Reflection of Dietary Iodine in the 24 h Urinary Iodine Concentration, Serum Iodine and Thyroglobulin as Biomarkers of Iodine Status: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of Screening and Baseline Testing
2.2. Participants
2.3. Baseline Measurements and Testing
2.4. Baseline Iodine Intake and Frequency of Intake of Iodine-Containing Foods
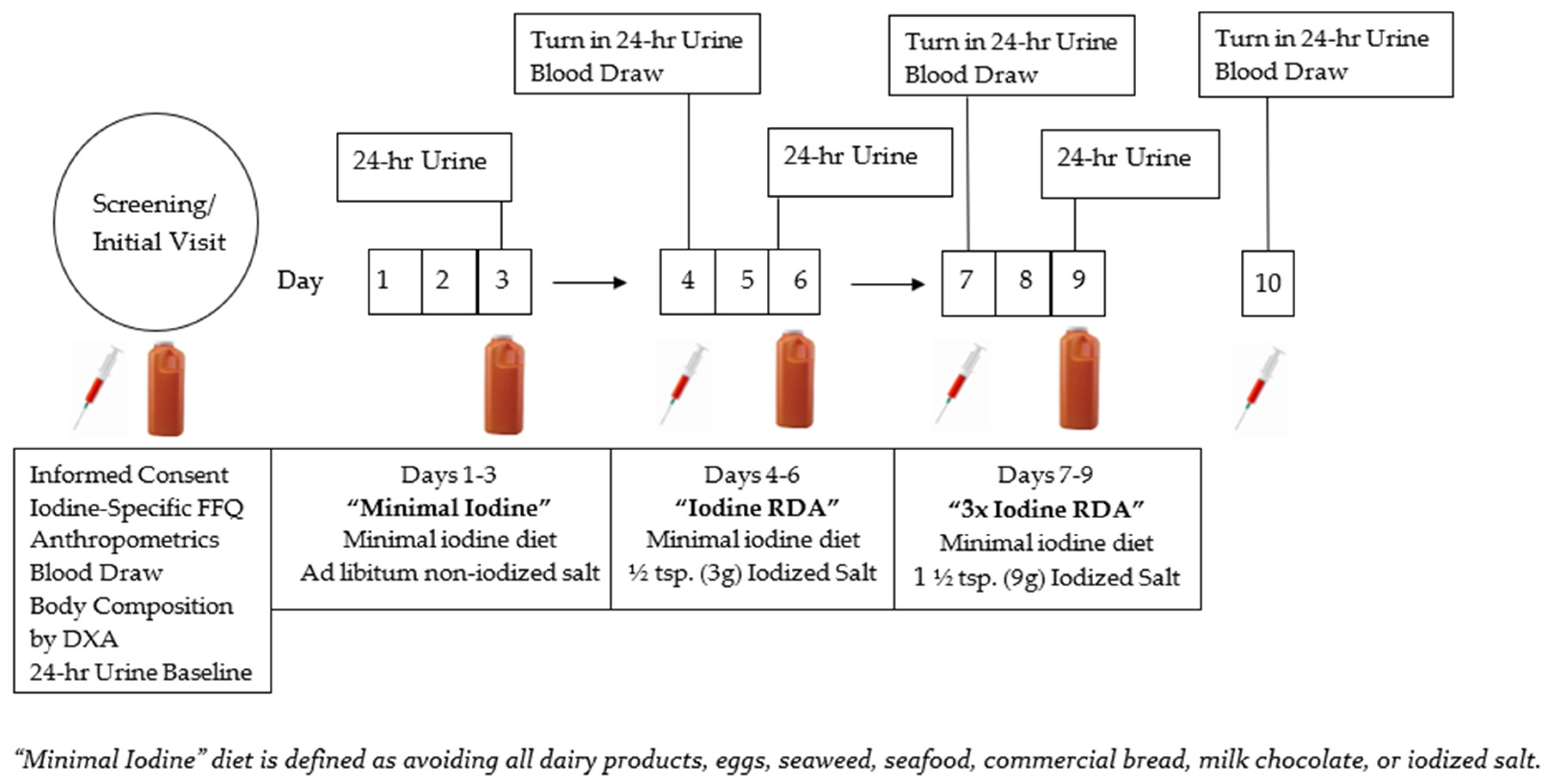
2.5. Iodine Titration Diet and Biomarker Protocol
2.6. The 24 h Urine Collections
2.7. Analysis of Iodine Status Biomarkers in Urine and Blood
2.8. Post-Study Questionnaire
2.9. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Baseline Daily Iodine Intake and Frequency of Intake of Iodine-Containing Foods
3.3. Baseline 24 h UIC and Iodine Status
3.4. Relationship between Baseline Iodine Intake and UIC
3.5. Effect of Titration Diet on Iodine Status Biomarkers
3.6. Post-Study Questionnaire Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Food Frequency Questionnaire (Ffq) For each food listed, check the box indicating how often on average you have used the amount specified during the last month | |||||||||
| Foods Consumed | Never or <1 per mo. | 1–3 per Month | 1 per Week | 2–4 per Week | 5–6 per Week | 1 per Day | 2–3 per Day | 4–5 per Day | 6+ per Day |
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| Multi Vitamin, 1 Tablet (Type and Brand) _________________ | |||||||||
| 3.Kelp (or other iodine supplement), 1 Tablet | |||||||||
| If you take any of the above, please list which you use, brand name, etc. | |||||||||
References
- Zimmermann, M.B. Iodine Deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef] [Green Version]
- Markel, H. When It Rains It Pours: Endemic Goiter, Iodized Salt, and David Murray Cowie, MD. Public Health Then Now 1987, 77, 219–229. [Google Scholar] [CrossRef]
- Caldwell, K.; Makhmudov, A.; Ely, E.; Jones, R.; Wang, R.Y. Iodine Status of the U.S. Population, National Health and Nutrition Examination Survery, 2005–2006 and 2007–2008. Thyroid 2011, 21, 419–427. [Google Scholar] [CrossRef]
- Panth, P.; Guerin, G.; DiMarco, N.M. A Review of Iodine Status of Women of Reproductive Age in the USA. Biol. Trace Elem. Res. 2019, 188, 208–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldwell, K.L.; Pan, Y.; Mortensen, M.E.; Makhmudov, A.; Merrill, L.; Moye, J. Iodine Status in Pregnant Women in the National Children’s Study and in U.S. Women (15–44 Years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013, 23, 927–937. [Google Scholar] [CrossRef]
- Hynes, K.L.; Seal, J.A.; Otahal, P.; Oddy, W.H.; Burgess, J.R. Women Remain at Risk of Iodine Deficiency during Pregnancy: The Importance of Iodine Supplementation before Conception and throughout Gestation. Nutrients 2019, 11, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, A.M.; LaMar, A.; He, X.; Braverman, L.E.; Pearce, E.N. Iodine Status and Thyroid Function of Boston-Area Vegetarians and Vegans. J. Clin. Endocrinol. Metab. 2011, 96, 1303–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groufh-Jacobsen, S.; Hess, S.Y.; Aakre, I.; Gjengedal, E.L.F.; Pettersen, K.B.; Henjum, S. Vegans, Vegetarians and Pescatarians Are at Risk of Iodine Deficiency in Norway. Nutrients 2020, 12, 3555. [Google Scholar] [CrossRef]
- Eveleigh, E.R.; Coneyworth, L.J.; Avery, A.; Welham, S.J.M. Vegans, Vegetarians, and Omnivores: How Does Dietary Choice Influence Iodine Intake? A Systematic Review. Nutrients 2020, 12, 1606. [Google Scholar] [CrossRef]
- Remer, T.; Neubert, A.; Manz, F. Increased Risk of Iodine Deficiency with Vegetarian Nutrition. Br. J. Nutr. 1999, 81, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Krajcovicova-Kudlackova, M.; Bučková, K.; Klimeš, I.; Šeboková, E. Iodine Deficiency in Vegetarians and Vegans. Ann. Nutr. Metab. 2003, 47, 183–185. [Google Scholar] [CrossRef]
- Pearce, E.N.; Pino, S.; He, X.; Bazrafshan, H.R.; Lee, S.L.; Braverman, L.E. Sources of Dietary Iodines Bread, Cows’ Milk, and Infant Formula in the Boston Area. J. Clin. Endocrinol. Metab. 2004, 89, 3421–3424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO; UNICEF; ICCIDD. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination; WHO: Geneva, Switerland, 2007; pp. 1–107. [Google Scholar]
- Tayie, F.A.K.; Jourdan, K. Hypertension, Dietary Salt Restriction, and Iodine Deficiency among Adults. Am. J. Hypertens. 2010, 23, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuge, R.; Johnson, C.C. The Geochemistry of Iodine—A Review. Br. Geol. Surv. 1958, 1957, 31–51. [Google Scholar] [CrossRef] [PubMed]
- USDA. Dietary Guidelines for Americans 2020–2025; U.S. Department of Agriculture and U.S. Department of Health and Human Services: Washington, DC, USA, 2020; p. 164.
- Henney, J.E.; Taylor, C.L.; Boon, C.S. Strategies to Reduce Sodium Intake in the United States; The National Academies Press: Washington, DC, USA, 2010; pp. 29–63. [Google Scholar] [CrossRef]
- Appel, L.J.; Frohlich, E.D.; Hall, J.E.; Pearson, T.A.; Sacco, R.L.; Seals, D.R.; Sacks, F.M.; Smith, S.C.; Vafiadis, D.K.; Van Horn, L.V. The Importance of Population-Wide Sodium Reduction as a Means to Prevent Cardiovascular Disease and Stroke: A Call to Action from the American Heart Association. Circulation 2011, 123, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Hetzel, B.S.; Dunn, J.T. The Iodine Deficiency Disorders: Their Nature and Prevention. Annu. Rev. Nutr. 1989, 9, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Haap, M.; Roth, H.J.; Huber, T.; Dittmann, H.; Wahl, R. Urinary Iodine: Comparison of a Simple Method for Its Determination in Microplates with Measurement by Inductively-Coupled Plasma Mass Spectrometry. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, P.; Cook, P. The Assessment of Iodine Status—Populations, Individuals and Limitations. Ann. Clin. Biochem. 2019, 56, 7–14. [Google Scholar] [CrossRef]
- Rasmussen, L.B.; Ovesen, L.; Christiansen, E. Day-to-Day and within-Day Variation in Urinary Iodine Excretion. Eur. J. Clin. Nutr. 1999, 53, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Remer, T.; Fonteyn, N.; Alexy, U.; Berkemeyer, S. Longitudinal Examination of 24-h Urinary Iodine Excretion in Schoolchildren as a Sensitive, Hydration Status-Independent Research Tool for Studying Iodine Status. Am. J. Clin. Nutr. 2006, 83, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Manousou, S.; Stål, M.; Larsson, C.; Mellberg, C.; Lindahl, B.; Eggertsen, R.; Hulthén, L.; Olsson, T.; Ryberg, M.; Sandberg, S.; et al. A Paleolithic-Type Diet Results in Iodine Deficiency: A 2-Year Randomized Trial in Postmenopausal Obese Women. Eur. J. Clin. Nutr. 2018, 72, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jiang, P.; Liu, L.; Jia, Q.; Liu, P.; Meng, F.; Zhang, X.; Guan, Y.; Pang, Y.; Lu, Z.; et al. The Application of Serum Iodine in Assessing Individual Iodine Status. Clin. Endocrinol. 2017, 87, 807–814. [Google Scholar] [CrossRef]
- Ma, Z.F.; Skeaff, S.A. Thyroglobulin as a Biomarker of Iodine Deficiency: A Review. Thyroid 2014, 24, 1195–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, A. Pathways of Iodine Metabolism. Am. J. Med. 1956, 20, 653–669. [Google Scholar]
- Mao, F.; Ko, Y.C.; Chen, M.-L. Stability of Iodine in Human Sweat. Jpn. J. Physiol. 1990, 40, 693–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gostas, D.E.; Larson-Meyer, D.E.; Yoder, H.A.; Huffman, A.E.; Johnson, E.C. Dietary Relationship with 24-Hour Urinary Iodine Concentrations of Young Adults in the Mountainous West Region of the United States. Nutrients 2020, 12, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliday, T.M.; Peterson, N.J.; Thomas, J.J.; Kleppinger, K.; Hollis, B.W.; Larson-Meyer, D.E. Vitamin D Status Relative to Diet, Lifestyle, Injury, and Illness in College Athletes. Med. Sci. Sports Exerc. 2011, 43, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Gerow, K.; Bozeman, B.; Grossman, G.D. Response Feature Analysis for Repeated Measures in Ecological Research. Bull. Ecol. Soc. Am. 2021, 102, 1–9. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Methods to Assess Iron and Iodine Status. Br. J. Nutr. 2008, 99 (Suppl. 3), 2–9. [Google Scholar] [CrossRef] [PubMed]
- Censi, S.; Watutantrige-Fernando, S.; Groccia, G.; Manso, J.; Plebani, M.; Faggian, D.; Mion, M.M.; Venturini, R.; Andrisani, A.; Casaro, A.; et al. The Effects of Iodine Supplementation in Pregnancy on Iodine Status, Thyroglobulin Levels and Thyroid Function Parameters: Results from a Randomized Controlled Clinical Trial in a Mild-to-Moderate Iodine Deficiency Area. Nutrients 2019, 11, 2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, M.B.; De Benoist, B.; Corigliano, S.; Jooste, P.L.; Molinari, L.; Moosa, K.; Pretell, E.A.; Al-Dallal, Z.S.; Wei, Y.; Chen, Z.P.; et al. Assessment of Iodine Status Using Dried Blood Spot Thyroglobulin: Development of Reference Material and Establishment of an International Reference Range in Iodine-Sufficient Children. J. Clin. Endocrinol. Metab. 2006, 91, 4881–4887. [Google Scholar] [CrossRef] [Green Version]
- Reinhardt, W.; Luster, M.; Rudorff, K.H.; Heckmann, C.; Petrasch, S.; Lederbogen, S.; Haase, R.; Saller, B.; Reiners, C.; Reinwein, D.; et al. Effect of Small Doses of Iodine on Thyroid Function in Patients with Hashimoto’s Thyroiditis Residing in an Area of Mild Iodine Deficiency. Eur. J. Endocrinol. 1998, 139, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Huda, S.N.; Grantham-McGregor, S.M.; Tomkins, A. Cognitive and Motor Functions of Iodine-Deficient but Euthyroid Children in Bangladesh Do Not Benefit from Iodized Poppy Seed Oil (Lipiodol). J. Nutr. 2001, 131, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Tonglet, R.; Bourdoux, P.; Minga, T.; Ermans, A.-M. Efficacy of Low Oral Doses of Iodized Oil in the Control of Iodine Deficiency in Zaire. N. Engl. J. Med. 1992, 329, 977–986. [Google Scholar] [CrossRef]
- Phillips, D.I.W.; Osmond, C. Iodine Supplementation with Oral or Intramuscular Iodized Oil. A Two-Year Follow-up of a Comparative Trial. Int. J. Epidemiol. 1989, 18, 907–910. [Google Scholar] [CrossRef]
- Rohner, F.; Zimmermann, M.B.; Jooste, P.L.; Pandav, C.; Caldwell, K.; Raghavan, R.; Raiten, D.J. Biomarkers of Nutrition for Development Iodine Review. J. Nutr. 2014, 144, 1322S–1342S. [Google Scholar] [CrossRef] [Green Version]
- Hetzel, B.S.; Zimmermann, M.B. The Iodine Deficiency Disorders. Iodine Defic. Eur. 1993, 25–31. [Google Scholar] [CrossRef]
- Andersen, S.; Karmisholt, J.; Pedersen, K.M.; Laurberg, P. Reliability of Studies of Iodine Intake and Recommendations for Number of Samples in Groups and in Individuals. Br. J. Nutr. 2008, 99, 813–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soldin, O.P. Controversies in Urinary Iodine Determinations. Clin. Biochem. 2002, 35, 575–579. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Reyes, R.; Carpentier, Y.A.; MacOurs, P.; Gulbis, B.; Corvilain, B.; Glinoer, D.; Goldman, S. Seasons but Not Ethnicity Influence Urinary Iodine Concentrations in Belgian Adults. Eur. J. Nutr. 2011, 50, 285–290. [Google Scholar] [CrossRef]
- Als, C.; Helbling, A.; Peter, K.; Haldimann, M.; Zimmerli, B.; Gerber, H. Urinary Iodine Concentration Follows a Circadian Rhythm: A Study with 3023 Spot Urine Samples in Adults and Children. J. Clin. Endocrinol. Metab. 2000, 85, 1367–1369. [Google Scholar] [CrossRef]
- Zou, Y.; Li, H.; Pang, J.; Liu, X.; Tian, L.; Yu, S.; Wang, D.; Hou, L.; Yin, Y.; Ma, C.; et al. An Evaluation of Urine and Serum Iodine Status in the Population of Tibet, China: No Longer an Iodine-Deficient Region. Nutrition 2021, 82, 111033. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, D.; Cheng, X.; Zhang, Q.; Wang, M.; Guo, H.; Yu, B.; Zhang, X.; Xia, L.; Sun, D.; et al. Establishing Reference Intervals for Urine and Serum Iodine Levels: A Nationwide Multicenter Study of a Euthyroid Chinese Population. Clin. Chim. Acta 2020, 502, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Næss, S.; Aakre, I.; Kjellevold, M.; Dahl, L.; Nerhus, I.; Midtbø, L.K.; Markhus, M.W. Validation and Reproducibility of a New Iodine Specific Food Frequency Questionnaire for Assessing Iodine Intake in Norwegian Pregnant Women. Nutr. J. 2019, 18, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brantsæter, A.L.; Haugen, M.; Julshamn, K.; Alexander, J.; Meltzer, H.M. Evaluation of Urinary Iodine Excretion as a Biomarker for Intake of Milk and Dairy Products in Pregnant Women in the Norwegian Mother and Child Cohort Study (MoBa). Eur. J. Clin. Nutr. 2009, 63, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.M.; Charlton, K.E.; Tan, S.Y.; Ma, G.; Batterham, M. Validity and Reproducibility of an Iodine-Specific Food Frequency Questionnaire to Estimate Dietary Iodine Intake in Older Australians. Nutr. Diet. 2013, 70, 71–78. [Google Scholar] [CrossRef]
- Michalke, B.; Witte, H. Characterization of a Rapid and Reliable Method for Iodide Biomonitoring in Serum and Urine Based on Ion Chromatography-ICP-Mass Spectrometry. J. Trace Elem. Med. Biol. 2015, 29, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.B.; Ovesen, L.; Knudsen, N.; Laurberg, P.; Perrild, H. Relations between Various Measures of Iodine Intake and Thyroid Volume, Thyroid Nodularity, and Serum Thyroglobulin. Am. J. Clin. Nutr. 2002, 76, 1069–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vejbjerg, P.; Knudsen, N.; Perrild, H.; Laurberg, P.; Carlé, A.; Pedersen, I.B.; Rasmussen, L.B.; Ovesen, L.; Jørgensen, T. Thyroglobulin as a Marker of Iodine Nutrition Status in the General Population. Eur. J. Endocrinol. 2009, 161, 475–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Yan Hui, G.; Zhuo Ying, F.; Fan Gang, M.; Li Jun, F.; Dian Jun, S. Serum Thyroglobulin—A Sensitive Biomarker of Iodine Nutrition Status and Affected by Thyroid Abnormalities and Disease in Adult Populations. Biomed. Environ. Sci. 2017, 30, 508–516. [Google Scholar] [CrossRef]
- Lee, K.W.; Shin, D.; Cho, M.S.; Song, W.O. Food Group Intakes as Determinants of Iodine Status among US Adult Population. Nutrients 2016, 8, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Reijden, O.L.; Zimmermann, M.B.; Galetti, V. Iodine in Dairy Milk: Sources, Concentrations and Importance to Human Health. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Pennington, J.A.T. Iodine Concentrations in US Milk: Variation Due to Time, Season, and Region. J. Dairy Sci. 1990, 73, 3421–3427. [Google Scholar] [CrossRef]
- Eckhoff, K.M.; Maage, A. Iodine Content in Fish and Other Food Products from East Africa Analyzed by ICP-MS. J. Food Compos. Anal. 1997, 10, 270–282. [Google Scholar] [CrossRef]

| Mean ± SEM | ||
|---|---|---|
| Male | Female | |
| Age | 26.6 ± 1.1 | 36.0 ± 7.4 |
| Height (cm) | 183.2 ± 2.8 | 166.4 ± 2.6 |
| Weight (kg) | 82.8 ± 4.4 | 69.5 ± 11.3 |
| BMI (kg/M2) | 24.7 ± 1.1 | 24.8 ± 3.4 |
| Body Fat (%) | 20.7 ± 3.8 | 31.3 ± 4.5 |
| Hemoglobin (g/dL) | 16.5 ± 0.2 | 14.6 ± 0.3 |
| Hematocrit (%) | 49.0 ± 0.6 | 43.5 ± 1.1 |
| TSH (mcIU/mL) | 2.0 ± 0.3 | 1.5 ± 0.2 |
| Thyroglobulin (ng/mL) | 17.5 ± 5.2 | 16.9 ± 4.2 |
| 24 h UIC (μg/L) | 126.6 ± 28.7 | 142.4 ± 29.6 |
| Iodine Marker | Minimal Iodine | RDA | 3× RDA | Normal Range |
|---|---|---|---|---|
| 24 h UIC (μg/L) | 19.1 ± 6.0 | 31.2 ± 9.9 | 173.9 ± 55.0 | 100–199 μg/L |
| 24 h UIE (μg/day) | 82.7 ± 8.5 | 174.1 ± 15.2 | 401.4 ± 63.8 | N/A |
| Serum Iodine (ng/mL) | 62.1 ± 3.3 | 61.5 ± 3.6 | 66.8 ± 3.8 | 40–92 ng/mL |
| Tg (ng/mL) | 17.2 ± 3.2 | 17.1 ± 2.9 | 17.8 ± 3.1 | ≤33 ng/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlucny, K.; Alexander, B.M.; Gerow, K.; Larson-Meyer, D.E. Reflection of Dietary Iodine in the 24 h Urinary Iodine Concentration, Serum Iodine and Thyroglobulin as Biomarkers of Iodine Status: A Pilot Study. Nutrients 2021, 13, 2520. https://doi.org/10.3390/nu13082520
Hlucny K, Alexander BM, Gerow K, Larson-Meyer DE. Reflection of Dietary Iodine in the 24 h Urinary Iodine Concentration, Serum Iodine and Thyroglobulin as Biomarkers of Iodine Status: A Pilot Study. Nutrients. 2021; 13(8):2520. https://doi.org/10.3390/nu13082520
Chicago/Turabian StyleHlucny, Katelyn, Brenda M. Alexander, Ken Gerow, and D. Enette Larson-Meyer. 2021. "Reflection of Dietary Iodine in the 24 h Urinary Iodine Concentration, Serum Iodine and Thyroglobulin as Biomarkers of Iodine Status: A Pilot Study" Nutrients 13, no. 8: 2520. https://doi.org/10.3390/nu13082520
APA StyleHlucny, K., Alexander, B. M., Gerow, K., & Larson-Meyer, D. E. (2021). Reflection of Dietary Iodine in the 24 h Urinary Iodine Concentration, Serum Iodine and Thyroglobulin as Biomarkers of Iodine Status: A Pilot Study. Nutrients, 13(8), 2520. https://doi.org/10.3390/nu13082520





