Low Bone Mineral Density and Risk for Osteoporotic Fractures in Patients with Chronic Pancreatitis
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Definitions
2.3. Statistics
2.4. Ethics
3. Results
4. Discussion
| Author | Year | Country | N | Age | Sex | Aetiology/Results | Comments |
|---|---|---|---|---|---|---|---|
| Moran [17] | 1997 | Argentina | 14 | 56 | Not mentioned | Alcohol: 71.4% Idiopathic 28.6% Osteopathy: 92.8% Osteopenia: 71.4% Osteoporosis: 21.4% | All included patients with severe PEI. |
| Haaber [18] | 2000 | Denmark | 58 | 54 | 55.2% male | Alcohol: 79% 56% of patients in the group without PEI and 69% in the group with PEI had Z-scores of the BMD < –1 | Conclusions: patients with CP, particularly patients with advanced disease and steatorrhea, are at risk of developing significant bone loss |
| Mann [19] | 2003 | Germany | 42 | 51.1 | All males | Not described | Conclusion: connection between the inflammatory destruction of the pancreas (Cambridge classification), exocrine pancreatic insufficiency (faecal elastase 1), altered levels of vitamin D metabolites and loss of skeletal mass |
| Dujsikova [13] | 2008 | Czech Republic | 73 | 46.6 | 76.7% males | Osteopathy: 39%. Osteopenia: 26%, Osteoporosis: 5%, Osteomalacia: 8% | CP diagnosed with EUS and defined as mild, moderate and severe (more than half of the patients had mild CP) |
| Sudeep [20] | 2011 | India | 31 | 35.8 | All males | Tropical pancreatitis: 65% Idiopathic: 35% 29% had a T-score of less than −2.5 | Conclusion: Patients with chronic pancreatitis and a T-score of < −2.5 had a significantly lower BMI. No correlation was found between 25(OH)D levels and BMD |
| Joshi [15] | 2011 | India | 72 (DXA in 60) | 31 | 53% males | All patients with tropical calcific pancreatitis | Conclusion: despite their young age, patients with tropical calcific pancreatitis have significantly low BMD |
| Duggan [9] | 2012 | Ireland | 53 | 48.7 | 75.5% males | Alcohol was the cause of disease in 38.7% of patients. (just over 6% of both patients and controls were never drinkers). Osteopathy: 73.6% Osteoporosis: 34% Osteopenia: 39.6% | Conclusion: a third of the patients with CP had osteoporosis, which was more than triple the rate in the matched control group |
| Sikkens [21] | 2013 | The Netherlands | 40 | 52 | 57% males | Alcohol: 50% Idiopathic: 43% Other: 7% Osteoporosis: 10% Osteopenia: 45% | Deficiencies of fat-soluble vitamins and a decreased BMD are frequently present in CP, even in exocrine-sufficient patients. Consequently, all patients with CP should be routinely screened for fat-soluble vitamin deficiencies and a decreased BMD |
| Duggan [8] | 2014 | Ireland | 29 | 44.3 | 58.6% male | Alcohol: 62.1% Idiopathic: 27.6% Other: 10.3% Osteoporosis: 31% Osteopenia: 44.8% | Conclusion: both bone formation and bone resorption were raised in patients with CP compared to controls. This finding indicates that bone turnover was elevated in CP. Those with alcohol-induced disease did not have lower BMD than those with CP of other aetiologies |
| Prabhakaran [12] | 2014 | India | 103 | 38.6 | All males | Alcohol: 70%, Idiopathic: 29.1%; (one patient had post-traumatic chronic Pancreatitis) Osteoporosis: 30.1%, Osteopenia: 39.8% | Conclusion: most patients with both alcoholic and idiopathic had low BMD and the frequency of bone changes was similar between calcific and non-calcific groups, diabetics and nondiabetics, patients with and without a history of steatorrhea, patients with and without vitamin D deficiency and across different pancreatitis severity groups |
| Min [11] | 2014 | USA | 91 | 48.6 | 62.6% females | Toxic/metabolic: 59.3% Idiopathic: 18.6% Hereditary: 14.3% Autoimmune: 5.5% Osteopenia: 46.7% Osteoporosis: 22.2% | Conclusion: There is a high prevalence of fat-soluble vitamin deficiencies, osteopathy and malnutrition in CP patients, which is underestimated due to the lack of effective diagnosis and suboptimal therapies for EPI |
| Haas [10] | 2015 | Germany | 15 | 45.2 | all males | Alcohol: 72% Osteoporosis: 16% Osteopenia: 76% | Conclusion: no correlation between bone metabolism and elastase |
| Stigliano [22] | 2018 | European multicentric | 211 | 60 | 67% males | Alcoholic: 43% Idiopathic: 19% Hereditary: 4% Obstructive: 5.7% Osteopenia: 42% Osteoporosis: 22% | Conclusion: vitamin K deficiency is the only factor associated with osteoporosis in male patients |
| Present study | 2021 | Sweden | 118 | 53.1 | 58.5% males | Alcohol and smoking: 33.9% Smoking only: 11% Alcohol only: 5.9% Hereditary: 11.8% Immunological: 14.4% Efferent duct factors: 9.3% Low BMD: 53.4% | Conclusion: Low BMD was found in 58% of patients with CP with a high prevalence of fractures (53%). Most of the fractures occurred in patients with low BMD but also occurred in patients with normal DXA. Previous treatment with either vitamin D or PERT demonstrated a significantly lower risk for fractures in all patient groups |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Löhr, J.M.; Dominguez-Munoz, E.; Rosendahl, J.; Besselink, M.; Mayerle, J.; Lerch, M.M.; Haas, S.; Akisik, F.; Kartalis, N.; Iglesias-Garcia, J.; et al. United european gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (hapaneu). United Eur. Gastroenterol. J. 2017, 5, 153–199. [Google Scholar] [CrossRef]
- Peck, W.A. Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef]
- Duggan, S.N.; Smyth, N.D.; Murphy, A.; Macnaughton, D.; O’Keefe, S.J.; Conlon, K.C. High prevalence of osteoporosis in patients with chronic pancreatitis: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, A.; Vipperla, K.; Papachristou, G.I.; Brand, R.E.; Slivka, A.; Whitcomb, D.C.; Yadav, D. Bone health assessment in clinical practice is infrequenty performed in patients with chronic pancreatitis. Pancreatology 2020, 20, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tignor, A.S.; Wu, B.U.; Whitlock, T.L.; Lopez, R.; Repas, K.; Banks, P.A.; Conwell, D. High prevalence of low-trauma fracture in chronic pancreatitis. Am. J. Gastroenterol. 2010, 105, 2680–2686. [Google Scholar] [CrossRef]
- Schneider, A.; Löhr, J.M.; Singer, M.V. The m-annheim classification of chronic pancreatitis: Introduction of a unifying classification system based on a review of previous classifications of the disease. J. Gastroenterol. 2007, 42, 101–119. [Google Scholar] [CrossRef]
- Duggan, S.N.; Purcell, C.; Kilbane, M.; O’Keane, M.; McKenna, M.; Gaffney, P.; Ridgway, P.F.; Boran, G.; Conlon, K.C. An association between abnormal bone turnover, systemic inflammation, and osteoporosis in patients with chronic pancreatitis: A case-matched study. Am. J. Gastroenterol. 2015, 110, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.N.; O’Sullivan, M.; Hamilton, S.; Feehan, S.M.; Ridgway, P.F.; Conlon, K.C. Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas 2012, 41, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.; Krins, S.; Knauerhase, A.; Löhr, M. Altered bone metabolism and bone density in patients with chronic pancreatitis and pancreatic exocrine insufficiency. Jop 2015, 16, 58–62. [Google Scholar]
- Min, M.; Patel, B.; Han, S.; Bocelli, L.; Kheder, J.; Vaze, A.; Wassef, W. Exocrine pancreatic insufficiency and malnutrition in chronic pancreatitis: Identification, treatment, and consequences. Pancreas 2018, 47, 1015–1018. [Google Scholar] [CrossRef]
- Prabhakaran, A.; Bhasin, D.K.; Rana, S.S.; Bhadada, S.K.; Bhansali, A.; Rao, C.; Gupta, R.; Khandelwal, N. Bone mineral metabolism and bone mineral density in alcohol related and idiopathic chronic pancreatitis. Trop. Gastroenterol. 2014, 35, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Dujsikova, H.; Dite, P.; Tomandl, J.; Sevcikova, A.; Precechtelova, M. Occurrence of metabolic osteopathy in patients with chronic pancreatitis. Pancreatology 2008, 8, 583–586. [Google Scholar] [CrossRef]
- Bang, U.C.; Benfield, T.; Bendtsen, F.; Hyldstrup, L.; Beck Jensen, J.E. The risk of fractures among patients with cirrhosis or chronic pancreatitis. Clin. Gastroenterol. Hepatol. 2014, 12, 320–326. [Google Scholar] [CrossRef]
- Joshi, A.; Reddy, S.V.; Bhatia, V.; Choudhuri, G.; Singh, R.K.; Singh, N.; Bhatia, E. High prevalence of low bone mineral density in patients with tropical calcific pancreatitis. Pancreas 2011, 40, 762–767. [Google Scholar] [CrossRef]
- Hirschfeld, H.P.; Kinsella, R.; Duque, G. Osteosarcopenia: Where bone, muscle, and fat collide. Osteoporos. Int. 2017, 28, 2781–2790. [Google Scholar] [CrossRef]
- Morán, C.E.; Sosa, E.G.; Martinez, S.M.; Geldern, P.; Messina, D.; Russo, A.; Boerr, L.; Bai, J.C. Bone mineral density in patients with pancreatic insufficiency and steatorrhea. Am. J. Gastroenterol. 1997, 92, 867–871. [Google Scholar] [PubMed]
- Haaber, A.B.; Rosenfalck, A.M.; Hansen, B.; Hilsted, J.; Larsen, S. Bone mineral metabolism, bone mineral density, and body composition in patients with chronic pancreatitis and pancreatic exocrine insufficiency. Int. J. Pancreatol. 2000, 27, 21–27. [Google Scholar] [CrossRef]
- Mann, S.T.; Stracke, H.; Lange, U.; Klör, H.U.; Teichmann, J. Alterations of bone mineral density and bone metabolism in patients with various grades of chronic pancreatitis. Metabolism 2003, 52, 579–585. [Google Scholar] [CrossRef]
- Sudeep, K.; Chacko, A.; Thomas, N.; Selvakumar, R.; George, B.; Paul, T.V.; Seshadri, M.S. Predictors of osteodystrophy in patients with chronic nonalcoholic pancreatitis with or without diabetes. Endocr. Pract. 2011, 17, 897–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikkens, E.C.; Cahen, D.L.; Koch, A.D.; Braat, H.; Poley, J.W.; Kuipers, E.J.; Bruno, M.J. The prevalence of fat-soluble vitamin deficiencies and a decreased bone mass in patients with chronic pancreatitis. Pancreatology 2013, 13, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Stigliano, S.; Waldthaler, A.; Martinez-Moneo, E.; Lionetto, L.; Robinson, S.; Malvik, M.; Hedstrom, A.; Kaczka, A.; Scholdei, M.; Haas, S.; et al. Vitamins d and k as factors associated with osteopathy in chronic pancreatitis: A prospective multicentre study (p-bone study). Clin. Transl. Gastroenterol. 2018, 9, 197. [Google Scholar] [CrossRef] [PubMed]


| Total (n = 118) | Low BMD (n = 63) | Normal BMD (n = 55) | p-Value | |
|---|---|---|---|---|
| Female sex, n (%) | 49 (41.5) | 32 (50.7) | 17 (30.9) | <0.05 |
| Follow-up (years), mean (SD) | 7.6 (7.5) | 7.1 (6.8) | 8.2 (8.1) | 0.424 |
| Age at CP diagnosis (years), mean (SD) | 53.1 (16.3) | 59.8 (11.8) | 45.5 (17.5) | <0.001 |
| Diabetes at diagnosis, n (%) | 28 (23.7) | 16 (25.4) | 12 (21.8) | 0.774 |
| Age group, n (%) | - | - | - | - |
| - <45 years | 35 (29.6) | 6 (9.5) | 29 (52.7) | - |
| - 45–65 years | 51 (43.2) | 35 (55.6) | 16 (29.0) | - |
| - ≥65 years | 32 (27.1) | 22 (35.9) | 10 (18.1) | <0.005 |
| BMI, mean (SD) | 23.9 (4.4) | 23.06 (4.05) | 24.9 (4.6) | <0.05 |
| - BMI <20.0, n (%) | 25 (21.2) | 15 (23.8) | 10 (1.1) | - |
| - 20.1 < BMI < 25.0, n (%) | 50 (42.4) | 33 (52.4) | 17 (30.9) | - |
| - 25.1 < BMI < 30.0, n (%) | 30 (25.4) | 10 (15.9) | 20 (36.3) | - |
| - 30.1 < BMI, n (%) | 13 (11.0) | 5 (7.9) | 8 (14.5) | <0.05 |
| Smoking status | - | - | - | - |
| - Current smoker, n (%) | 42 (35.6) | 28 (44.4) | 14 (25.5) | - |
| - Former smoker, n (%) | 34 (28.8) | 19 (30.2) | 15 (27.2) | - |
| - Never smoker, n (%) | 42 (35.6) | 16 (25.4) | 26 (47.2) | <0.05 |
| Alcohol status | ||||
| - Non-drinker | 64 (54.7) | 28 (44.4) | 36 (66.7) | |
| - Drinker | 53 (45.2) | 35 (55.6) | 18 (33.3) | <0.01 |
| - Data not available | 1 | 0 | 1 | |
| Aetiology of CP | - | - | - | - |
| - Alcohol and nicotine, n (%) | 40 (33.9) | 27 (46.6) | 13 (26.5) | - |
| - Nicotine, n (%) | 13 (11.0) | 9 (15.5) | 4 (8.1) | - |
| - Alcohol, n (%) | 7 (5.9) | 4 (6.9) | 3 (6.1) | - |
| - Hereditary, n (%) | 14 (11.8) | 3 (5.2) | 11 (22.4) | - |
| - Immunological, n (%) | 17 (14.4) | 9 (15.5) | 8 (16.3) | - |
| - Immunological factors and nicotine, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| - Efferent duct | 11 (9.3) | 6 (10.3) | 5 (10.2) | - |
| - Misc./Other, n (%) | 8 (6.7) | 3 (5.1) | 5 (10.2) | <0.05 |
| - Data not available | 8 | 2 | 6 | - |
| PEI at diagnosis | - | - | - | - |
| - No n (%) | 35 (39.3) | 20 (40.0) | 15 (38.5) | |
| - Yes n (%) | 54 (60.7) | 30 (60.0) | 24 (61.5) | 1.0 |
| - Missing data | 29 | 13 | 16 | - |
| DXA results, median (quartiles) | - | - | - | - |
| - T-score hip | −1.47 (−2.36, 0.81) | −2.18 (−2.6, 1.7) | −0.3 (−0.85, 0.2) | <0.005 |
| - T-score lower back | −0.71 (−2.0, 0.55) | −1,55 (−2.78, −0.5) | 0.69 (0.1, 1.2) | <0.005 |
| Median time from CP diagnosis to DXA, years (IQR) | 2.8 (7.4) | 2.8 (8.0) | 2.7 (6.5) | 0.696 |
| N | Event | >Person-Years | Incidence | Crude HR [CI 95%] | aHR * [CI 95%] | |
|---|---|---|---|---|---|---|
| Normal BMD | 55 | 5 | 451.4 | 1.1 | 1.0 [ref.] | 1.0 [ref.] |
| Low BMD | 63 | 28 | 446.2 | 6.3 | 5.5 [2.1, 14.2] | 3.4 [1.2, 9.6] |
| - Osteopenia | 33 | 10 | 248.6 | 4.0 | 3.5 [1.2, 10.4] | 2.2 [0.7, 6.8] |
| - Osteoporosis | 30 | 18 | 197.6 | 9.2 | 7.8 [2.9, 21.01] | 5.5 [1.9, 15.8] |
| Low BMD | Normal BMD | |||||
|---|---|---|---|---|---|---|
| Event | Person-Years | Incidence | Event | Person-Years | Incidence | |
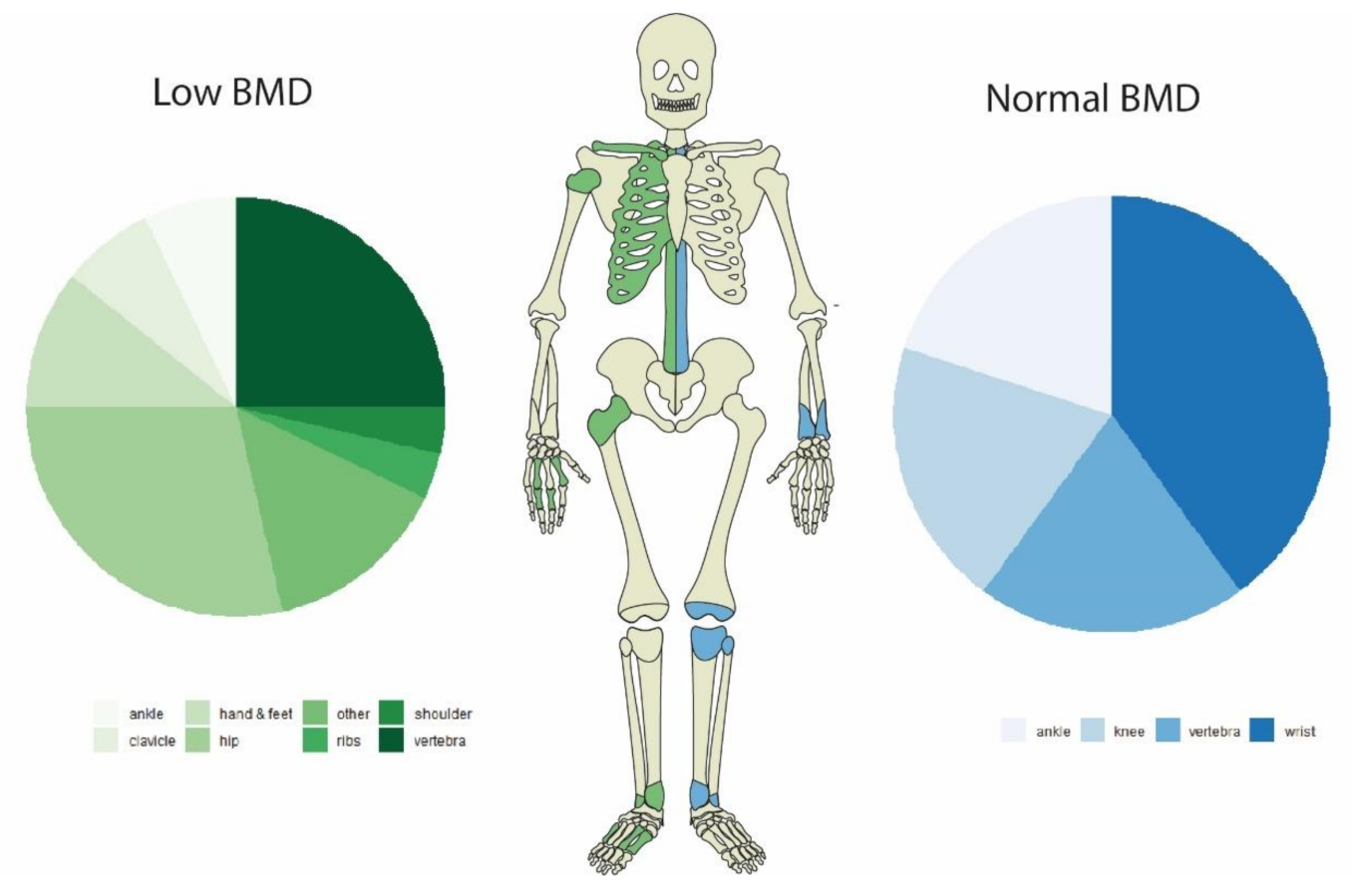
| Hip | 8 | 446.2 | 1.8 | 0 | 451.4 | 0.0 |
| Vertebrae | 7 | 446.2 | 1.6 | 1 | 451.4 | 0.22 |
| Rib | 1 | 446.2 | 0.22 | 0 | 451.4 | 0 |
| Ankle | 2 | 446.2 | 0.45 | 1 | 451.4 | 0.22 |
| Shoulder | 1 | 446.2 | 0.22 | 0 | 451.4 | 0 |
| Wrist | 0 | 446.2 | 0.0 | 2 | 451.4 | 0.44 |
| Clavicle | 2 | 446.2 | 0.45 | 0 | 451.4 | 0.0 |
| Hand & feet | 3 | 446.2 | 0.67 | 0 | 451.4 | 0.0 |
| Knee | 1 | 446.2 | 0.22 | 1 | 451.4 | 0.22 |
| Other | 3 | 446.2 | 0.67 | 0 | 451.4 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vujasinovic, M.; Nezirevic Dobrijevic, L.; Asplund, E.; Rutkowski, W.; Dugic, A.; Kahn, M.; Dahlman, I.; Sääf, M.; Hagström, H.; Löhr, J.-M. Low Bone Mineral Density and Risk for Osteoporotic Fractures in Patients with Chronic Pancreatitis. Nutrients 2021, 13, 2386. https://doi.org/10.3390/nu13072386
Vujasinovic M, Nezirevic Dobrijevic L, Asplund E, Rutkowski W, Dugic A, Kahn M, Dahlman I, Sääf M, Hagström H, Löhr J-M. Low Bone Mineral Density and Risk for Osteoporotic Fractures in Patients with Chronic Pancreatitis. Nutrients. 2021; 13(7):2386. https://doi.org/10.3390/nu13072386
Chicago/Turabian StyleVujasinovic, Miroslav, Lorena Nezirevic Dobrijevic, Ebba Asplund, Wiktor Rutkowski, Ana Dugic, Mashroor Kahn, Ingrid Dahlman, Maria Sääf, Hannes Hagström, and Johannes-Matthias Löhr. 2021. "Low Bone Mineral Density and Risk for Osteoporotic Fractures in Patients with Chronic Pancreatitis" Nutrients 13, no. 7: 2386. https://doi.org/10.3390/nu13072386






