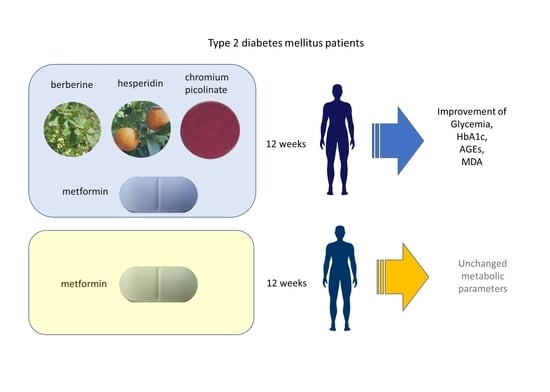
Effect of a New Formulation of Nutraceuticals as an Add-On to Metformin Monotherapy for Patients with Type 2 Diabetes and Suboptimal Glycemic Control: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Nutraceutical Formulation
2.3. Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, H.; Venkatesan, V. Beta-cell Management in Type 2 Diabetes: Beneficial Role of Nutraceuticals. Endocr. Metab. Immune Disord. Drug Targets 2016, 16, 89–98. [Google Scholar] [CrossRef]
- Piarulli, F.; Sartore, G.; Ceriello, A.; Ragazzi, E.; Reitano, R.; Nollino, L.; Cosma, C.; Fedele, D.; Lapolla, A. Relationship between glyco-oxidation, antioxidant status and microalbuminuria in type 2 diabetic patients. Diabetologia 2009, 52, 1419–1425. [Google Scholar] [CrossRef] [Green Version]
- Cook, M.N.; Girman, C.J.; Stein, P.P.; Alexander, C.M. Initial monotherapy with either metformin or sulphonylureas often fails to achieve or maintain current glycaemic goals in patients with Type 2 diabetes in UK primary care. Diabet. Med. 2007, 24, 350–358. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, L.-H.; Zhou, Q.; Zhao, T.-Y.; Wang, H.; Gu, C.-J.; Tong, X.-L. Application of Berberine on Treating Type 2 Diabetes Mellitus. Int. J. Endocrinol. 2015, 2015, 905749. [Google Scholar] [CrossRef] [Green Version]
- Dai, P.; Wang, J.; Lin, L.; Zhang, Y.; Wang, Z. Renoprotective effects of berberine as adjuvant therapy for hypertensive patients with type 2 diabetes mellitus: Evaluation via biochemical markers and color Doppler ultrasonography. Exp. Ther. Med. 2015, 10, 869–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metab. Clin. Exp. 2008, 57, 712–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of Type 2 Diabetes and Dyslipidemia with the Natural Plant Alkaloid Berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Liu, L.; Wang, X.; Liu, X.; Xie, L.; Wang, G. Modulation of glucagon-like peptide-1 release by berberine: In vivo and in vitro studies. Biochem. Pharmacol. 2010, 79, 1000–1006. [Google Scholar] [CrossRef]
- Lu, S.-S.; Yu, Y.-L.; Zhu, H.-J.; Liu, X.-D.; Liu, L.; Liu, Y.-W.; Wang, P.; Xie, L.; Wang, G.-J. Berberine promotes glucagon-like peptide-1 (7–36) amide secretion in streptozotocin-induced diabetic rats. J. Endocrinol. 2009, 200, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derosa, G.; Maffioli, P.; Cicero, A.F.G. Berberine on metabolic and cardiovascular risk factors: An analysis from preclinical evidences to clinical trials. Expert Opin. Biol. Ther. 2012, 12, 1113–1124. [Google Scholar] [CrossRef]
- Singh, I.P.; Mahajan, S. Berberine and its derivatives: A patent review (2009–2012). Expert Opin. Ther. Pat. 2013, 23, 215–231. [Google Scholar] [CrossRef]
- Han, J.; Lin, H.; Huang, W. Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med. Sci. Monit. 2011, 17, RA164-167. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Gu, D.; Li, J.; Cui, K.; Zhang, Y. Effects and Action Mechanisms of Berberine and Rhizoma coptidis on Gut Microbes and Obesity in High-Fat Diet-Fed C57BL/6J Mice. PLoS ONE 2011, 6, e24520. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhao, Y.; Zhang, M.; Pang, X.; Xu, J.; Kang, C.; Li, M.; Zhang, C.; Zhang, Z.; Zhang, Y.; et al. Structural Changes of Gut Microbiota during Berberine-Mediated Prevention of Obesity and Insulin Resistance in High-Fat Diet-Fed Rats. PLoS ONE 2012, 7, e42529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.-S.; Zheng, Y.-R.; Zhang, Y.-F.; Long, X.-Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef]
- Fratter, A.; De Servi, B. New oral delivery system to improve absorption of Berberine: Likely interaction of cationized chitosan with PG-P pump. Int. J. Drug Deliv. Technol. 2014, 5, 33–42. [Google Scholar]
- Johnsen, S.P.; Overvad, K.; Stripp, C.; Tjonneland, A.; E Husted, S.; Sørensen, H.T. Intake of fruit and vegetables and the risk of ischemic stroke in a cohort of Danish men and women. Am. J. Clin. Nutr. 2003, 78, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Dauchet, L.; Ferrieres, J.; Arveiler, D.; Yarnell, J.W.; Gey, F.; Ducimetière, P.; Ruidavets, J.-B.; Haas, B.; Evans, A.; Bingham, A.; et al. Frequency of fruit and vegetable consumption and coronary heart disease in France and Northern Ireland: The PRIME study. Br. J. Nutr. 2004, 92, 963–972. [Google Scholar] [CrossRef]
- Guarnieri, S.; Riso, P.; Porrini, M. Orange juice vs vitamin C: Effect on hydrogen peroxide-induced DNA damage in mononuclear blood cells. Br. J. Nutr. 2007, 97, 639–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morand, C.; Dubray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin contributes to the vascular pro-tective effects of orange juice: A randomized crossover study in healthy volunteers. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.M.J. Citrus poly-phenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ashour, M.B.; Abdel-Moneim, A.; Ahmed, O.M. Hesperidin and naringin attenuate hyperglycemia-mediated ox-idative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabetes Complicat. 2012, 26, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, S.; Katsumata, S.-I.; Suzuki, K.; Nakaya, Y.; Ishimi, Y.; Uehara, M. Hypoglycemic and Hypolipidemic Effects of Hesperidin and Cyclodextrin-Clathrated Hesperetin in Goto-Kakizaki Rats with Type 2 Diabetes. Biosci. Biotechnol. Biochem. 2009, 73, 2779–2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Althuis, M.D.; Jordan, N.E.; Ludington, E.A.; Wittes, J.T. Glucose and insulin responses to dietary chromium supplements: A me-ta-analysis. Am. J. Clin. Nutr. 2002, 76, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Balk, E.M.; Tatsioni, A.; Lichtenstein, A.H.; Lau, J.; Pittas, A.G. Effect of chromium supplementation on glucose metabolism and lipids: A systematic review of randomized controlled trials. Diabetes Care 2007, 30, 2154–2163. [Google Scholar] [CrossRef] [Green Version]
- Broadhurst, C.L.; Domenico, P. Clinical Studies on Chromium Picolinate Supplementation in Diabetes Mellitus—A Review. Diabetes Technol. Ther. 2006, 8, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Agrawal, R.P.; Choudhary, M.; Jain, S.; Goyal, S.; Agarwal, V. Beneficial effect of chromium supplementation on glucose, HbA1C and lipid variables in individuals with newly onset type-2 diabetes. J. Trace Elem. Med. Biol. 2011, 25, 149–153. [Google Scholar] [CrossRef]
- Jain, S.K.; Rains, J.L.; Croad, J.L. Effect of chromium niacinate and chromium picolinate supplementation on lipid peroxidation, TNF-alpha, IL-6, CRP, glycated hemoglobin, triglycerides, and cholesterol levels in blood of streptozotocin-treated diabetic rats. Free Radic. Biol. Med. 2007, 43, 1124–1131. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Xu, X.; Yin, M.; Zhang, Y.; Huang, L.; Chen, R.; Ni, J. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: A systematic literature review and a meta-analysis. Endocr. J. 2019, 66, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Gulfraz, M.; Mehmood, S.; Ahmad, A.; Fatima, N.; Praveen, Z.; Williamson, E.M. Comparison of the antidiabetic activity of Berberis lyceum root extract and berberine in alloxan-induced diabetic rats. Phytother. Res. 2008, 22, 1208–1212. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberine and barberry (Berberis vulgaris): A clinical review. Phytother. Res. 2019, 33, 504–523. [Google Scholar] [CrossRef]
- Zhong, Q.; Wang, I.; William, T.C. Current concepts about chromium supplementation in type 2 diabetes and insulin resistance. Curr. Diabetes Rep. 2010, 10, 145–151. [Google Scholar]
- Cefalu, W.T.; Rood, J.; Pinsonat, P.; Qin, J.; Sereda, O.; Levitan, L.; Anderson, R.A.; Zhang, X.H.; Martin, J.M.; Martin, C.K.; et al. Characterization of the metabolic and physiologic response to chromium supplementation in subjects with type 2 diabetes mellitus. Metabolism 2010, 59, 755–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homayouni, F.; Haidari, F.; Hedayati, M.; Zakerkish, M.; Ahmadi, K. Hesperidin supplementation alleviates oxidative DNA damage and lipid peroxidation in type 2 diabetes: A randomized double-blind placebo-controlled clinical trial. Phytother. Res. 2017, 31, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Khangholi, S.; Majid, F.A.A.; Berwary, N.J.A.; Ahmad, F.; Aziz, R.B.A. The Mechanisms of Inhibition of Advanced Glycation End Products Formation through Polyphenols in Hyperglycemic Condition. Planta Medica 2016, 82, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Tsukushi, S.; Katsuzaki, T.; Aoyama, I.; Takayama, F.; Miyazaki, T.; Shimokata, K.; Niwa, T. Increased erythrocyte 3-DG and AGEs in diabetic hemodialysis patients: Role of the polyol pathway. Kidney Int. 1999, 55, 1970–1976. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.-H.; Wang, Y.-F.; Dai, F.-Y.; Zhao, J.-H.; Li, P. The protective effects of Berberine and Hesperidin on inflammatory factor-stimulating cardiac fibroblasts. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5468–5476. [Google Scholar] [PubMed]
| Parameter | Add-On Group (n = 20) | Control Group (n = 20) | p† |
|---|---|---|---|
| Age, years | 67.2 ± 6.3 | 66.9 ± 6.1 | ns |
| Gender, M/F | 15/5 | 17/3 | ns |
| Duration of diabetes, years | 8.3 ± 3.5 | 7.9 ± 6.1 | ns |
| Smoker, Yes/No | 3/17 | 4/15 | ns |
| Metformin dose, mg/day | 1295 ± 538 | 1450 ± 777 | ns |
| Antihypertensive therapy, Yes/No | 14/6 | 15/5 | ns |
| Statins, Yes/No | 13/7 | 13/7 | ns |
| Parameter | Add-On Group (n = 20) | Control Group (n = 20) | p† (Between Groups) | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| IFCC-HbA1c, mmol/mol (DCCT-HbA1c, %) | 53.5 ± 3.7 (7.0 ± 2.5) | 49.5 ± 5.1 * (6.7 ± 2.6) | 53.9 ± 4.3 (7.1 ± 2.5) | 56.4 ± 4.3 (7.3 ± 2.5) | ns | <0.01 |
| FBG, mg/dL | 145 ± 20 | 128 ± 23 * | 150 ± 32 | 152 ± 35 | ns | <0.05 |
| AGEs, μg/mL | 9.34 ± 7.61 | 6.75 ± 6.13 * | 9.02 ± 5.37 | 12.79 ± 7.71 | ns | <0.05 |
| s-RAGEs, pg/mL | 597 ± 188 | 815 ± 805 | 566 ± 139 | 535 ± 145 | ns | ns |
| Total cholesterol, mg/dL | 166 ± 41 | 167 ± 32 | 158 ± 29 | 159 ± 38 | ns | ns |
| HDL cholesterol, mg/dL | 50 ± 12 | 48 ± 11 | 52 ± 18 | 52 ± 18 | ns | ns |
| LDL cholesterol, mg/dL | 92 ± 37 | 83 ± 40 | 80 ± 23 | 77 ± 33 | ns | ns |
| Triglycerides, mg/dL | 123 ± 63 | 136 ± 79 | 133 ± 108 | 129 ± 112 | ns | ns |
| Fasting C-peptide, nmol/L | 3.3 ± 1.2 | 3.4 ± 1.5 | 3.6 ± 2.0 | 3.5 ± 1.7 | ns | ns |
| Fasting serum Insulin, pmol/L | 17.0 ± 15.1 | 27.6 ± 43.6 | 16.7 ± 14.7 | 15.0 ± 9.3 | ns | ns |
| HOMA -IR | 5.58 ± 4.62 | 8.27 ± 12.19 | 6.91 ± 10.05 | 5.03 ± 3.52 | ns | ns |
| MDA, μmol/L | 1.7 ± 0.15 | 1.4 ± 0.25* | 1.7 ± 0.21 | 1.7 ± 0.29 | ns | <0.005 |
| IL-1, pg/mL | 1.78 ± 0.64 | 2.09 ± 0.83 | 1.78 ± 0.62 | 2.16 ± 0.45 | ns | ns |
| IL-6, pg/mL | 3.5 ± 2.1 | 4.9 ± 4.6 | 2.8 ± 0.6 | 2.9 ± 0.9 | ns | ns |
| TNFα, pg/mL | 8.6 ± 5.1 | 7.8 ± 2.9 | 6.8 ± 1.6 | 7.2 ± 1.6 | ns | ns |
| hsCRP, mg/L | 1.46 ± 1.25 | 1.39 ± 2.33 | 1.18 ± 0.78 | 1.64 ± 1.26 | ns | ns |
| Systolic blood pressure, mmHg | 136 ± 12 | 136 ± 12 | 138 ± 10 | 137 ± 9 | ns | ns |
| Diastolic blood pressure, mmHg | 82 ± 8 | 82 ± 8 | 81 ± 7 | 81 ± 11 | ns | ns |
| BMI, kg/m2 | 29.1 ± 5.2 | 29.5 ± 5.3 | 28.7 ± 4.2 | 28.8 ± 4.2 | ns | ns |
| Waist circumference, cm | 102 ± 9 | 102 ± 9 | 101 ± 10 | 101 ± 9 | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartore, G.; Ragazzi, E.; Antonello, G.; Cosma, C.; Lapolla, A. Effect of a New Formulation of Nutraceuticals as an Add-On to Metformin Monotherapy for Patients with Type 2 Diabetes and Suboptimal Glycemic Control: A Randomized Controlled Trial. Nutrients 2021, 13, 2373. https://doi.org/10.3390/nu13072373
Sartore G, Ragazzi E, Antonello G, Cosma C, Lapolla A. Effect of a New Formulation of Nutraceuticals as an Add-On to Metformin Monotherapy for Patients with Type 2 Diabetes and Suboptimal Glycemic Control: A Randomized Controlled Trial. Nutrients. 2021; 13(7):2373. https://doi.org/10.3390/nu13072373
Chicago/Turabian StyleSartore, Giovanni, Eugenio Ragazzi, Giulia Antonello, Chiara Cosma, and Annunziata Lapolla. 2021. "Effect of a New Formulation of Nutraceuticals as an Add-On to Metformin Monotherapy for Patients with Type 2 Diabetes and Suboptimal Glycemic Control: A Randomized Controlled Trial" Nutrients 13, no. 7: 2373. https://doi.org/10.3390/nu13072373
APA StyleSartore, G., Ragazzi, E., Antonello, G., Cosma, C., & Lapolla, A. (2021). Effect of a New Formulation of Nutraceuticals as an Add-On to Metformin Monotherapy for Patients with Type 2 Diabetes and Suboptimal Glycemic Control: A Randomized Controlled Trial. Nutrients, 13(7), 2373. https://doi.org/10.3390/nu13072373