Muscle Thickness and Echogenicity Measured by Ultrasound Could Detect Local Sarcopenia and Malnutrition in Older Patients Hospitalized for Hip Fracture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Outcomes
2.2.1. Nutritional and Anthropometric Assessment
2.2.2. Functional Assessment
2.2.3. Sarcopenia Evaluation
2.2.4. Muscle Ultrasound Exploration
2.3. Statistical Analyses
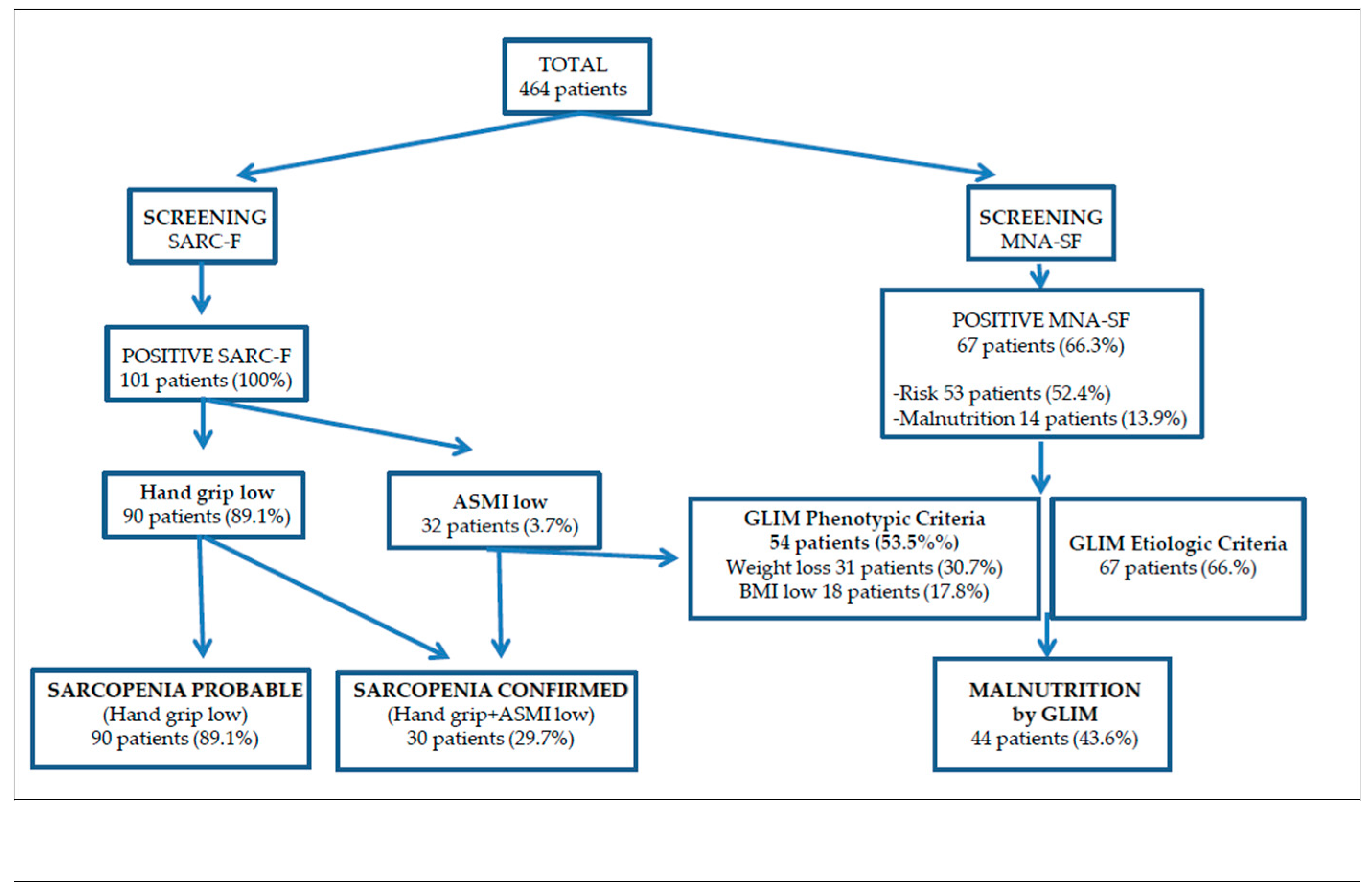
3. Results
3.1. Study Population
3.2. Interrelations of Thickness and Echogenicity of the Three Muscle Groups Studied by Ultrasound
3.3. Relations of Thickness and Echogenicity of the Three Muscle Groups Studied by Ultrasound with Age and Anthropometric Assessments
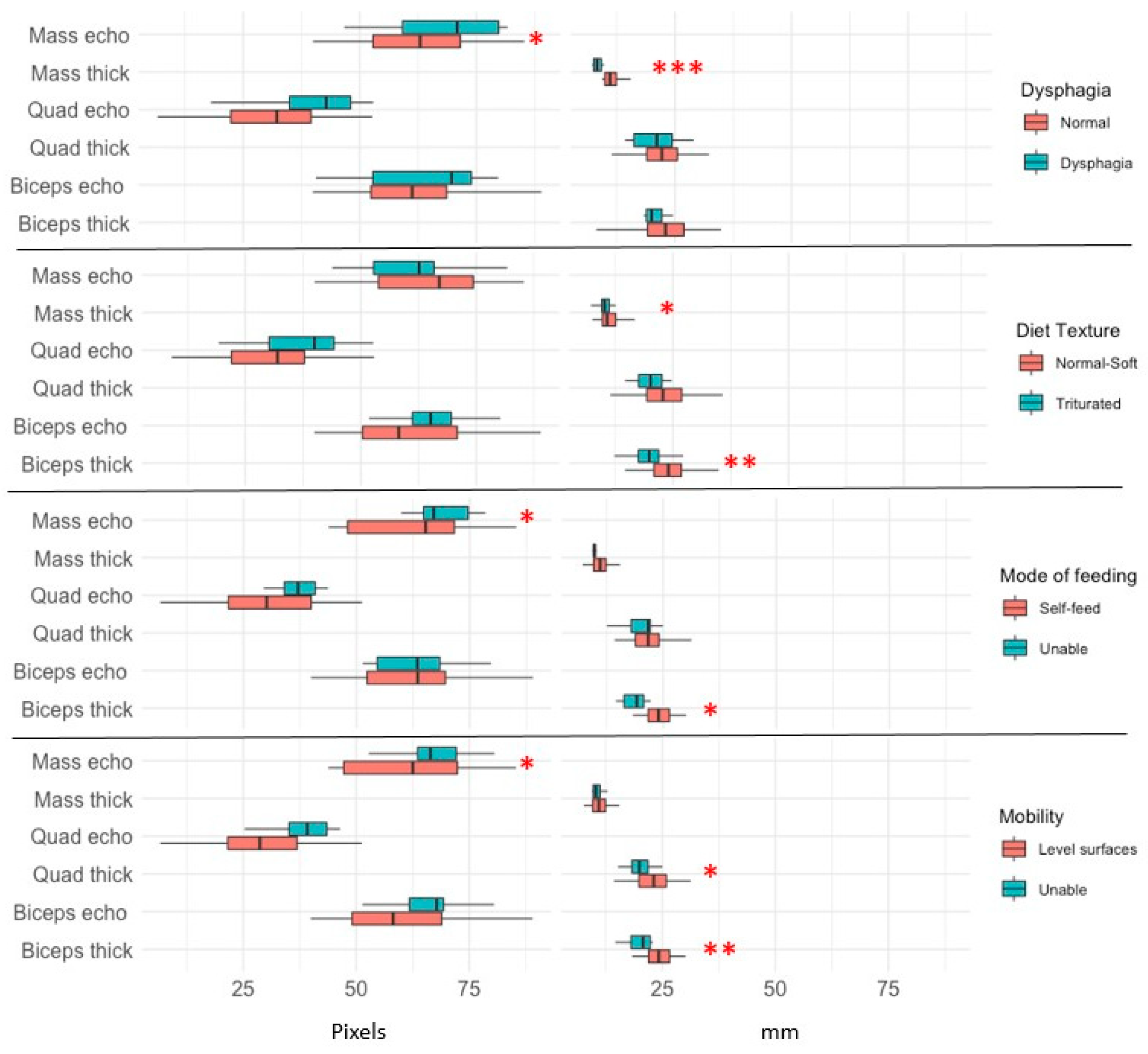
3.4. Associations of Thickness and Echogenicity of the Three Muscle Groups Studied by Ultrasound with Nutritional Assessment
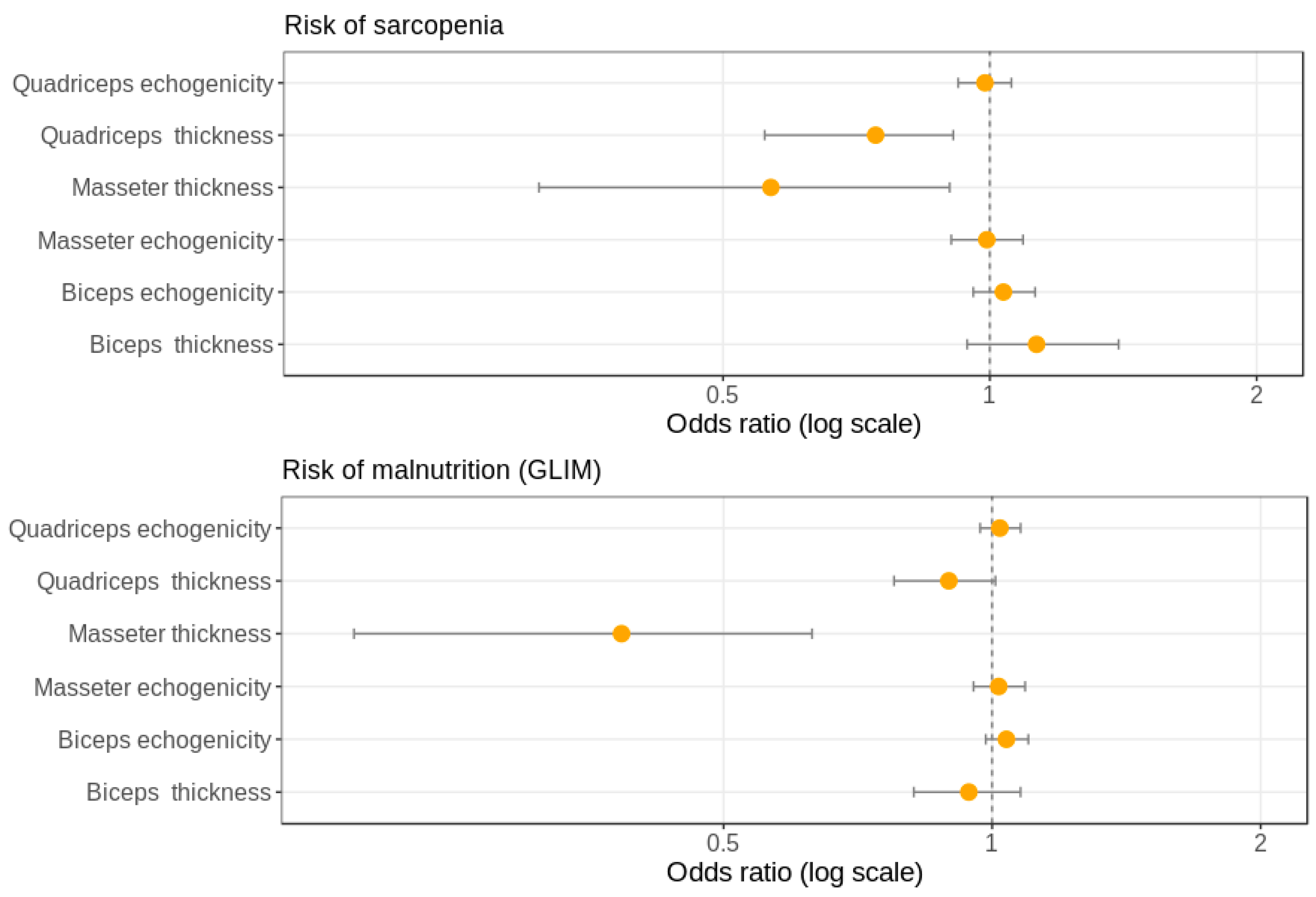
3.5. Associations of Thickness and Echogenicity of the Three Muscle Groups Studied by Ultrasound with Sarcopenia Diagnosis
3.6. Relations of Thickness and Echogenicity of the Three Muscle Groups Studied by Ultrasound with Functional Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veronese, N.; Maggi, S. Epidemiology and social costs of hip fracture. Injury 2018, 49, 1458–1460. [Google Scholar] [CrossRef]
- Maravic, M.; Taupin, P.; Landais, P.; Roux, C. Change in hip fracture incidence over the last 6 years in France. Osteoporos. Int. 2011, 22, 797–801. [Google Scholar] [CrossRef]
- Giversen, I.M. Time trends of age-adjusted incidence rates of first hip fractures: A register-based study among older people in Viborg County, Denmark, 1987–1997. Osteoporos. Int. 2006, 17, 552–564. [Google Scholar] [CrossRef]
- Malafarina, V.; Reginster, J.-Y.; Cabrerizo, S.; Bruyère, O.; Kanis, J.A.; Martinez, J.A.; Zulet, M.A. Nutritional Status and Nutritional Treatment Are Related to Outcomes and Mortality in Older Adults with Hip Fracture. Nutrients 2018, 10, 555. [Google Scholar] [CrossRef] [Green Version]
- Beaudart, C.; Sanchez-Rodriguez, D.; Locquet, M.; Reginster, J.-Y.; Lengelé, L.; Bruyère, O. Malnutrition as a Strong Predictor of the Onset of Sarcopenia. Nutrients 2019, 11, 2883. [Google Scholar] [CrossRef] [Green Version]
- Mareschal, J.; Achamrah, N.; Norman, K.; Genton, L. Clinical Value of Muscle Mass Assessment in Clinical Conditions Associated with Malnutrition. J. Clin. Med. 2019, 8, 1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, T.; Maeda, K.; Nagano, A.; Shimizu, A.; Ueshima, J.; Murotani, K.; Sato, K.; Tsubaki, A. Undernutrition, Sarcopenia, and Frailty in Fragility Hip Fracture: Advanced Strategies for Improving Clinical Outcomes. Nutrients 2020, 12, 3743. [Google Scholar] [CrossRef] [PubMed]
- Ceniccola, G.D.; Castro, M.G.; Piovacari, S.M.F.; Horie, L.M.; Corrêa, F.G.; Barrere, A.P.N.; Toledo, D.O. Current technologies in body composition assessment: Advantages and disadvantages. Nutrients 2019, 62, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Rustani, K.; Kundisova, L.; Capecchi, P.L.; Nante, N.; Bicchi, M. Ultrasound measurement of rectus femoris muscle thickness as a quick screening test for sarcopenia assessment. Arch. Gerontol. Geriatr. 2019, 83, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strasser, E.M.; Draskovits, T.; Praschak, M.; Quittan, M.; Graf, A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age 2013, 35, 2377–2388. [Google Scholar] [CrossRef] [Green Version]
- Hestermann, U.; Backenstrass, M.; Gekle, I.; Hack, M.; Mundt, C.; Oster, P.; Thomas, C. Validation of a German Version of the Confusion Assessment Method for Delirium Detection in a Sample of Acute Geriatric Patients with a High Prevalence of Dementia. Psychopathology 2009, 42, 270–276. [Google Scholar] [CrossRef]
- Jiang, L.; Chou, A.; Nadkarni, N.; Ng, C.E.Q.; Chong, Y.S.; Howe, T.S.; Koh, J.S.B. Charlson Comorbidity Index Predicts 5-Year Survivorship of Surgically Treated Hip Fracture Patients. Geriatr. Orthop. Surg. Rehabil. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the elderly at risk for malnutrition: The Mini Nutritional Assessment. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knauf, T.; Buecking, B.; Hack, J.; Barthel, J.; Bliemel, C.; Aigner, R.; Ruchholtz, S.; Eschbach, D. Development of the Barthel Index 5 years after hip fracture: Results of a prospective study. Geriatr. Gerontol. Int. 2019, 19, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Burgos, R.; Sarto, B.; Segurola, H.; Romagosa, A.; Puiggrós, C.; Vázquez, C.; Cárdenas, G.; Barcons, N.; Araujo, K.; Pérez-Portabella, C. Traducción y validación de la versiónenespañol de la escala EAT-10 (Eating Assessment Tool-10) para el despistaje de la disfagia [Translation and validation of the Spanish version of the EAT-10 (Eating Assessment Tool-10) for the screening of dysphagia]. Nutr. Hosp. 2012, 27, 2048–2054. [Google Scholar] [PubMed]
- Bahat, G.; Yilmaz, O.; Durmazoglu, S.; Kilic, C.; Tascioglu, C.; Karan, M.A. Association between Dysphagia and Frailty in Community Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 571–577. [Google Scholar] [CrossRef]
- Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. Am. J. Clin. Nutr. 1996, 64, 524S–532S. [CrossRef]
- Sergi, G.; De Rui, M.; Veronese, N.; Bolzetta, F.; Berton, L.; Carraro, S.; Bano, G.; Coin, A.; Manzato, E.; Perissinotto, E. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin. Nutr. 2015, 34, 667–673. [Google Scholar] [CrossRef]
- Sabatino, A.; Regolisti, G.; Delsante, M.; Di Motta, T.; Cantarelli, C.; Pioli, S.; Grassi, G.; Batini, V.; Gregorini, M.; Fiaccadori, E. Noninvasive evaluation of muscle mass by ultrasonography of quadriceps femoris muscle in End-Stage Renal Disease patients on hemodialysis. Clin. Nutr. 2019, 38, 1232–1239. [Google Scholar] [CrossRef]
- Abe, T.; Loenneke, J.P.; Thiebaud, R.S.; Loftin, M. Morphological and functional relationships with ultrasound measured muscle thickness of the upper extremity and trunk. Ultrasound 2014, 22, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillen, S.; Tak, R.O.; Zwarts, M.J.; Lammens, M.; Verrijp, K.N.; Arts, I.M.; van der Laak, J.A.; Hoogerbrugge, P.M.; van Engelen, B.G.; Verrips, A. Skeletal Muscle Ultrasound: Correlation Between Fibrous Tissue and Echo Intensity. Ultrasound Med. Biol. 2009, 35, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Walowski, C.O.; Braun, W.; Maisch, M.J.; Jensen, B.; Peine, S.; Norman, K.; Müller, M.J.; Bosy-Westphal, A. Reference Values for Skeletal Muscle Mass—Current Concepts and Methodological Considerations. Nutrients 2020, 12, 755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello-Quiroz, M.; López-González, D.; Montiel-Ojeda, D.; Klunder-Klunder, M.; Clark, P. Correlation of appendicular muscle mass measured with dual-energy X-ray absorptiometry and anthropometry in a healthy population of pediatric and adolescent subjects. Bol. Med. Hosp. Infant. Mex. 2020, 77, 28–33. [Google Scholar] [PubMed] [Green Version]
- Doxey, G.E. Assessing arm muscularity with anthropometric and real time ultrasonic techniques. J. Strength Cond. Res. 1989, 3, 1–6. [Google Scholar]
- Mateos-Angulo, A.; Galán-Mercant, A.; Cuesta-Vargas, A.I. Ultrasound Muscle Assessment and Nutritional Status in Institutionalized Older Adults: A Pilot Study. Nutrients 2019, 11, 1247. [Google Scholar] [CrossRef] [Green Version]
- Fraiz, G.M.; Gallo, L.H.; Rabito, E.I.; Gomes, A.R.S.; Schieferdecker, M.E.M. Relationship between muscle thickness and calf circumference in healthy older women. Arch. Gerontol. Geriatr. 2020, 86, 103942. [Google Scholar] [CrossRef]
- Ticinesi, A.; Meschi, T.; Narici, M.V.; Lauretani, F.; Maggio, M. Muscle Ultrasound and Sarcopenia in Older Individuals: A Clinical Perspective. J. Am. Med. Dir. Assoc. 2017, 18, 290–300. [Google Scholar] [CrossRef]
- Probert, N.; Lööw, A.; Akner, G.; Wretenberg, P.; Andersson, Å.G. A Comparison of Patients with Hip Fracture, Ten Years Apart: Morbidity, Malnutrition and Sarcopenia. J. Nutr. Health Aging 2020, 24, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Yoshida, M.; Sato, A.; Fujimoto, Y.; Minematsu, T.; Sugama, J.; Sanada, H. Temporal muscle thickness as a new indicator of nutritional status in older individuals. Geriatr. Gerontol. Int. 2019, 19, 135–140. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, Y.H.; Cho, D.H.; Kim, M.; Lee, D.-S.; Cho, H.J. Applicability of the masseter muscle as a nutritional biomarker. Medicine 2020, 99, e19069. [Google Scholar] [CrossRef]
- González-Montalvo, J.I.; Alarcón, T.; Gotor, P.; Queipo, R.; Velasco, R.; Hoyos, R.; Pardo, A.; Otero, A. Prevalence of sarcopenia in acute hip fracture patients and its influence on short-term clinical outcome. Geriatr. Gerontol. Int. 2016, 16, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Steihaug, O.M.; Gjesdal, C.G.; Bogen, B.; Kristoffersen, M.H.; Lien, G.; Ranhoff, A.H. Sarcopenia in patients with hip fracture: A multicenter cross-sectional study. PLoS ONE 2017, 12, e0184780. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.M.; Martin, D.S.; Ploutz-Snyder, R.; Matz, T.; Caine, T.; Downs, M.; Hackney, K.; Buxton, R.; Ryder, J.W.; Ploutz-Snyder, L. Panoramic ultrasound: A novel and valid tool for monitoring change in muscle mass. J. Cachexia Sarcopenia Muscle 2017, 8, 475–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groenendijk, I.; Kramer, C.S.; Boeft, L.M.D.; Hobbelen, H.S.M.; Van Der Putten, G.-J.; De Groot, L.C. Hip Fracture Patients in Geriatric Rehabilitation Show Poor Nutritional Status, Dietary Intake and Muscle Health. Nutrients 2020, 12, 2528. [Google Scholar] [CrossRef]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Nishio, N.; Abe, Y.; Kakehi, T.; Fujimoto, J.; Tanaka, T.; Ohji, S.; Otobe, Y.; et al. Differential Characteristics of Skeletal Muscle in Community-Dwelling Older Adults. J. Am. Med. Dir. Assoc. 2017, 18, 807.e9–807.e16. [Google Scholar] [CrossRef]
- Ata, A.M.; Kara, M.; Kaymak, B.; Gürçay, E.; Çakır, B.; Ünlü, H.; Akıncı, A.; Özçakar, L. Regional and total muscle mass, muscle strength and physical performance: The potential use of ultrasound imaging for sarcopenia. Arch. Gerontol. Geriatr. 2019, 83, 55–60. [Google Scholar] [CrossRef]
- Nagano, A.; Maeda, K.; Shimizu, A.; Nagami, S.; Takigawa, N.; Ueshima, J.; Suenaga, M. Association of Sarcopenic Dysphagia with Underlying Sarcopenia Following Hip Fracture Surgery in Older Women. Nutrients 2020, 12, 1365. [Google Scholar] [CrossRef] [PubMed]
- Gaszynski, T.; Gaszynska, E.; Godala, M.; Szatko, F. Masseter muscle tension, chewing ability, and selected parameters of physical fitness in elderly care home residents in Lodz, Poland. Clin. Interv. Aging 2014, 9, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- Umeki, K.; Watanabe, Y.; Hirano, H.; Edahiro, A.; Ohara, Y.; Yoshida, H.; Obuchi, S.; Kawai, H.; Murakami, M.; Takagi, D.; et al. The relationship between masseter muscle thickness and appendicular skeletal muscle mass in Japanese community-dwelling elders: A cross-sectional study. Arch. Gerontol. Geriatr. 2018, 78, 18–22. [Google Scholar] [CrossRef]
- Murakami, M.; Hirano, H.; Watanabe, Y.; Sakai, K.; Kim, H.; Katakura, A. Relationship between chewing ability and sarcopenia in Japanese community-dwelling older adults. Geriatr. Gerontol. Int. 2015, 15, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Madsen, G.; Kristoffersen, S.M.; Westergaard, M.R.; Gjødvad, V.; Jessen, M.M.; Melgaard, D. Prevalence of Swallowing and Eating Difficulties in an Elderly Postoperative Hip Fracture Population—A Multi-Center-Based Pilot Study. Geriatrics 2020, 5, 52. [Google Scholar] [CrossRef]
- González-Fernández, M.; Arbones-Mainar, J.M.; Ferrer-Lahuerta, E.; Perez-Nogueras, J.; Serrano-Oliver, A.; Torres-Anoro, E.; Sanz-Paris, A. Ultrasonographic Measurement of Masseter Muscle Thickness Associates with Oral Phase Dysphagia in Institutionalized Elderly Individuals. Dysphagia 2021, 1–9. [Google Scholar] [CrossRef]
- Abe, T.; Counts, B.R.; Barnett, B.E.; Dankel, S.J.; Lee, K.; Loenneke, J.P. Associations between Handgrip Strength and Ultrasound-Measured Muscle Thickness of the Hand and Forearm in Young Men and Women. Ultrasound Med. Biol. 2015, 41, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, H.; Hu, Y.; Zhu, S.; Xu, Z.; Zhang, Q.; Yang, Y.; Wang, Z.; Xu, J. Ultrasound for Measuring the Cross-Sectional Area of Biceps Brachii Muscle in Sarcopenia. Int. J. Med. Sci. 2020, 17, 2947–2953. [Google Scholar] [CrossRef]
- Nijholt, W.; Scafoglieri, A.; Jager-Wittenaar, H.; Hobbelen, J.S.M.; Van Der Schans, C.P. The reliability and validity of ultrasound to quantify muscles in older adults: A systematic review. J. Cachex Sarcopenia Muscle 2017, 8, 702–712. [Google Scholar] [CrossRef] [PubMed]
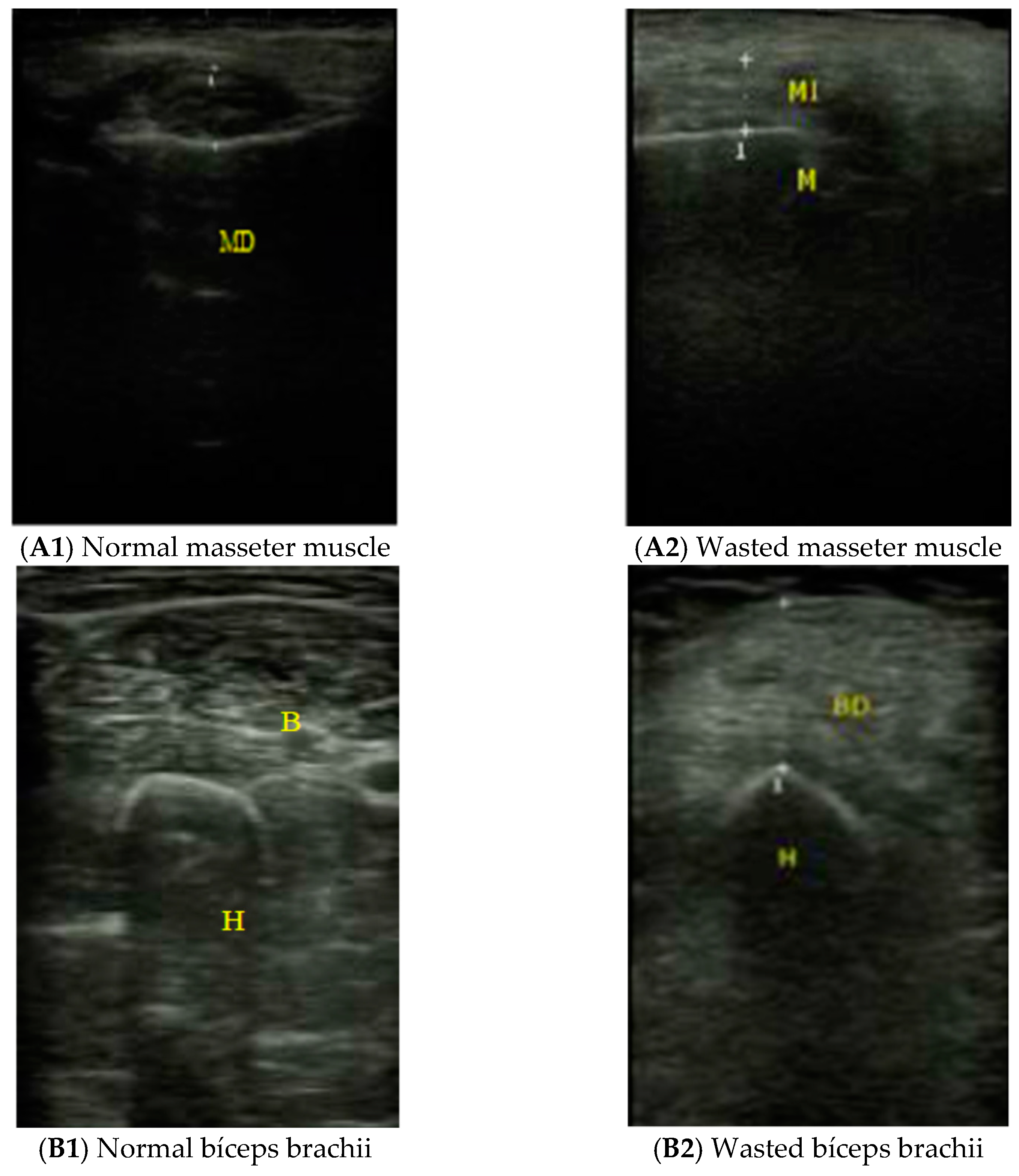
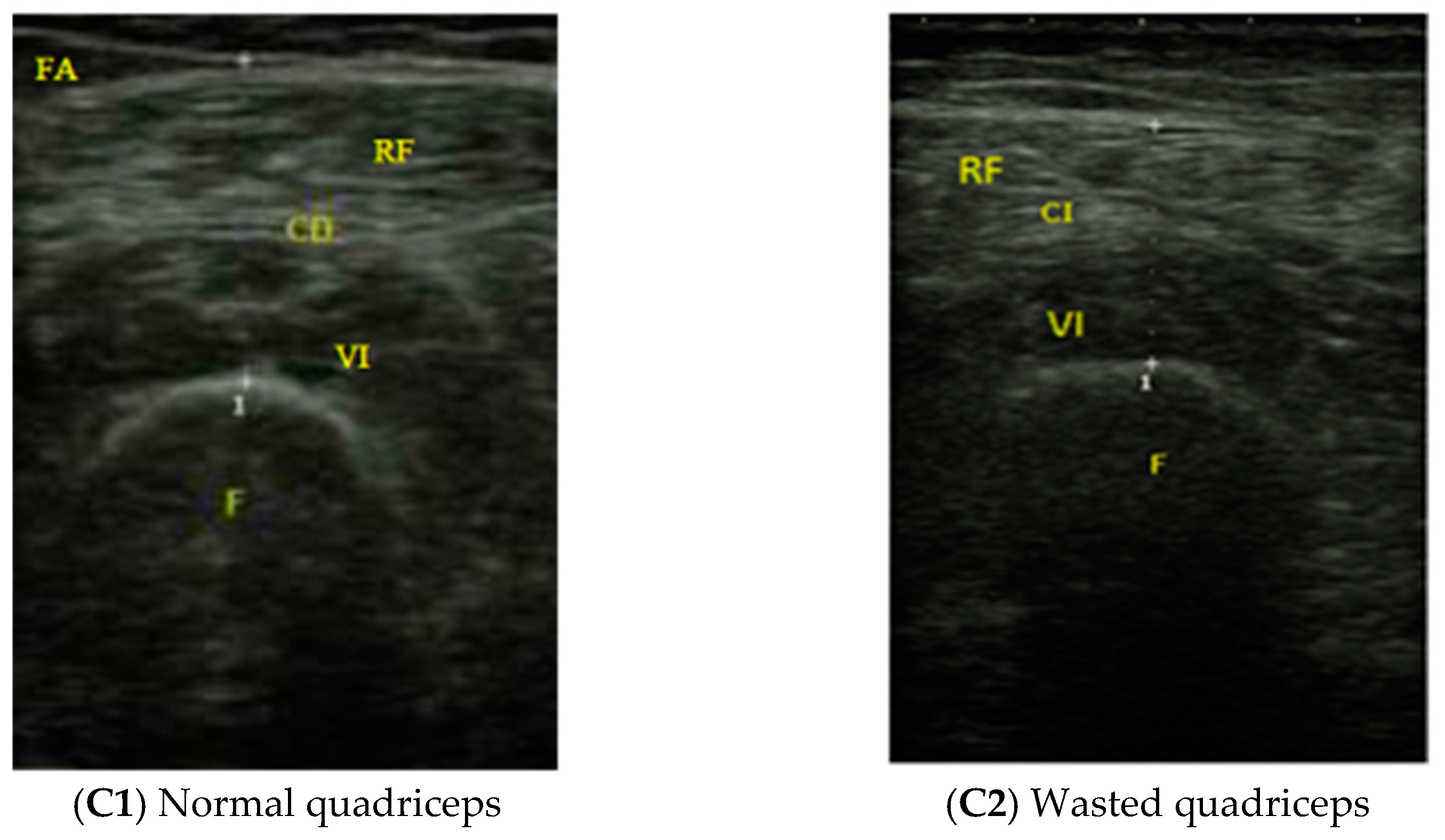
| Parameter | Total (n: 101) | Female (n: 71) | Male (n: 30) | P |
|---|---|---|---|---|
| Age (years) | 86 (9) | 86 (10) | 86.5 (5) | 0.9 |
| Masseter thickness (mm) | 10.4 (2.73) | 10.35 (2.05) | 11.5 (3.2) | 0.3 |
| Masseter echogenicity | 32.4 (11.7) | 35.6 (10.5) | 24.5 (11.2) | 0.001 |
| Biceps thickness (mm) | 22.3 (7.2) | 22.2 (7.07) | 23.5 (8.4) | 0.1 |
| Biceps echogenicity | 61.8 (12.2) | 62.8 (12.6) | 59.3 (11.3) | 0.3 |
| Quadriceps thickness (mm) | 22.1 (6.45) | 22.1 (6.3) | 22.2 (6.98) | 0.6 |
| Quadriceps echogenicity | 63.7 (12.4) | 64.8 (11.9) | 61 (13.5) | 0.3 |
| Xc (ohm) | 45.6 (12.2) | 45.3 (12.4) | 46.4 (11.9) | 0.7 |
| Rz (ohm) | 509.1 (67.2) | 509.1 (65.4) | 509.5 (72.8) | 0.9 |
| PA (grades) | 5.1 (2.3) | 5.1 (1.3) | 5.1 (1.2) | 0.9 |
| ASMI (Kg/m2) | 6.7 (0.86) | 6.6 (0.88) | 6.9 (0.75) | 0.1 |
| ASMI low (%) | 31.7 | 19.7 | 60 | 0.0001 |
| Hand grip (Kg) | 13.05 (7.53) | 11.9 (6.5) | 14.6 (11.65) | 0.07 |
| Sarcopenia probable (%) (Hand grip low) | 89.1 | 84.5 | 100 | 0.028 |
| Sarcopenia confirmed (%) (Hand grip low + ASMI low) | 29.7 | 16.9 | 60 | 0.0001 |
| BMI (Kg/m2) | 25.39 (4.67) | 24.78 (5.69) | 26.04 (2.87) | 0.09 |
| Arm circumference (cm) | 26 (5.9) | 26 (6.3) | 26.5 (5.5) | 0.8 |
| Calf circumference (cm) | 31 (4) | 31 (4) | 31 (5.6) | 0.4 |
| Malnutrition risk by MNA-SF (%) | 66.3 | 73.2 | 50 | 0.025 |
| Malnutrition by GLIM (%) | 43.6 | 43.7 | 43.3 | 0.5 |
| Charlson index | 7 (3) | 6 (3) | 7 (2) | 0.1 |
| Barthel index | 85 (24) | 85 (23) | 85 (40) | 0.7 |
| Triturated diet (%) | 26.8 | 27.5 | 25 | 0.5 |
| Dysphagia (>3 score EAT-10) (%) | 13.9 | 14.1 | 13.3 | 0.6 |
| Dysphagia (>15 score EAT-0) (%) | 9.9 | 8.5 | 13.3 | 0.3 |
| Feed themselves (%) | 71.7 | 75.6 | 63.2 | 0.2 |
| Mobility on level surfaces (%) | 60 | 61 | 57.9 | 0.5 |
| Climb stairs (%) | 31.7 | 26.8 | 42.1 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz-Paris, A.; González-Fernandez, M.; Hueso-Del Río, L.E.; Ferrer-Lahuerta, E.; Monge-Vazquez, A.; Losfablos-Callau, F.; Sanclemente-Hernández, T.; Sanz-Arque, A.; Arbones-Mainar, J.M. Muscle Thickness and Echogenicity Measured by Ultrasound Could Detect Local Sarcopenia and Malnutrition in Older Patients Hospitalized for Hip Fracture. Nutrients 2021, 13, 2401. https://doi.org/10.3390/nu13072401
Sanz-Paris A, González-Fernandez M, Hueso-Del Río LE, Ferrer-Lahuerta E, Monge-Vazquez A, Losfablos-Callau F, Sanclemente-Hernández T, Sanz-Arque A, Arbones-Mainar JM. Muscle Thickness and Echogenicity Measured by Ultrasound Could Detect Local Sarcopenia and Malnutrition in Older Patients Hospitalized for Hip Fracture. Nutrients. 2021; 13(7):2401. https://doi.org/10.3390/nu13072401
Chicago/Turabian StyleSanz-Paris, Alejandro, Mikel González-Fernandez, Luis Enrique Hueso-Del Río, Eduardo Ferrer-Lahuerta, Alejandra Monge-Vazquez, Francisco Losfablos-Callau, Teresa Sanclemente-Hernández, Alejandro Sanz-Arque, and Jose Miguel Arbones-Mainar. 2021. "Muscle Thickness and Echogenicity Measured by Ultrasound Could Detect Local Sarcopenia and Malnutrition in Older Patients Hospitalized for Hip Fracture" Nutrients 13, no. 7: 2401. https://doi.org/10.3390/nu13072401
APA StyleSanz-Paris, A., González-Fernandez, M., Hueso-Del Río, L. E., Ferrer-Lahuerta, E., Monge-Vazquez, A., Losfablos-Callau, F., Sanclemente-Hernández, T., Sanz-Arque, A., & Arbones-Mainar, J. M. (2021). Muscle Thickness and Echogenicity Measured by Ultrasound Could Detect Local Sarcopenia and Malnutrition in Older Patients Hospitalized for Hip Fracture. Nutrients, 13(7), 2401. https://doi.org/10.3390/nu13072401







