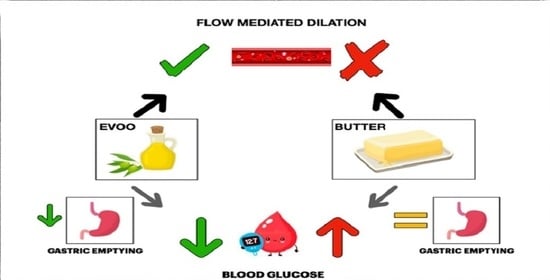
Effect of Extra Virgin Olive Oil and Butter on Endothelial Function in Type 1 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meigs, J.B.; Nathan, D.M.; D’Agostino, R.B.; Wilson, P.W.F. Fasting and post challenge glycemia and cardiovascular disease risk: The Framingham offspring study. Diabetes Care 2002, 25, 1845–1850. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Gong, C.; Cao, B.; Peng, X.; Wu, D.; Gu, Y.; Wei, L.; Liang, X.; Liu, M.; Li, W.; et al. Glucose fluctuations in association with oxidative stress among children with T1DM: Comparison of different phases. J. Clin. Endocrinol. Metab. 2015, 100, 1828–1836. [Google Scholar] [CrossRef] [Green Version]
- Monnier, L.; Lapinski, H.; Colette, C. Contributions of fasting and post-prandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: Variations with increasing levels of HbA1c. Diabetes Care 2003, 26, 881–885. [Google Scholar] [CrossRef] [Green Version]
- Lupoli, R.; Pisano, F.; Capaldo, B. Postprandial Glucose Control in Type 1 Diabetes: Importance of the gastric emptying rate. Nutrients 2019, 11, 1559. [Google Scholar] [CrossRef] [Green Version]
- Bell, K.J.; Smart, C.E.; Steil, G.M.; Brand-Miller, J.C.; King, B.; Wolpert, H.A. Impact of fat, protein, and glycemic index on post-prandial glucose control in type 1diabetes: Implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care 2015, 38, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, S.J.; Lundman, P.; Harmer, J.A.; Cutri, B.; Griffiths, K.A.; Rye, K.A.; Barter, P.J.; Celermajer, D.S. Consumption of saturated fat impairs the anti-inflammatory properties of high-density lipoproteins and endothelial function. J. Am. Coll. Cardiol. 2006, 48, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Tentolouris, N.; Arapostathi, C.; Perrea, D.; Kyriaki, D.; Revenas, C.; Katsilambros, N. Differential effects of two isoenergetic meals rich in saturated or monounsaturated fat on endothelial function in subjects with type 2 diabetes. Diabetes Care 2008, 31, 2276–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keefe, J.H.; Bell, D.S.H. Postprandial hyperglycemia/hyperlipidemia (Postprandial Dysmetabolism) is a cardiovascular risk factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of olive oil on markers of inflammation and endothelial function—A systematic review and meta-analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [CrossRef] [Green Version]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, A.; Sanchez-Rodriguez, E.; Vargas, F.; Montoro-Molina, S.; Romero, M.; Espejo-Calvo, J.A.; Vilchez, P.; Jaramillo, S.; Olmo-García, L.; Carrasco-Pancorbo, A.; et al. Cardioprotective effect of a virgin olive oil enriched with bioactive compounds in spontaneously hypertensive rats. Nutrients 2019, 11, 1728. [Google Scholar] [CrossRef] [Green Version]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Bozzetto, L.; Alderisio, A.; Giorgini, M.; Barone, F.; Giacco, A.; Riccardi, G.; Rivellese, A.A.; Annuzzi, G. Extra-virgin olive oil reduces glycemic response to a high-glycemic index meal in patients with type 1 diabetes: A randomized controlled trial. Diabetes Care 2016, 39, 518–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celermajer, D.S.; Sorensen, K.E.; Gooch, V.M.; Spiegelhalter, D.J.; Miller, O.I.; Sullivan, I.D.; Lloyd, J.K.; Deanfield, J. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992, 340, 1111–1115. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Tremblay, J.C.; Pyke, K.E. Flow-mediated dilation stimulated by sustained increases in shear stress: A useful tool for assessing endothelial function in humans? Am. J. Physiol. Hear. Circ. Physiol. 2018, 314, H508–H520. [Google Scholar] [CrossRef] [PubMed]
- Lavi, T.L.; Karasik, A.; Koren-Morag, N.; Kanety, H.; Feinberg, M.S.; Shechter, M. The acute effect of various glycemic index dietary carbohydrates on endothelial function in non-diabetic overweight and obese subjects. J. Am. Coll. Cardiol. 2009, 53, 2283–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Standard Italiani per la Cura del Diabete Mellito 2018. Available online: https://www.siditalia.it/clinica/standard-di-cura-amd-sid (accessed on 16 May 2018).
- Wolever, T.M.S.; Jenkins, D.J.A.; Ocana, A.M.; Rao, V.A.; Collier, G.R. Second-meal effect: Low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am. J. Clin. Nutr. 1988, 48, 1041–1047. [Google Scholar] [CrossRef]
- Bozzetto, L.; Alderisio, A.; Clemente, G.; Giorgini, M.; Barone, F.; Griffo, E.; Costabile, G.; Vetrani, C.; Cipriano, P.; Giacco, A.; et al. Gastrointestinal effects of extra-virgin olive oil associated with lower post-prandial glycemia in type 1 diabetes. Clin. Nutr. 2019, 38, 2645–2651. [Google Scholar] [CrossRef]
- Daenen, S.; Sola-Gazagnes, A.; M’Bemba, J.; Dorange-Breillard, C.; Defer, F.; Elgrably, F.; Larger, E.; Slama, G. Peak-time determination of post-meal glucose excursions in insulin-treated diabetic patients. Diabetes Metab. 2010, 36, 165–169. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.C.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef]
- Hashimoto, M.; Akishita, M.; Eto, M.; Ishikawa, M.; Kozaki, K.; Toba, K.; Sagara, Y.; Taketani, Y.; Orimo, H.; Ouchi, Y. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation 1995, 92, 3431–3435. [Google Scholar] [CrossRef]
- Irace, C.; Tschakovsky, M.E.; Carallo, C.; Cortese, C.; Gnasso, A. Endothelial Dysfunction or dysfunctions? Identification of three different FMD responses in males with type 2 diabetes. Atherosclerosis 2008, 200, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Irace, C.; Padilla, J.; Carallo, C.; Scavelli, F.; Gnasso, A. Delayed vasodilation is associated with cardiovascular risk. Eur. J. Clin. Invest. 2014, 44, 549–556. [Google Scholar] [CrossRef]
- Cutruzzolà, A.; Irace, C.; Frazzetto, M.; Sabatino, J.; Gullace, R.; De Rosa, S.; Spaccarotella, C.; Concolino, D.; Indolfi, C.; Gnasso, A. Functional and morphological cardiovascular alterations associated with neurofibromatosis 1. Sci. Rep. 2020, 10, 12070. [Google Scholar] [CrossRef] [PubMed]
- Pyke, K.E.; Jazuli, F. Impact of repeated increases in shear stress via reactive hyperemia and handgrip exercise: No evidence of systematic changes in brachial artery FMD. Am. J. Physiol. Hear. Circ. Physiol. 2011, 300, 1078–1089. [Google Scholar] [CrossRef]
- Grzelak, P.; Olszycki, M.; Majos, A.; Czupryniak, L.; Strzelczyk, J.; Stefańczyk, L. Hand exercise test for the assessment of endothelium-dependent vasodilation in subjects with type 1 diabetes. Diabetes Technol. Ther. 2010, 8, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Irvine, E.J.; Tougas, G.; Lappainen, R.; Bathurst, N.C. Reliability and interobserver variability of ultrasonographic measurement of gastric emptying rate. Dig. Dis. Sci. 1993, 38, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Perlas, A.; Chan, V.W.S.; Lupu, C.M.; Mitsakakis, N.; Hanbidge, A. Ultrasound assessment of gastric content and volume. Anesthesiology 2009, 111, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Arora, R.C.; Hiebert, B.M.; Lerner, B.; Szwajcer, A.; McDonald, K.; Rigatto, C.; Komenda, P.; Sood, M.M.; Tangri, N. Non-invasive endothelial function testing and the risk of adverse outcomes: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 736–746. [Google Scholar] [CrossRef] [Green Version]
- Ras, R.T.; Streppel, M.T.; Draijer, R.; Zock, P.L. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. Int. J. Cardiol. 2013, 168, 344–351. [Google Scholar] [CrossRef]
- Kim, F.; Tysseling, K.A.; Rice, J.; Pham, M.; Haji, L.; Gallis, B.M.; Baas, A.S.; Paramsothy, P.; Giachelli, C.M.; Corson, M.A.; et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKβ. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 989–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Li, H.; Bao, Y.; Zhang, X.; Yu, Y. Free fatty acids induce endothelial dysfunction and activate protein kinase C and nuclear factor-κB pathway in rat aorta. Int. J. Cardiol. 2011, 152, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferrè, M.; Tysseling, K.A.; Rice, J.; Pham, M.; Haji, L.; Gallis, B.M.; Baas, A.S.; Paramsothy, P.; Giachelli, C.M.; Corson, M.A.; et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whisner, C.M.; Angadi, S.S.; Weltman, N.Y.; Weltman, A.; Rodriguez, J.; Patrie, J.T.; Gaesser, G.A. Effects of low-fat and high-fat meals, with and without dietary fiber, on post-prandial endothelial function, triglyceridemia, and glycemia in adolescents. Nutrients 2019, 11, 2626. [Google Scholar] [CrossRef] [Green Version]
- Violi, F.; Loffredo, L.; Pignatelli, P.; Angelico, F.; Bartimoccia, S.; Nocella, C.; Cangemi, R.; Petruccioli, A.; Monticolo, R.; Pastori, D.; et al. Extra-virgin olive oil use is associated with improved post-prandial blood glucose and LDL cholesterol in healthy subjects. Nutr. Diabetes 2015, 5, e172. [Google Scholar] [CrossRef] [Green Version]
- Carnevale, R.; Pignatelli, P.; Nocella, C.; Loffredo, L.; Pastori, D.; Vicario, T.; Petruccioli, A.; Bartimoccia, S.; Violi, F. Extra-virgin olive oil blunts post-prandial oxidative stress via NOX2 down regulation. Atherosclerosis 2014, 235, 649–658. [Google Scholar] [CrossRef]
- Aigner, L.; Becker, B.; Gerken, S.; Quast, D.R.; Meier, J.J.; Nauck, M.A. Day-to-day variations in fasting plasma glucose do not influence gastric emptying in subjects with type 1 Diabetes. Diabetes Care 2021, 44, 479–488. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Dehghan, M.; Mente, A.; Bangdiwala, S.I.; Rangarajan, S.; Srichaikul, K.; Mohan, V.; Avezum, A.; Díaz, R.; Rosengren, A.; et al. Glycemic index, glycemic load, and cardiovascular disease and mortality. N. Engl. J. Med. 2021, 384, 1312–1322. [Google Scholar] [CrossRef]



| Test Meal Composition | EVOO | Butter |
|---|---|---|
| Energy (kcal) | 900 | 900 |
| Carbohydrates (g) | 100.1 | 100.5 |
| Starches (g) | 90.1 | 90.1 |
| Proteins (g) | 38.5 | 40.9 |
| Fiber (g) | 4.0 | 4.0 |
| Fat (g) | 41.1 | 39.7 |
| Cholesterol (mg) | 99.4 | 206.5 |
| MUFA (g) | 28.0 | 11.6 |
| PUFA (g) | 4.6 | 2.6 |
| SFA (g) | 7.7 | 21.7 |
| Glycemic Index (%) | 76 | 76 |
| Type 1 Diabetes (N 10) | Controls (N 6) | p | |
|---|---|---|---|
| Age (years) | 28 ± 8 | 25 ± 3 | 0.38 |
| Male n (%) | 7 (70) | 6 (100) | 0.25 |
| Waist (cm) | 82 ± 10 | 81 ± 5 | 0.91 |
| BMI (kg/m2) | 24.2 ± 3.0 | 22.3 ± 1.8 | 0.18 |
| Systolic BP (mmHg) | 122 ± 10 | 110 ± 10 | 0.04 |
| Diastolic BP (mmHg) | 73 ± 8 | 71 ± 10 | 0.68 |
| HR (beats/min) | 72 ± 10 | 70 ± 8 | 0.69 |
| Smokers n (%) | 1 (10) | 3 (50) | 0.12 |
| Pre-meal glucose, (mmol/L) * | 7.3 ± 2.9 | 4.5 ± 0.3 | 0.04 |
| Pre-meal triglycerides (mmol/L) * | 0.71 ± 0.24 | 0.98 ± 0.21 | 0.04 |
| Pre-meal total cholesterol (mmol/L) * | 4.07 ± 0.67 | 3.76 ± 0.65 | 0.38 |
| Pre-meal HDL-cholesterol (mmol/L) * | 1.17 ± 0.28 | 1.19 ± 0.31 | 0.90 |
| Pre-meal LDL-cholesterol (mmol/L) * | 2.43 ± 0.41 | 2.20 ± 0.36 | 0.92 |
| Disease duration (years) | 12 ± 6 | - | - |
| HbA1c (%) | 7.4 ± 0.8 | - | - |
| Insulin daily dose (unit/kg) | 0.56 ± 0.21 | - | - |
| Type 1 Diabetes | Pre Meal | 1 h | 2 h | 3 h | 4 h | 5 h | p |
|---|---|---|---|---|---|---|---|
| EVOO total cholesterol (mmol/L) | 4.05 ± 0.69 | 3.80 ± 0.56 | 3.89 ± 0.62 | 3.97 ± 0.72 | 3.89 ± 0.68 | 3.92 ± 0.65 | p = 0.66 |
| Butter total cholesterol (mmol/L) | 4.00 ± 0.54 | 3.81 ± 0.55 | 3.82 ± 0.61 | 3.87 ± 0.53 | 3.96 ± 0.57 | 3.95 ± 0.57 | |
| EVOO HDL-cholesterol (mmol/L) | 1.15 ± 0.29 | 1.14 ± 0.27 | 1.12 ± 0.27 | 1.15 ± 0.29 | 1.13 ± 0.28 | 1.16 ± 0.28 | p = 0.81 |
| Butter HDL-cholesterol (mmol/L) | 1.22 ± 0.25 | 1.17 ± 0.25 | 1.15 ± 0.26 | 1.17 ± 0.25 | 1.19 ± 0.25 | 1.18 ± 0.27 | |
| EVOO LDL-cholesterol (mmol/L) | 2.57 ± 0.58 | 2.27 ± 0.39 | 2.38 ± 0.49 | 2.39 ± 0.53 | 2.30 ± 0.44 | 2.29 ± 0.41 | p = 0.23 |
| Butter LDL-cholesterol (mmol/L) | 2.43 ± 0.34 | 2.25 ± 0.34 | 2.24 ± 0.39 | 2.25 ± 0.31 | 2.28 ± 0.36 | 2.28 ± 0.37 | |
| EVOO triglycerides (mmol/L) | 0.72 ± 0.24 | 0.87 ± 0.26 | 0.87 ± 0.27 | 0.95 ± 0.31 | 1.02 ± 0.39 | 1.04 ± 0.49 | p = 0.92 |
| Butter triglycerides (mmol/L) | 0.78 ± 0.32 | 0.86 ± 0.28 | 0.93 ± 0.35 | 0.99 ± 0.42 | 1.09 ± 0.47 | 1.06 ± 0.42 | |
| Control Subjects | Pre Meal | 1 h | 2 h | 3 h | 4 h | 5 h | |
| EVOO total cholesterol (mmol/L) | 3.75 ± 0.65 | 3.78 ± 0.51 | 3.71 ± 0.66 | 3.76 ± 0.60 | 3.71 ± 0.60 | 3.79 ± 0.61 | p = 0.85 |
| Butter total cholesterol (mmol/L) | 3.72 ± 0.45 | 3.64 ± 0.44 | 3.64 ± 0.41 | 3.65 ± 0.40 | 3.66 ± 0.47 | 3.77 ± 0.47 | |
| EVOO HDL-cholesterol (mmol/L) | 1.18 ± 0.30 | 1.16 ± 0.27 | 1.16 ± 0.29 | 1.18 ± 0.29 | 1.16 ± 0.30 | 1.19 ± 0.32 | p = 0.86 |
| Butter HDL-cholesterol (mmol/L) | 1.24 ± 0.29 | 1.18 ± 0.32 | 1.19 ± 0.30 | 1.19 ± 0.30 | 1.20 ± 0.32 | 1.24 ± 0.37 | |
| EVOO LDL-cholesterol (mmol/L) | 2.11 ± 0.39 | 2.11 ± 0.19 | 2.08 ± 0.42 | 2.08 ± 0.41 | 2.07 ± 0.41 | 2.11 ± 0.36 | p = 0.90 |
| Butter LDL-cholesterol (mmol/L) | 2.04 ± 0.44 | 1.94 ± 0.42 | 1.95 ± 0.39 | 1.87 ± 0.36 | 1.94 ± 0.41 | 1.99 ± 0.31 | |
| EVOO triglycerides (mmol/L) | 0.99 ± 0.22 | 1.12 ± 0.38 | 1.05 ± 0.09 | 1.10 ± 0.16 | 1.04 ± 0.19 | 1.07 ± 0.28 | p = 0.69 |
| Butter triglycerides (mmol/L) | 0.98 ± 0.28 | 1.13 ± 0.29 | 1.09 ± 0.36 | 1.26 ± 0.99 | 1.12 ± 0.38 | 1.17 ± 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutruzzolà, A.; Parise, M.; Vallelunga, R.; Lamanna, F.; Gnasso, A.; Irace, C. Effect of Extra Virgin Olive Oil and Butter on Endothelial Function in Type 1 Diabetes. Nutrients 2021, 13, 2436. https://doi.org/10.3390/nu13072436
Cutruzzolà A, Parise M, Vallelunga R, Lamanna F, Gnasso A, Irace C. Effect of Extra Virgin Olive Oil and Butter on Endothelial Function in Type 1 Diabetes. Nutrients. 2021; 13(7):2436. https://doi.org/10.3390/nu13072436
Chicago/Turabian StyleCutruzzolà, Antonio, Martina Parise, Rosarina Vallelunga, Francesco Lamanna, Agostino Gnasso, and Concetta Irace. 2021. "Effect of Extra Virgin Olive Oil and Butter on Endothelial Function in Type 1 Diabetes" Nutrients 13, no. 7: 2436. https://doi.org/10.3390/nu13072436
APA StyleCutruzzolà, A., Parise, M., Vallelunga, R., Lamanna, F., Gnasso, A., & Irace, C. (2021). Effect of Extra Virgin Olive Oil and Butter on Endothelial Function in Type 1 Diabetes. Nutrients, 13(7), 2436. https://doi.org/10.3390/nu13072436