2005; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Haas, J.D.; Brownlie, T., IV. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J. Nutr. 2001, 131, 676S–690S. [Google Scholar] [CrossRef] [Green Version]
- Low, M.S.Y.; Speedy, J.; Styles, C.E.; De-Regil, L.M.; Pasricha, S.R. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database Syst. Rev. 2016, 2016. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Daily Iron Supplementation in Adult Women and Adolescent Girls; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Low Estrogen Birth Control|Lo Loestrin® Fe. Available online: https://www.loloestrin.com/ (accessed on 4 June 2021).
- Lo Minastrin Fe. Available online: https://allergan-web-us-prod.azurewebsites.net/assets/pdf/lo-minastrin-fe-_pi (accessed on 4 June 2021).
- FDA. CDER Lo/Ovral-28* and Ferrous Fumarate Tablets (Norgestrel and Ethinyl Estradiol Tablets and Ferrous Fumarate Tablets). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/017612s043,018206s021lbl.pdf (accessed on 4 June 2021).
- FDA. CDER Taytulla. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/204426s004lbl.pdf (accessed on 4 June 2021).
- FDA. CDER ESTROSTEP Fe. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020130s018lbl.pdf (accessed on 4 June 2021).
- Balcoltra®. Available online: https://www.balcoltra.com/ (accessed on 4 June 2021).
- Hailey Fe 1/20—FDA Prescribing Information, Side Effects and Uses. Available online: https://www.drugs.com/pro/hailey-fe-1-20.html (accessed on 4 June 2021).
- MICROGESTIN® (Norethindrone Acetate and Ethinyl Estradiol Tablets, USP). Available online: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a56c0da1-1a72-4765-9dc4-09127a1e3505&type=display (accessed on 4 June 2021).
- Norminest® Fe Tablets. Available online: http://labeling.pfizer.com/ShowLabeling.aspx?id=9403 (accessed on 4 June 2021).
- Loestrin® 24 Fe. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021871lbl.pdf (accessed on 4 June 2021).
- FDA. CDER MinastrinTM 24 Fe. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204426s000lbl.pdf (accessed on 4 June 2021).
- Suvida—The Best Oral Contraceptive Pill—EskagPharma. Available online: http://eskag.in/suvida-the-best-oral-contraceptive-pill/ (accessed on 4 June 2021).
- Government of India. Reference Manual for Oral Contraceptive Pills Family Planning; Division Ministry of Health and Family Welfare: New Delhi, India, 2016.
- Incepta Pharmaceuticals|A Leading Pharmaceutical Company in Bangladesh. Available online: http://www.inceptapharma.com/product-details.php?pid=505 (accessed on 4 June 2021).
- Teva’s Generic of Loestrin® 21 Tablets: Junel® 1/20 (Norethindrone Acetate and Ethinyl Estradiol Tablets, USP). Available online: https://www.tevagenerics.com/product/junel-1-20-norethindrone-acetate-and-ethinyl-estradiol-tablets-usp (accessed on 4 June 2021).
- Prescription Medications—Charlize®. Available online: https://trust.ph/prescription/ (accessed on 4 June 2021).
- Trust Pill Full Prescribing Information, Dosage & Side Effects|MIMS Philippines. Available online: https://www.mims.com/philippines/drug/info/trust%20pill?type=full#:~:text=Ethinyl%20Estradiol%20%2B%20Levonorgestrel%20%2B%20Ferrous%20Fumarate,symptoms%2C%20and%20excessive%20uterine%20bleeding (accessed on 4 June 2021).
- World Health Organization. Zinnia F: Patient Information Leaflet; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Levofem. Available online: https://www.rxnigeria.com/en/items?task=view&id=4707#brand_name (accessed on 4 June 2021).
- Lasserson, T.J.; Thomas, J.; Higgins, J.P.T. Starting a review. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; pp. 3–12. ISBN 978-1-11953-660-4. [Google Scholar]
- Con fer: Contraception plus iron. Drug Ther. Bull. 1974, 12, 55–56.
- Lydia Rosa Full Prescribing Information, Dosage & Side Effects|MIMS Myanmar. Available online: https://www.mims.com/myanmar/drug/info/lydia%20rosa?type=full (accessed on 4 June 2021).
- DKT International, Inc. 2020 Monthly Sales Report v2.16.2021.xlsx; DKT International, Inc.: Washington, DC, USA, 2021. [Google Scholar]
- Rivera, R.; Almonte, H.; Arreola, M.; Iqez, F.; Mcnarrez, G.; Navarro, C.; Perkin, G.W.; Ruiz, R. The effects of three different regimens of oral contraceptives and three different intrauterine devices on the levels of hemoglobin, serum iron and iron binding capacity in anemic women. Contraception 1983, 27, 311–327. [Google Scholar] [CrossRef]
- Task Force for Epidemiological Research on Reproductive Health. Effects of contraceptives on hemoglobin and ferritin. Contraception 1998, 58, 261–273. [Google Scholar] [CrossRef]
- Richards-Brandt, M. Twenty-eight-day oral contraceptives: Physician and user attitudes. Clin. Ther. 1988, 10, 259–262. [Google Scholar]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Gebremedhin, S.; Asefa, A. Association between type of contraceptive use and haemoglobin status among women of reproductive age in 24 sub-Saharan Africa countries. BMJ Sex. Reprod. Health 2019, 45, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Shere, M.; Bapat, P.; Nickel, C.; Kapur, B.; Koren, G. The effectiveness of folate-fortified oral contraceptives in maintaining optimal folate levels to protect against neural tube defects: A systematic review. J. Obstet. Gynaecol. Can. 2015, 37, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Otten, J.J.; Hellwig, J.P.; Meyers, L.D. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006; ISBN 978-0-309-15742-1. [Google Scholar]
- Schümann, K.; Kroll, S.; Weiss, G.; Frank, J.; Biesalski, H.K.; Daniel, H.; Friel, J.; Solomons, N.W. Monitoring of hematological, inflammatory and oxidative reactions to acute oral iron exposure in human volunteers: Preliminary screening for selection of potentially-responsive biomarkers. Toxicology 2005, 212, 10–23. [Google Scholar] [CrossRef]
- Schümann, K.; Ettle, T.; Szegner, B.; Elsenhans, B.; Solomons, N.W. On risks and benefits of iron supplementation recommendations for iron intake revisited. J. Trace Elem. Med. Biol. 2007, 21, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Brissot, P.; Ropert, M.; Le Lan, C.; Loréal, O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochim. Biophys. Acta 2011, 169, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Mollet, I.G.; Dilipkumar, P.; Govani, F.S.; Giess, A.; Paschalaki, K.; Periyasamy, M.; Lidington, E.C.; Mason, J.C.; Jones, M.D.; Game, L.; et al. Low dose iron treatments induce a DNA damage response in human endothelial cells within minutes. PLoS ONE 2016, 11, e0147990. [Google Scholar] [CrossRef]
- Aksu, B.Y.; Hasbal, C.; Himmetoglu, S.; Dincer, Y.; Koc, E.E.; Hatipoglu, S.; Akcay, T. Leukocyte DNA damage in children with iron deficiency anemia: Effect of iron supplementation. Eur. J. Pediatr. 2010, 169, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Reichmann, H. Role of iron in neurodegenerative diseases. J. Neural Transm. 2016, 123, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.B.; Moen, I.W.; Mandrup-Poulsen, T. Iron: The hard player in diabetes pathophysiology. Acta Physiol. 2014, 210, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Lind, T.; Seswandhana, R.; Persson, L.-Å.; Lönnerdal, B. Iron supplementation of iron-replete Indonesian infants is associated with reduced weight-for-age. Acta Paediatr. Int. J. 2008, 97, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B. Iron deficiency and child development. Food Nutr. Bull. 2007, 28, S560–S571. [Google Scholar] [CrossRef]
- Lozoff, B.; Castillo, M.; Clark, K.M.; Smith, J.B. Iron-fortified vs low-iron infant formula: Developmental outcome at 10 years. Arch. Pediatr. Adolesc. Med. 2012, 166, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Dewey, K.G.; Domellöf, M.; Cohen, R.J.; Rivera, L.L.; Hernell, O.; Lönnerdal, B. Iron supplementation affects growth and morbidity of breast-fed infants: Results of a randomized trial in Sweden and Honduras. J. Nutr. 2002, 132, 3249–3255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sazawal, S.; Black, R.E.; Ramsan, M.; Chwaya, H.M.; Stoltzfus, R.J.; Dutta, A.; Dhingra, U.; Kabole, I.; Deb, S.; Othman, M.K.; et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: Community-based, randomised, placebo-controlled trial. Lancet 2006, 367, 133–143. [Google Scholar] [CrossRef]
- Jaeggi, T.; Kortman, G.A.M.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef]
- Paganini, D.; Zimmermann, M.B. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: A review. Am. J. Clin. Nutr. 2017, 106, 1688S–1693S. [Google Scholar] [CrossRef] [Green Version]
- Paganini, D.; Uyoga, M.A.; Kortman, G.A.M.; Cercamondi, C.I.; Moretti, D.; Barth-Jaeggi, T.; Schwab, C.; Boekhorst, J.; Timmerman, H.M.; Lacroix, C.; et al. Prebiotic galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: A randomised controlled study in Kenyan infants. Gut 2017, 66, 1956–1967. [Google Scholar] [CrossRef] [Green Version]
- Dostal, A.; Baumgartner, J.; Riesen, N.; Chassard, C.; Smuts, C.M.; Zimmermann, M.B.; Lacroix, C. Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: A randomised, placebo-controlled intervention trial in South African children. Br. J. Nutr. 2014, 112, 547–556. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Fucharoen, S.; Winichagoon, P.; Sirankapracha, P.; Zeder, C.; Gowachirapant, S.; Judprasong, K.; Tanno, T.; Miller, J.L.; Hurrell, R.F. Iron metabolism in heterozygotes for hemoglobin E (HbE), α-thalassemia 1, or β-thalassemia and in compound heterozygotes for HbE/β-thalassemia. Am. J. Clin. Nutr. 2008, 88, 1026–1031. [Google Scholar] [CrossRef] [Green Version]
- Tondeur, M.C.; Schauer, C.S.; Christofides, A.L.; Asante, K.P.; Newton, S.; Serfass, R.E.; Zlotkin, S.H. Determination of iron absorption from intrinsically labeled microencapsulated ferrous fumarate (sprinkles) in infants with different iron and hematologic status by using a dual-stable-isotope method. Am. J. Clin. Nutr. 2004, 80, 1436–1444. [Google Scholar] [CrossRef]
- Youssef, A.M.; Shata, A.F.; Kamal, H.M.; El-saied, Y.; Ali, O.F. A comparative study of efficacy, tolerability, and compliance of oral iron preparations for iron deficiency anemia in pregnant women. Am. J. Med. Med. Sci. 2014, 4, 244–249. [Google Scholar] [CrossRef]
- Milman, N.; Jønsson, L.; Dyre, P.; Pedersen, P.L.; Larsen, L.G. Ferrous bisglycinate 25 mg iron is as effective as ferrous sulfate 50 mg iron in the prophylaxis of iron deficiency and anemia during pregnancy in a randomized trial. J. Perinat. Med. 2014, 42, 197–206. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO|Essential Medicines. Available online: https://www.who.int/medicines/services/essmedicines_def/en/ (accessed on 4 June 2021).
- Roche, M.L.; Samson, K.L.I.; Green, T.J.; Karakochuk, C.D.; Martinez, H. Perspective: Weekly iron and folic acid supplementation (WIFAS): A critical review and rationale for inclusion in the essential medicines list to accelerate anemia and neural tube defects reduction. Adv. Nutr. 2021, 12, 334–342. [Google Scholar] [CrossRef] [PubMed]
| Brand Name | Pharmaceutical Company | Reported Country of Use | Marketed Iron Dose (mg) | Elemental Iron Dose (mg) | Number of Iron Tablet Days/Total Number of Tablets in Monthly Package | Form of Iron | Relevant Information Found in Package Insert Regarding the Inclusion of Iron |
|---|---|---|---|---|---|---|---|
| Lo Loestrin® Fe [24] Lo Minastrin™ Fe [25] | AbbVie Inc. | United States | 75 | ~25 | 2/28 | Ferrous Fumarate | The ferrous fumarate tablets do not serve any therapeutic purpose. |
| Taytulla™ [27] ESTROSTEP Fe™ [28] | AbbVie Inc. | United States | 75 | ~25 | 4/28 | Ferrous Fumarate | (Ferrous fumarate) does not serve any therapeutic purpose. |
| Balcoltra® [29] | Avion Pharmaceuticals | United States | 36.5 | 10 | 7/28 | Ferrous Bisglycinate | The ferrous bisglycinate tablets do not serve any therapeutic purpose. |
| Levofem® [42] | DKT International Nigeria | Nigeria | 75 | ~25 | 7/28 | Ferrous Fumarate | Contains ferrous fumarate for blood forming or repair ingredient by stimulating the formation of red blood cells. |
| Charlize® [39] Trust Pill® [40] | DKT Philippines Inc. | Philippines | 75 | 24.75 | 7/28 | Ferrous Fumarate | Ferrous fumarate is an iron supplement that helps improve the hemoglobin content of blood during menstruation. |
| Suvida® [35] | Eskag Pharma | India | 60 | ~25 | 7/28 | Ferrous Fumarate | None listed. |
| Hailey™ Fe [30] | Glenmark Pharmaceuticals | United States | 75 | ~25 | 7/28 | Ferrous Fumarate | The ferrous fumarate tablets are present to facilitate ease of drug administration via a 28-day regimen, are non-hormonal, and do not serve any therapeutic purpose. |
| Lyta-28® [37] | Incepta Pharmaceuticals | Bangladesh | 75 | ~25 | 7/28 | Ferrous Fumarate | None listed. |
| Microgestin® Fe [31] | Mayne Pharma | United States | 75 | ~25 | 7/28 | Ferrous Fumarate | The ferrous fumarate tablets are present to facilitate ease of drug administration via a 28-day regimen, are non-hormonal, and do not serve any therapeutic purpose. |
| Zinnia-F [41] | Mylan Laboratories Limited | Cambodia | 75 | ~25 | 7/28 | Ferrous Fumarate | The other tablets (7 brown tablets) are hormone-free. |
| Norminest® Fe [32] Norquest® Fe [32] | Pfizer | United States | 75 | ~25 | 7/28 | Ferrous Fumarate | The iron tablets are not included for any therapeutic purpose but to provide a daily tablet regimen for days 22 through 28 of the cycle. |
| Junel® 1/20 [38] | Teva Generics | Israel | 75 | ~25 | 7/28 | Ferrous Fumarate | The ferrous fumarate tablets are present to facilitate ease of drug administration via a 28-day regimen, are non-hormonal, and do not serve any therapeutic purpose. |
| Loestrin® 24 Fe [33] | Warner Chilcott Company | United States | 75 | ~25 | 7/28 | Ferrous Fumarate | The ferrous fumarate tablets are present to facilitate ease of drug administration via a 28-day regimen, are non-hormonal, and do not serve any therapeutic purpose. |
| Minastrin™ Fe [34] | Warner Chilcott Company | United States | 75 | ~25 | 4/24 | Ferrous Fumarate | The ferrous fumarate capsules do not serve any therapeutic purpose. |
| Lo/Ovral®-28 [26] Ferrous Fumarate [26] | Wyeth Pharmaceuticals | United States | 75 | ~25 | 7/28 | Ferrous Fumarate | The hormone-free tablets containing ferrous fumarate should not be used in women with iron storage disorders, such as hemochromatosis and hemosiderosis. |
| Mala-N [36] | None reported | India | 75 | ~25 | 7/28 | Ferrous Fumarate | None listed. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, J.A.J.; Sasai, C.S.; Karakochuk, C.D. Iron-Containing Oral Contraceptives and Their Effect on Hemoglobin and Biomarkers of Iron Status: A Narrative Review. Nutrients 2021, 13, 2340. https://doi.org/10.3390/nu13072340
Fischer JAJ, Sasai CS, Karakochuk CD. Iron-Containing Oral Contraceptives and Their Effect on Hemoglobin and Biomarkers of Iron Status: A Narrative Review. Nutrients. 2021; 13(7):2340. https://doi.org/10.3390/nu13072340
Chicago/Turabian StyleFischer, Jordie A. J., Carolina S. Sasai, and Crystal D. Karakochuk. 2021. "Iron-Containing Oral Contraceptives and Their Effect on Hemoglobin and Biomarkers of Iron Status: A Narrative Review" Nutrients 13, no. 7: 2340. https://doi.org/10.3390/nu13072340
APA StyleFischer, J. A. J., Sasai, C. S., & Karakochuk, C. D. (2021). Iron-Containing Oral Contraceptives and Their Effect on Hemoglobin and Biomarkers of Iron Status: A Narrative Review. Nutrients, 13(7), 2340. https://doi.org/10.3390/nu13072340






