Supplementation of Morin Restores the Altered Bone Histomorphometry in Hyperglycemic Rodents via Regulation of Insulin/IGF-1 Signaling
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leidig-Bruckner, G.; Grobholz, S.; Bruckner, T.; Scheidt-Nave, C.; Nawroth, P.; Schneider, J.G. Prevalence and determinants of osteoporosis in patients with type 1 and type 2 diabetes mellitus. BMC Endocr. Disord. 2014, 14, 33. [Google Scholar] [CrossRef] [Green Version]
- Wongdee, K.; Charoenphandhu, N. Osteoporosis in diabetes mellitus: Possible cellular and molecular mechanisms. World J. Diabetes 2011, 2, 41–48. [Google Scholar] [CrossRef]
- Garay-Sevilla, M.E.; Nava, L.E.; Malacara, J.M.; Wrobel, K.; Wrobel, K.; Perez, U. Advanced glycosylation end products (AGEs), insulin-like growth factor-1 (IGF-1) and IGF-binding protein-3 (IGFBP-3) in patients with type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2000, 16, 106–113. [Google Scholar] [CrossRef]
- Raisingani, M.; Preneet, B.; Kohn, B.; Yakar, S. Skeletal growth and bone mineral acquisition in type 1 diabetic children; abnormalities of the GH/IGF-1 axis. Growth Horm. IGF Res. 2017, 34, 13–21. [Google Scholar] [CrossRef]
- Ardawi, M.S.; Akhbar, D.H.; Alshaikh, A.; Ahmed, M.M.; Qari, M.H.; Rouzi, A.A.; Ali, A.Y.; Abdulrafee, A.A.; Saeda, M.Y. Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone 2013, 56, 355–362. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, N.; Cheng, G.; Zhang, Q.; He, Y.; Shen, Y.; Zhang, Q.; Zhu, B.; Zhang, Q.; Qin, L. Rehmannia glutinosa Libosch Extracts Prevent Bone Loss and Architectural Deterioration and Enhance Osteoblastic Bone Formation by Regulating the IGF-1/PI3K/mTOR Pathway in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2019, 20, 3964. [Google Scholar] [CrossRef] [Green Version]
- An, J.L.; Zhang, W.; Zhang, J.; Lian, L.C.; Shen, Y.; Ding, W.Y. Vitamin D improves the content of TGF-beta and IGF-1 in intervertebral disc of diabetic rats. Exp. Biol. Med. 2017, 242, 1254–1261. [Google Scholar] [CrossRef] [Green Version]
- Adil, M.; Khan, R.A.; Kalam, A.; Venkata, S.K.; Kandhare, A.D.; Ghosh, P.; Sharma, M. Effect of anti-diabetic drugs on bone metabolism: Evidence from preclinical and clinical studies. Pharmacol. Rep. 2017, 69, 1328–1340. [Google Scholar] [CrossRef]
- Caselli, A.; Cirri, P.; Santi, A.; Paoli, P. Morin: A Promising Natural Drug. Curr. Med. Chem. 2016, 23, 774–791. [Google Scholar] [CrossRef]
- Vanitha, P.; Uma, C.; Suganya, N.; Bhakkiyalakshmi, E.; Suriyanarayanan, S.; Gunasekaran, P.; Sivasubramanian, S.; Ramkumar, K.M. Modulatory effects of morin on hyperglycemia by attenuating the hepatic key enzymes of carbohydrate metabolism and beta-cell function in streptozotocin-induced diabetic rats. Environ. Toxicol. Pharmacol. 2014, 37, 326–335. [Google Scholar] [CrossRef]
- Paoli, P.; Cirri, P.; Caselli, A.; Ranaldi, F.; Bruschi, G.; Santi, A.; Camici, G. The insulin-mimetic effect of Morin: A promising molecule in diabetes treatment. Biochim. Biophys. Acta 2013, 1830, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Hsu, C.C.; Lin, J.; Cheng, J.T.; Wu, M.C. Investigation of morin-induced insulin secretion in cultured pancreatic cells. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wan, X.; Li, Y.; Zhang, H.; Zhang, L. Morin protects glucocorticoid-induced osteoporosis through regulating the mitogen-activated protein kinase signaling pathway. J. Nat. Med. 2018, 72, 929–936. [Google Scholar] [CrossRef]
- Sultana, F.; Rasool, M. A novel therapeutic approach targeting rheumatoid arthritis by combined administration of morin, a dietary flavanol and non-steroidal anti-inflammatory drug indomethacin with reference to pro-inflammatory cytokines, inflammatory enzymes, RANKL and transcription factors. Chem. Biol. Interact. 2015, 230, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Abuohashish, H.M.; Al-Rejaie, S.S.; Al-Hosaini, K.A.; Parmar, M.Y.; Ahmed, M.M. Alleviating effects of morin against experimentally-induced diabetic osteopenia. Diabetol. Metab. Syndr. 2013, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kalu, D.N.; Liu, C.C.; Hardin, R.R.; Hollis, B.W. The aged rat model of ovarian hormone deficiency bone loss. Endocrinology 1989, 124, 7–16. [Google Scholar] [CrossRef]
- Tamagaki, K.; Yuan, Q.; Ohkawa, H.; Imazeki, I.; Moriguchi, Y.; Imai, N.; Sasaki, S.; Takeda, K.; Fukagawa, M. Severe hyperparathyroidism with bone abnormalities and metastatic calcification in rats with adenine-induced uraemia. Nephrol. Dial. Transplant. 2006, 21, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar]
- Londzin, P.; Kisiel-Nawrot, E.; Kocik, S.; Janas, A.; Trawczyński, M.; Cegieła, U.; Folwarczna, J. Effects of diosgenin on the skeletal system in rats with experimental type 1 diabetes. Biomed. Pharmacother. 2020, 129, 110342. [Google Scholar] [CrossRef] [PubMed]
- Rivoira, M.; Rodríguez, V.; Picotto, G.; Battaglino, R.; De Talamoni, N.T. Naringin prevents bone loss in a rat model of type 1 Diabetes mellitus. Arch. Biochem. Biophys. 2018, 637, 56–63. [Google Scholar] [CrossRef]
- Subash, S.; Subramanian, P. Morin a flavonoid exerts antioxidant potential in chronic hyperammonemic rats: A biochemical and histopathological study. Mol. Cell. Biochem. 2009, 327, 153–161. [Google Scholar] [CrossRef]
- Higashi, Y.; Sukhanov, S.; Anwar, A.; Shai, S.Y.; Delafontaine, P. Aging, atherosclerosis, and IGF-1. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 626–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekbote, V.H.; Khadilkar, V.V.; Khadilkar, A.V.; Mughal, Z.; Chiplonkar, S.A.; Palande, S.A.; Phanse-Gupte, S.S.; Patwardhan, V.G.; Shilvant, D.S. Relationship of insulin-like growth factor 1 and bone parameters in 7–15 years old apparently, healthy Indian children. Indian J. Endocrinol. Metab. 2015, 19, 770–774. [Google Scholar] [CrossRef]
- Yakar, S.; Canalis, E.; Sun, H.; Mejia, W.; Kawashima, Y.; Nasser, P.; Courtland, H.W.; Williams, V.; Bouxsein, M.; Rosen, C.; et al. Serum IGF-1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J. Bone Miner. Res. 2009, 24, 1481–1492. [Google Scholar] [CrossRef] [Green Version]
- Ola, M.S.; Aleisa, A.M.; Al-Rejaie, S.S.; Abuohashish, H.M.; Parmar, M.Y.; Alhomida, A.S.; Ahmed, M.M. Flavonoid, morin inhibits oxidative stress, inflammation and enhances neurotrophic support in the brain of streptozotocin-induced diabetic rats. Neurol. Sci. 2014, 35, 1003–1008. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]

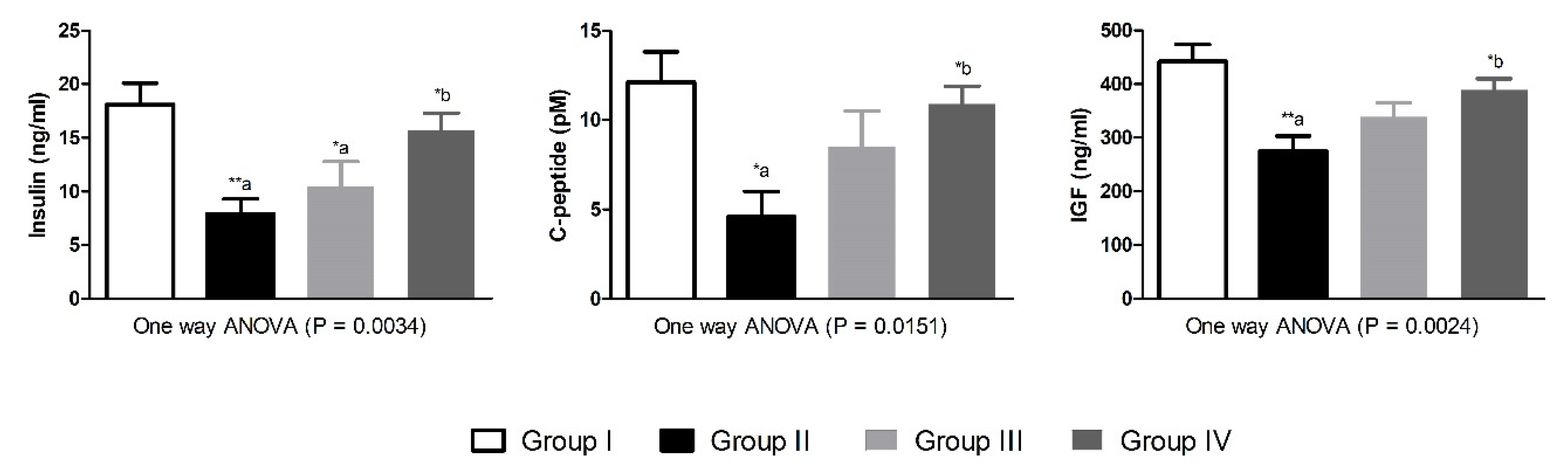

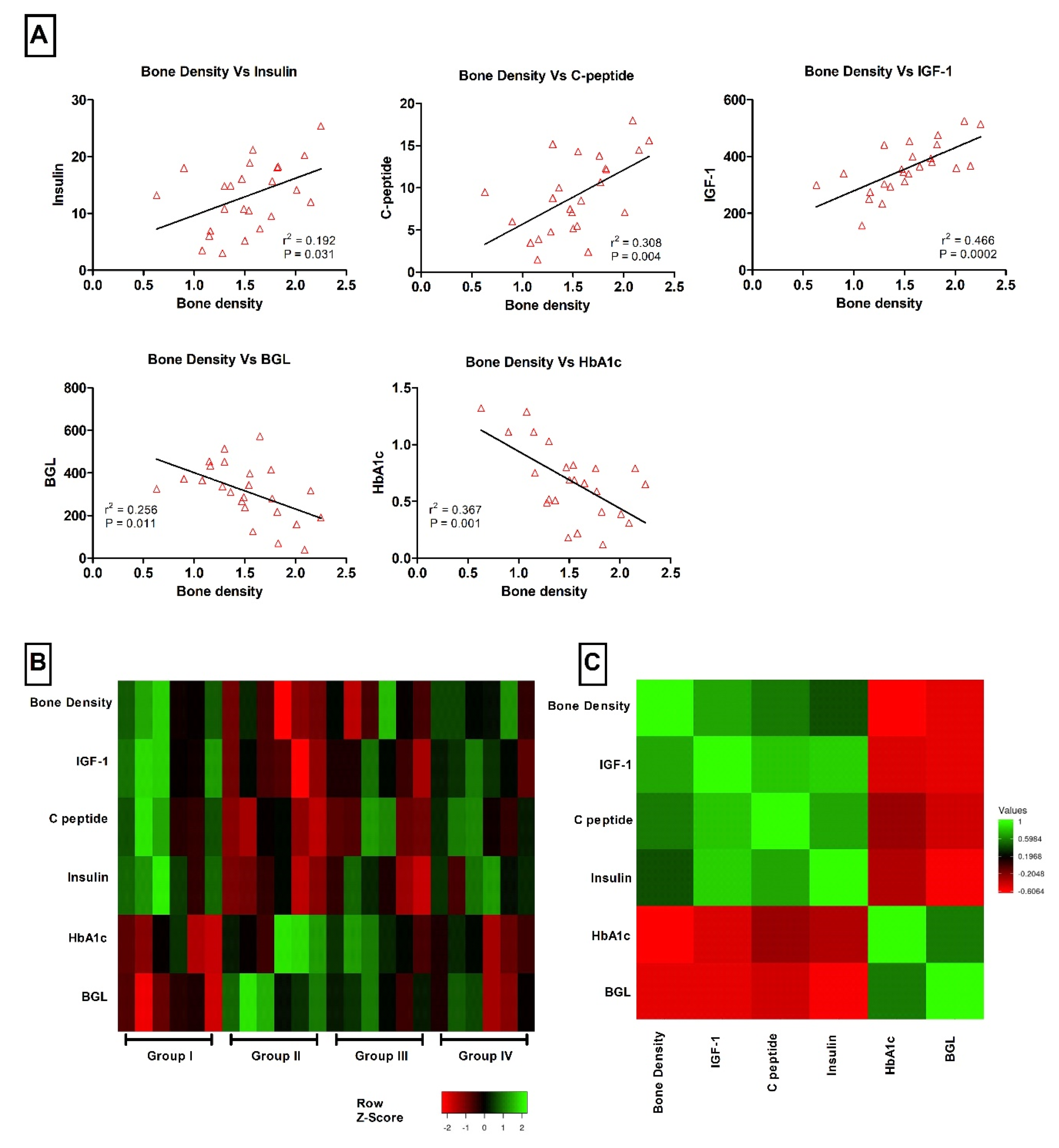
| Abbreviation | Unit | Group-I | Group-II | Group-III | Group-IV | One Way ANOVA p Value | |
|---|---|---|---|---|---|---|---|
| Initial Body weight | IBW | g | 275 ± 4.8 | 286 ± 6.2 | 283 ± 6.1 | 268 ± 5.5 | 0.1396 |
| Final Body weight | FBW | g | 321 ± 8.9 | 209 ± 12 ***,a | 233 ± 10 ***,a | 266 ± 9 **,a,b | <0.0001 |
| Bone weight | BW | g | 0.59 ± 0.03 | 0.44 ± 0.03 **,a | 0.48 ± 0.02 | 0.54 ± 0.03 *,b | 0.0058 |
| Bone density | BMD | g/cm3 | 1.82 ± 0.12 | 1.16 ± 0.13 **,a | 1.44 ± 0.16 | 1.67 ± 0.09 *,b | 0.0088 |
| Bone weight/100 g final body weight | BW/100FBW | % | 0.184 ± 0.019 | 0.234 ± 0.031 | 0.221 ± 0.017 | 0.218 ± 0.027 | 0.5202 |
| Biochemical Parameters | Abbreviation | Unit | Group-I | Group-II | Group-III | Group-IV | One Way ANOVA p Value |
|---|---|---|---|---|---|---|---|
| Glycated hemoglobin | HbA1c | % | 0.41 ± 0.11 | 0.95 ± 0.14 *,a | 0.82 ± 0.09 | 0.53 ± 0.08 | 0.0068 |
| Blood glucose level | BGL | mg/dL | 178 ± 42 | 444 ± 37 ***,a | 343 ± 28 *,a | 280 ± 49 *,b | 0.0012 |
| Alkaline Phosphatase | ALP | U/L | 206 ± 47 | 1183 ± 388 *,a | 813 ± 157 | 652 ± 169 | 0.0462 |
| Alanine aminotransferase | ALT | U/L | 43.35 ± 4.46 | 66.62 ± 11.41 | 47.50 ± 6.10 | 55.16 ± 7.28 | 0.1912 |
| Aspartate Aminotransferase | AST | U/L | 78.03 ± 10 | 108.99 ± 13 | 83.57 ± 11 | 89.50 ± 8 | 0.2204 |
| Blood urea nitrogen | BUN | µm/L | 66.31 ± 22 | 183.30 ± 51 | 129.85 ± 15 | 131.67 ± 27 | 0.1114 |
| Creatinine | Cr | µm/L | 64.71 ± 22 | 85.97 ± 6 | 54.58 ± 28 | 61.71 ± 15 | 0.7018 |
| Histomorphometry Parameters | Abbreviation | Unit | Group-I | Group-II | Group-III | Group-IV | One Way ANOVA p Value |
|---|---|---|---|---|---|---|---|
| Osteoclast surface/bone surface | Oc.S/BS | % | 1.9 ± 0.09 | 3.2 ± 0.4 **,a | 2.8 ± 0.3 | 2.1 ± 0.1 *,b | 0.0065 |
| Porosity area/cortical bone area | Po.Ar/Ct.Ar | % | 2.1 ± 0.5 | 4.2 ± 0.4 *,a | 3.4 ± 0.4 | 2.6 ± 0.4 | 0.0126 |
| Osteoid volume/cortical bone area | OV/Ct.Ar | % | 1.4 ± 0.3 | 3.6 ± 0.5 **,a | 2.9 ± 0.55 | 2 ± 0.3 | 0.0084 |
| Mineralizing surface/osteoid surface | MS/BS | % | 57.1 ± 6 | 18.9 ± 4 ***,a | 28.6 ± 5 **,a | 38.4 ± 6 | 0.0004 |
| Fibrosis tissue volume/cortical bone area | Fb.V/Ct.Ar | % | 0.02 ± 0.009 | 0.2 ± 0.04 ***,a | 0.1 ± 0.03 | 0.08 ± 0.02 *,b | 0.0015 |
| Histomorphometry Parameters | Abbreviation | Unit | Group-I | Group-II | Group-III | Group-IV | One Way ANOVA p Value |
|---|---|---|---|---|---|---|---|
| Osteoclast surface/bone surface | Oc.S/BS | (%) | 5.4 ± 2.1 | 19.2 ± 3.1 **,a | 12.4 ± 2.4 | 9.5 ± 1.6 *,b | 0.0042 |
| Trabecular thickness | Tb.Th | (µm) | 69.4 ± 3 | 30.4 ± 6 ***,a | 43.5 ± 5 **,a | 51.2 ± 4 *,b | <0.0001 |
| Trabecular number | Tb.N | (mm) | 3.5 ± 0.14 | 2.5 ± 0.2 **,a | 2.9 ± 0.12 | 3.2 ± 0.2 *,b | 0.0032 |
| Osteoid volume/bone volume | OV/BV | (%) | 8.3 ± 1.6 | 37.4 ± 6.3 ***,a | 28.5 ± 5.5 *,a | 21.2 ± 2.9 | 0.0015 |
| Mineralizing surface/osteoid surface | MS/BS | (%) | 37.4 ± 4.3 | 17.4 ± 3.1 **,a | 27.3 ± 4.2 | 32.2 ± 3.5 | 0.0095 |
| Fibrosis tissue volume/tissue volume | Fb.V/TV | (%) | 0.1 ± 0.003 | 0.35 ± 0.07 **,a | 0.2 ± 0.04 | 0.12 ± 0.05 *,b | 0.0054 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuohashish, H.M.; AlAsmari, A.F.; Mohany, M.; Ahmed, M.M.; Al-Rejaie, S.S. Supplementation of Morin Restores the Altered Bone Histomorphometry in Hyperglycemic Rodents via Regulation of Insulin/IGF-1 Signaling. Nutrients 2021, 13, 2365. https://doi.org/10.3390/nu13072365
Abuohashish HM, AlAsmari AF, Mohany M, Ahmed MM, Al-Rejaie SS. Supplementation of Morin Restores the Altered Bone Histomorphometry in Hyperglycemic Rodents via Regulation of Insulin/IGF-1 Signaling. Nutrients. 2021; 13(7):2365. https://doi.org/10.3390/nu13072365
Chicago/Turabian StyleAbuohashish, Hatem M., Abdullah F. AlAsmari, Mohamed Mohany, Mohammed M. Ahmed, and Salim S. Al-Rejaie. 2021. "Supplementation of Morin Restores the Altered Bone Histomorphometry in Hyperglycemic Rodents via Regulation of Insulin/IGF-1 Signaling" Nutrients 13, no. 7: 2365. https://doi.org/10.3390/nu13072365
APA StyleAbuohashish, H. M., AlAsmari, A. F., Mohany, M., Ahmed, M. M., & Al-Rejaie, S. S. (2021). Supplementation of Morin Restores the Altered Bone Histomorphometry in Hyperglycemic Rodents via Regulation of Insulin/IGF-1 Signaling. Nutrients, 13(7), 2365. https://doi.org/10.3390/nu13072365









