Glucose Fluctuation and Severe Internal Carotid Artery Siphon Stenosis in Type 2 Diabetes Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
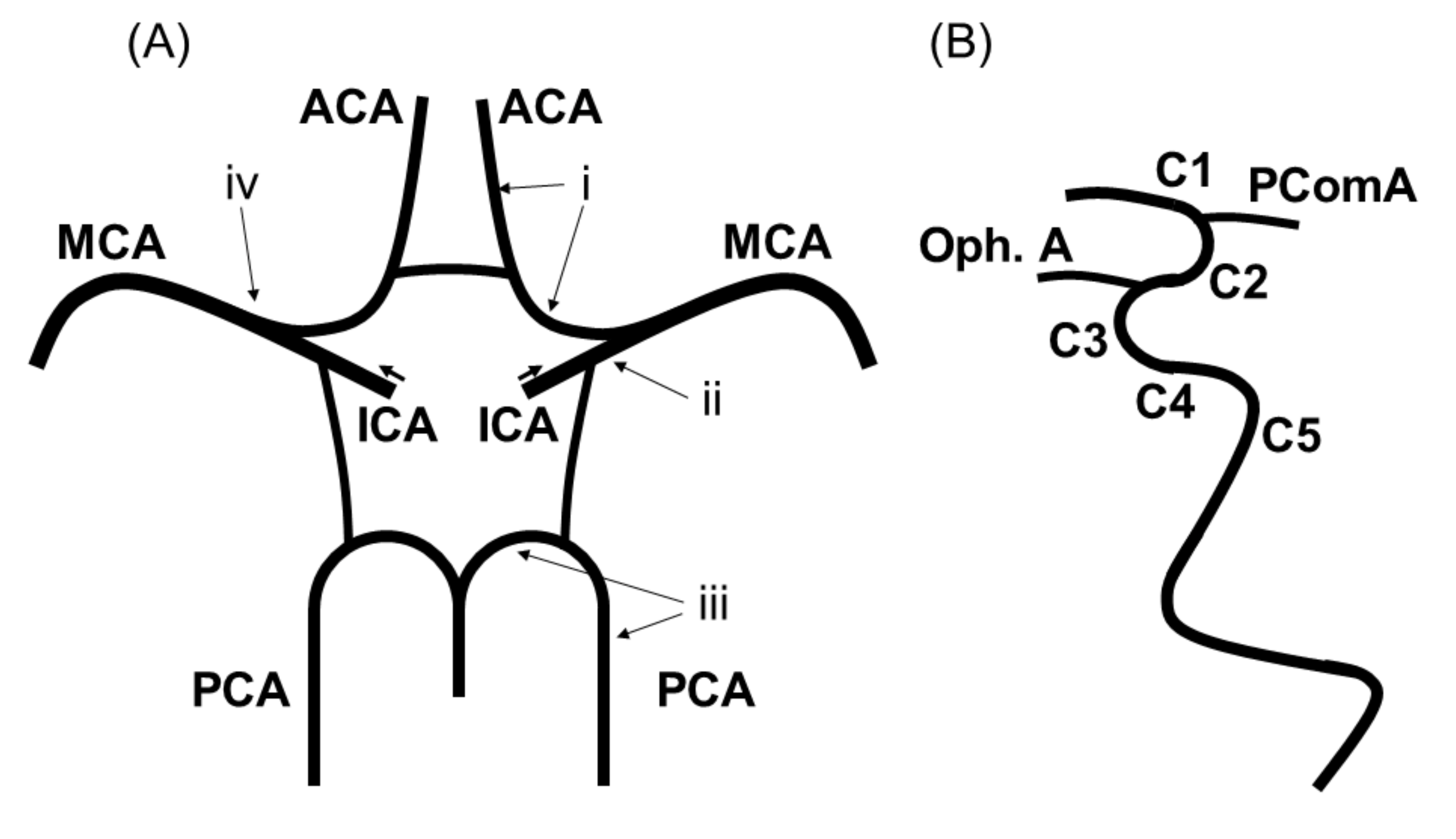
2.2. Imaging Protocol
2.3. Continuous Glucose Monitoring
2.4. Statistical Analyses
3. Results
3.1. Baseline Patient Characteristics
3.2. Head Magnetic Resonance Angiography Findings
3.3. Association between Glucose Fluctuation and Intracranial Artery Stenosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Fernandes, J.D.R.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Arenillas, J.F. Intracranial atherosclerosis: Current concepts. Stroke 2011, 42, S20–S23. [Google Scholar] [CrossRef] [Green Version]
- Kasner, S.E.; Chimowitz, M.I.; Lynn, M.J.; Howlett-Smith, H.; Stern, B.J.; Hertzberg, V.S.; Frankel, M.R.; Levine, S.R.; Chaturvedi, S.; Benesch, C.G.; et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006, 113, 555–563. [Google Scholar] [CrossRef]
- Shitara, S.; Fujiyoshi, A.; Hisamatsu, T.; Torii, S.; Suzuki, S.; Ito, T.; Arima, H.; Shiino, A.; Nozaki, K.; Miura, K.; et al. Intracranial artery stenosis and its association with conventional risk factors in a general popilation of Japanese men. Stroke 2019, 50, 2967–2969. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, Q.; Wang, X.; Ji, X.; Sang, S.; Shao, S.; Zhao, Y.; Xiang, Y.; Xue, Y.; Li, J.; et al. Prevalence and cardiovascular risk factors of asymptomatic intracranial arterial stenosis: The Kongcun town study in Shandong, China. Eur. J. Neurol. 2020, 27, 729–735. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Leng, X.-Y.; Dong, Y.; Hou, X.-H.; Tong, L.; Ma, Y.-H.; Xu, W.; Cui, M.; Dong, Q.; Tan, L.; et al. Fasting glucose and HbA1c levels as risk factors for the presence of intracranial atherosclerotic stenosis. Ann. Transl. Med. 2019, 7, 804. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Zhou, J.; Li, M.; Wang, Y.; Bao, Y.; Ma, X.; Li, D.; Lu, W.; Hu, C.; Li, M.; et al. Glycemic variability is associated with subclinical atherosclerosis in Chinese type 2 diabetic patients. Cardiovasc. Diabetol. 2013, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, F.R.; Gibson, L.C.; Halvorson, M.; Carpenter, S.; Fisher, L.K.; Pitukcheewanont, P. A pilot study of the continuous glucose monitoring system: Clinical decisions and glycemic control after its use in pediatric type 1 diabetic subjects. Diabetes Care 2001, 24, 2030–2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.M.; Zhang, Y.; Shen, X.P.; Huang, Q.; Ma, H.; Huang, Y.L.; Zhang, W.Q.; Wu, H.J. Correlation between glucose fluctuations and carotid intima-media thickness in type 2 diabetes. Diabetes Res. Clin. Pract. 2010, 90, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Gohbara, M.; Hibi, K.; Mitsuhashi, T.; Maejima, N.; Iwahashi, N.; Kataoka, S.; Akiyama, E.; Tsukahara, K.; Kosuge, M.; Ebina, T.; et al. Glycemic variability on continuous glucose monitoring system correlates with non-culprit vessel coronary plaque vulnerability in patients with first-episode acute coronary syndrome. Circ. J. 2016, 80, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Su, G.; Mi, S.; Tao, H.; Li, Z.; Yang, H.; Zheng, H.; Zhou, Y.; Ma, C. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc. Diabetol. 2011, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Iwahashi, N.; Kirigaya, J.; Kataoka, S.; Minamimoto, Y.; Gohbara, M.; Abe, T.; Okada, K.; Matsuzawa, Y.; Konishi, M.; et al. Glycemic variability determined with a continuous glucose monitoring system can predict prognosis after acute coronary syndrome. Cardiovasc. Diabetol. 2018, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, M.; Makino, H.; Washida, K.; Matsuo, M.; Koezuka, R.; Ohata, Y.; Tamanaha, T.; Honda-Kohmo, K.; Noguchi, M.; Tomita, T.; et al. A prospective longitudinal study on the relationship between glucose fluctuation and cognitive function in type 2 diabetes: PROPOSAL Study Protocol. Diabetes Ther. 2020, 11, 2729–2737. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Suzuki, H.; Yasunaga, M.; Sugiyama, M.; Ijuin, M.; Sakuma, N.; Inagaki, H.; Iwasa, H.; Ura, C.; Yatomi, N.; et al. Brief screening tool for mild cognitive impairment in older Japanese: Validation of the Japanese version of the Montreal Cognitive Assessment. Geriatr. Gerontol. Int. 2010, 10, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Trzepacz, P.T.; Hochstetler, H.; Wang, S.; Walker, B.; Saykin, A.J.; The Alzheimer’s Disease Neuroimaging Initiative. Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. 2015, 15, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991, 325, 445–453. [Google Scholar] [CrossRef]
- Marshall, R.S.; Pavol, M.A.; Cheung, Y.K.; Asllani, I.; Lazar, R.M. Cognitive impairment correlates linearly with mean flow velocity by transcranial doppler below a definable threshold. Cerebrovasc. Dis. Extra 2020, 10, 21–27. [Google Scholar] [CrossRef]
- Zwieten, A.; Wong, G.; Ruospo, M.; Palmer, S.C.; Barulli, M.R.; Iurillo, A.; Saglimbene, V.; Natale, P.; Gargano, L.; Murgo, M.; et al. Prevalence and patterns of cognitive impairment in adult hemodialysis patients: The COGNITIVE-HD study. Nephrol. Dial. Transplant. 2018, 33, 1197–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, E. Die Lageabweichungen de vorderen hirnarterie im gefaessbild. Zentralbl. Neurochir. 1938, 3, 300–313. [Google Scholar]
- Samuels, O.B.; Joseph, G.J.; Lynn, M.J.; Smith, H.A.; Chimowitz, M.I. A standardized method for measuring intracranial arterial stenosis. AJNR 2000, 21, 643–646. [Google Scholar]
- Baradaran, H.; Patel, P.; Gialdini, G.; Al-Dasuqi, K.; Giambrone, A.; Kamel, H.; Gupta, A. Quantifying intracranial internal carotid artery stenosis on MR angiography. AJNR 2017, 38, 986–990. [Google Scholar] [CrossRef] [Green Version]
- Turan, T.N.; Makki, A.A.; Tsappidi, S.; Cotsonis, G.; Lynn, M.J.; Cloft, H.J.; Chimowitz, M.I.; WASID investigators. Risk factors associated with severity and location of intracranial arterial stenosis. Stroke 2010, 41, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.; Bode, B.W.; Christiansen, M.P.; Klaff, L.J.; Alva, S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol. Ther. 2015, 17, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marics, G.; Lendvai, Z.; Lódi, C.; Koncz, L.; Zakariás, D.; Schuster, G.; Mikos, B.; Hermann, C.; Szabó, A.J.; Tóth-Heyn, P. Evaluation of an open access software for calculating glucose variability parameters of a continuous glucose monitoring system applied at pediatric intensive care unit. Biomed. Eng. Online 2015, 14, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuoka, A.; Hirota, Y.; Takeda, A.; Kishi, M.; Hashimoto, N.; Ohara, T.; Higo, S.; Yamada, H.; Nakamura, T.; Hamaguchi, T.; et al. Relationship between glycated hemoglobin level and duration of hypoglycemia in type 2 diabetes patients treated with sulfonylureas: A multicenter cross-sectional study. J. Diabetes Investig. 2020, 11, 417–425. [Google Scholar] [CrossRef]
- Inaba, Y.; Tsutsumi, C.; Haseda, F.; Fujisawa, R.; Mitsui, S.; Sano, H.; Terasaki, J.; Hanafusa, T.; Imagawa, A. Impact of glycemic variability on the levels of endothelial progenitor cells in patients with type 1 diabetes. Diabetol. Int. 2017, 9, 113–120. [Google Scholar] [CrossRef]
- Di Flaviani, A.; Picconi, F.; Di Stefano, P.; Giordani, I.; Malandrucco, I.; Maggio, P.; Palazzo, P.; Sgreccia, F.; Peraldo, C.; Farina, F.; et al. Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care 2011, 34, 1605–1609. [Google Scholar] [CrossRef] [Green Version]
- Otowa-Suematsu, N.; Sakaguchi, K.; Komada, H.; Nakamura, T.; Sou, A.; Hirota, Y.; Kuroda, M.; Shinke, T.; Hirata, K.-I.; Ogawa, W. Comparison of the relationship between multiple parameters of glycemic variability and coronary plaque vulnerability assessed by virtual histology-intravascular ultrasound. J. Diabetes Investig. 2017, 9, 610–615. [Google Scholar] [CrossRef]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of glycemic indices (Hyperglycemia, Glucose variability, and Hypoglycemia) with oxidative stress and diabetic complications. J. Diabetes Res. 2020, 7489795. [Google Scholar] [CrossRef]
- Katakami, N. Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Yin, H.; Wei, C.; Xie, L.; He, H.; Liu, X. Glucose variability for cardiovascular risk factors in type 2 diabetes: A meta-analysis. J. Diabetes Metab. Disord. 2017, 16, 45. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, M.; Rizzo, M.R.; Marfella, R.; Boccardi, V.; Esposito, A.; Pansini, A.; Paolisso, G. Decreased carotid atherosclerotic process by control of daily acute glucose fluctuations in diabetic patients treated by DPP-IV inhibitors. Atherosclerosis 2013, 227, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Watt, C.; Sanchez-Rangel, E.; Hwang, J.J. Glycemic variability and CNS inflammation: Reviewing the connection. Nutrients 2020, 12, 3906. [Google Scholar] [CrossRef]
- Sajja, R.K.; Cucullo, L. Altered glycaemia differentially modulates efflux transporter expression and activity in hCMEC/D3 cell line. Neurosci. Lett. 2015, 598, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, S.; Yoshimura, S.; Inoue, M.; Matsuki, T.; Arihiro, S.; Koga, M.; Kitazono, T.; Makino, H.; Hosoda, K.; Ihara, M.; et al. Outcome prediction in acute stroke patients by continuous glucose monitoring. J. Am. Heart Assoc. 2018, 7, e008744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livny, A.; Ravona-Springer, R.; Heymann, A.; Priess, R.; Kushnir, T.; Tsarfaty, G.; Rabinov, L.; Moran, R.; Hoffman, H.; Cooper, I.; et al. Long-term variability in glycemic control is associated with white matter hyperintensities in APOE4 genotype carriers with type 2 diabetes. Diabetes Care 2016, 39, 1056–1059. [Google Scholar] [CrossRef] [Green Version]
- Hui, J.; Zhang, J.; Mao, X.; Li, Z.; Li, X.; Wang, F.; Wang, T.; Yuan, Q.; Wang, S.; Pu, M.; et al. The initial glycemic variability is associated with early neurological deterioration in diabetic patients with acute ischemic stroke. Neurol. Sci. 2018, 39, 1571–1577. [Google Scholar] [CrossRef]
- Naess, H.; Thomassen, L.; Waje-Andreassen, U.; Glad, S.; Kvistad, C.E. High risk of neurological worsening of lacunar infarction. Acta Neurol. Scand. 2019, 139, 143–149. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, S.; Li, S.; Pu, F.; Deng, X.; Fan, Y.; Li, D. Flow patterns and wall shear stress distribution in human internal carotid arteries: The geometric effect on the risk for stenoses. J. Biomech. 2012, 45, 83–89. [Google Scholar] [CrossRef]
- Cecchi, E.; Giglioli, C.; Valente, S.; Lazzeri, C.; Gensini, G.F.; Abbate, R.; Mannini, L. Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis 2011, 214, 249–256. [Google Scholar] [CrossRef]
- Pu, Y.; Liu, L.; Wang, Y.; Zou, X.; Pan, Y.; Soo, Y.; Leung, T.; Zhao, X.; Wong, K.S.; Wang, Y.; et al. Geographic and sex difference in the distribution of intracranial atherosclerosis in China. Stroke 2013, 44, 2109–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton-Tyrrell, K.; Wildman, R.P.; Matthews, K.A.; Chae, C.; Lasley, B.L.; Brockwell, S.; Pasternak, R.C.; Lloyd-Jones, D.; Sowers, M.F.; Torréns, J.I.; et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Woman across the Nation (SWAN). Circulation 2005, 111, 1242–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Kim, Y.; Park, S.Y.; Kim, C.; Kim, Y.J.; Sohn, J.H. Pre-stroke glycemic variability estimated by glycated albumin is associated with early neurological deterioration and poor functional outcome in prediabetic patients with acute ischemic stroke. Cerebrovasc. Dis. 2021, 50, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Sissani, L.; Labreuche, J.; Ducrocq, G.; Lavallée, P.C.; Meseguer, E.; Guidoux, C.; Cabrejo, L.; Hobeanu, C.; Gongora-Rivera, F.; et al. Prevalence of Systemic Atherosclerosis Burdens and Overlapping Stroke Etiologies and Their Associations with Long-term Vascular Prognosis in Stroke with Intracranial Atherosclerotic Disease. JAMA Neurol. 2018, 75, 203–211. [Google Scholar] [CrossRef]



| Intracranial Internal Carotid Artery Siphon Stenosis | |||
|---|---|---|---|
| Variables | Severe (n = 8) | Non-Severe (n = 95) | p Value |
| Baseline demographics | |||
| Female (%) | 6 (75) | 26 (27) | 0.01 |
| Age, years | 76 ± 5 | 76 ± 5 | 0.63 |
| Cerebrovascular risk factors | |||
| Hypertension (%) | 6 (75) | 88 (93) | 0.14 |
| Dyslipidemia (%) | 8 (100) | 83 (87) | 0.59 |
| Current or past smoking (%) | 3 (38) | 54 (57) | 0.46 |
| Atrial fibrillation (%) | 1 (13) | 16 (17) | 1.00 |
| Medical history of percutaneous coronary intervention or coronary artery bypass grafting (%) | 4 (50) | 41 (44) | 0.73 |
| Ischemic stroke episode (%) | 3 (38) | 42 (45) | 1.00 |
| Diabetes mellitus | |||
| Duration, years | 26 ± 10 | 23 ± 11 | 0.32 |
| HbA1c at registration, % | 7.8 ± 0.9 | 7.5 ± 0.9 | 0.47 |
| Blood glucose at registration, mg/dL | 136 ± 42 | 150 ± 42 | 0.54 |
| Blood glucose (CGM average), mg/dL | 148 ± 25 | 136 ± 28 | 0.19 |
| SD, mg/dL | 53 ± 12 | 39 ± 10 | <0.01 |
| %CV | 36 ± 7 | 29 ± 6 | <0.01 |
| MAGE, mg/dL | 114 ± 18 | 90 ± 23 | <0.01 |
| SD (10 mg/dL) | %CV (/10) | MAGE (10 mg/dL) | ||||
|---|---|---|---|---|---|---|
| p Value | p Value | p Value | ||||
| Crude OR (95% CI) | 3.60 (1.60–8.08) | <0.01 | 7.85 (1.90–32.5) | <0.01 | 1.56 (1.11–2.20) | 0.01 |
| Adjusted OR (95% CI) | 3.00 (1.32–6.84) | <0.01 | 5.55 (1.23–25.2) | 0.03 | 1.52 (1.06–2.19) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eto, F.; Washida, K.; Matsubara, M.; Makino, H.; Takahashi, A.; Noda, K.; Hattori, Y.; Nakaoku, Y.; Nishimura, K.; Hosoda, K.; et al. Glucose Fluctuation and Severe Internal Carotid Artery Siphon Stenosis in Type 2 Diabetes Patients. Nutrients 2021, 13, 2379. https://doi.org/10.3390/nu13072379
Eto F, Washida K, Matsubara M, Makino H, Takahashi A, Noda K, Hattori Y, Nakaoku Y, Nishimura K, Hosoda K, et al. Glucose Fluctuation and Severe Internal Carotid Artery Siphon Stenosis in Type 2 Diabetes Patients. Nutrients. 2021; 13(7):2379. https://doi.org/10.3390/nu13072379
Chicago/Turabian StyleEto, Futoshi, Kazuo Washida, Masaki Matsubara, Hisashi Makino, Akio Takahashi, Kotaro Noda, Yorito Hattori, Yuriko Nakaoku, Kunihiro Nishimura, Kiminori Hosoda, and et al. 2021. "Glucose Fluctuation and Severe Internal Carotid Artery Siphon Stenosis in Type 2 Diabetes Patients" Nutrients 13, no. 7: 2379. https://doi.org/10.3390/nu13072379






