Abstract
The worldwide global increase in serum 25-hydroxyvitamin D (25(OH)D) measurements has led some countries to restrict reimbursement for certain clinical situations only. Another approach could consist in providing physicians with screening tools in order to better target blood test prescription. The objective of the SCOPYD study was to identify the best combination of predictors of serum VitD concentration among adults aged 18–70 years. Potential risk factors for VitD deficiency were collected using a comprehensive self-administered questionnaire. A multivariable linear regression was used to build a predictive model of serum 25(OH)D concentration. Among 2488 participants, 1080 (43.4%) had VitD deficiency (<50 nmol/L) and 195 (7.8%) had severe deficiency (<25 nmol/L). The final model included sunlight exposure in the preceding week and during the last holidays, month of blood sampling, age, sex, body mass index, skin phototype, employment, smoking, sport practice, latitude, and VitD supplementation in preceding year. The area under the curve was 0.82 (95% CI (0.78; 0.85)) for severe deficiency. The model predicted severe deficiency with a sensitivity of 77.9% (95% CI (69.1; 85.7)) and a specificity of 68.3% (95% CI (64.8; 71.9)). We identified a set of predictors of severe VitD deficiency that are easy to collect in routine that may help to better target patients for serum 25(OH)D concentration determination.
1. Introduction
Vitamin D deficiency has long been recognized as a cause of osteomalacia in adults and rickets in children. A low vitamin D concentration is also considered as a risk factor for bone fragility [1]. In the last decades, many observational studies have found that serum vitamin D concentration was inversely correlated with the occurrence of multiple chronic disorders (e.g., cancer, cardiovascular disease, diabetes, and autoimmune disorders). Due to this growing interest in the pleiotropic putative effects of vitamin D, a massive increase in the number of serum 25-hydroxyvitamin D (25(OH)D) measurements has been observed worldwide [2,3,4,5,6]. In this context, providing physicians with clinical tools to identify and discriminate between high-risk patients and those presenting a very low risk of vitamin D deficiency should help them to better target the patients for whom vitamin D concentration determination is indicated.
A large number of studies have been conducted to identify the risk factors of vitamin D deficiency and try to develop clinical scores. However, most studies have addressed this question only in specific sub-groups of the population such as post-menopausal women [7,8,9,10], older adults [11,12,13], or pregnant women [14], in restricted geographical areas [15,16,17], or without considering critical risk factors such as sun exposure [18,19] or other known factors of hypovitaminosis D [15,20,21]. Furthermore, studies that have addressed individual sun exposure have measured it incompletely or without precision regarding the surface exposed. Consequently, there is a need for further studies in the general population to address more comprehensively all the risk factors (and more precisely individual sun exposure) associated with vitamin D deficiency in order to further develop a diagnostic tool based on risk factors that would be easily assessed through a self-administered questionnaire.
The objective of the present study was to identify the best combination of factors to predict the concentration of serum vitamin D among adults aged 18 to 70 years drawn from the general population.
2. Materials and Methods
2.1. Study Design and Participants
A multicenter national cross-sectional study was performed. We used the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) [22] to guide the reporting.
Outpatients were recruited from rheumatology, dermatology, anesthesiology, occupational medicine, and sports medicine departments in 5 university hospitals (Lyon, Clermont-Ferrand, Saint Etienne, Nice, Lille) representing various geographic regions of France to take into account the vitamin D variability associated with latitude (43° to 50°). The inclusion period stretched over a whole year to take into account the seasonal variability of vitamin D concentration, and it eventually lasted 14 months (from September 2016 to November 2017) to meet the initial sample size calculation. Men and women aged 18 to 70 years old were included. Exclusion criteria were health disorders that could possibly impact the vitamin D status such as renal failure (severe renal impairment, dialysis, and kidney transplant), known hepatic impairment, gastrointestinal disorders (celiac disease, Crohn’s disease, ulcerative colitis, bariatric surgery, and gastrointestinal surgery with stoma), known primary hypo/hyperparathyroidism, and bone cancer/metastases current or within the last 2 years. Other exclusion criteria were current or recent (less than one year) participation in a study related to vitamin D, pregnancy, or breast-feeding, ongoing antiepileptic or antiretroviral treatment, ongoing long-term glucocorticoid treatment (>3 months), legal incapacity or limited legal capacity, no affiliation to the national French health insurance, or having received at least 80,000 IU vitamin D in the last 3 months in a single dose. Conversely, patients could be currently treated with low daily doses of vitamin D or could have received higher unique dose of vitamin D more than 3 months before the inclusion in the study.
2.2. Outcome
The primary objective was to identify the best combination of factors to predict the concentration of serum vitamin D, and the corresponding primary outcome was the circulating 25(OH)D concentration as a continuous variable and measured as described below. The secondary objective was to evaluate the performance of the model to identify people having a vitamin D deficiency and those having a severe vitamin D deficiency. The gold standard used to measure the sensitivity and specificity of the models was based on the result of the serum 25(OH)D measurements. A vitamin D deficiency was defined as a serum 25(OH)D concentration <50 nmol/L [23,24], and a severe vitamin D deficiency was defined as a serum 25(OH)D measurement <25 nmol/L [25].
2.3. Serum 25(OH)D Measurement
Blood was collected from all participants on the day of questionnaire completion, serum was separated and kept at −80 °C until assayed. Serum 25(OH)D concentration was determined by chemiluminescent immunoassay technology (Liaison XL®, DiaSorin, Saluggia, Italy) in duplicate. Measurements were performed blinded from the questionnaires results, in one of the three participating centers in Lyon (77%), Nice (14%), and Clermont-Ferrand (9%). The laboratories of these centers are affiliated to an external quality control program allowing inter-center standardization. The intra- and inter-assay coefficients of variation were <10%.
2.4. Risk Factors
For collecting data assessing potential risk factors for vitamin deficiency, a self-assessment questionnaire was developed in four steps. The first step was to gather all relevant information through a literature review of factors potentially associated with vitamin D concentration or hypovitaminosis D symptoms in order to build a template for the questionnaire. The second step was the validation of the questionnaire content by a multidisciplinary group of rheumatologists, biologists, and public health specialists. The third step was a test of the first version on 13 outpatients for evaluating the study feasibility, understandability, and the appropriateness of the questionnaire, and for changing its content accordingly. The fourth step was a test of the second version in real study conditions on 19 outpatients, which led to the final version after minor changes.
The self-administered questionnaire was provided to each participant upon arrival in the ward. A large number of potential risk factors for vitamin D deficiency were collected through this questionnaire, which contained six sections (Appendix A).
The first section was related to socio-economic characteristics (age, sex, country of birth, education level, employment status, and place of residence). The latitude of the place of residence was classified in three areas according to the French Lambert zone projection system [26]: North for latitudes of 48°15′ or above, Center for latitudes between 44°45′ and 48°14′, and South/Corsica for latitudes between 42°76′ and 44°44′.
The second section was composed of clinical characteristics (weight, height, skin phototype, current smoking status (Yes/No), physical activities, chronic muscle, joint, or bone pain with no known cause, and for women number of pregnancies, age at first pregnancy, year of birth of last child, menopausal status). Regarding the skin phototype, participants were asked to choose one of the 6 categories of the Fitzpatrick scale [27]. Then, they were grouped into 3 categories, (1) light-colored skin (type I to III), (2) tanned skin (type IV), and (3) dark skin (type V and VI). Physical activities were classified in 3 categories according to their intensity based on the Mitchell classification [28]. This classification has two dimensions (static and dynamic component of the activity), if the activity was rated as low on one dimension and low or moderate on the other, it was classified as “low-intensity sports”; if the activity was rated as moderate on both dimensions, it was classified as “medium-intensity sports”; if the activity was rated as high on at least one dimension, it was classified as “high-intensity sport”.
The third section included data on sun exposure. All situations of sun exposure were collected: usual sun exposure during work time, usual sun exposure during leisure time, recent exposure over the 7 days prior to the blood test, and sun exposure during holidays over the last 12 months. Regarding this last question, a “significant sun exposure” was defined as having exposed one’s bust during at least one period of holidays over the last 12 months (Yes/No).
The fourth section contained information regarding treatments: vitamin D prescribed by physicians, over-the-counter vitamin D supplements, non-steroidal anti-inflammatory drugs, diuretics, oral contraceptives, hormone replacement therapy, and other treatments regularly taken that may influence vitamin D concentration.
The fifth section was composed of dietary sources of vitamin D (food naturally rich in vitamin D or that contains added vitamin D), and the sixth section collected data regarding exposure to artificial ultraviolet radiations.
2.5. Statistical Analysis
2.5.1. Sample Size
The number of participants to be included in the sample used to construct the multiple linear model was determined so that the relative reduction of the model predictive ability for new participants, measured by the coefficient of determination (R-squared or R2), would not be more than 2.5%. Previously published predictive models had a R2 between 0.13 and 0.42 [7,11,18,29,30]. We hypothesized that the predictive ability of the model would be at least 0.4. For a number of 10 predictors included in the model, and a relative difference between the R2 and the corrected R2 of 2.5% (from 0.4 to 0.39), the number of participants to be included was about 1300 [31,32]. Thus, we planned to include 2500 participants, so that the model could be constructed using a randomly selected sample of 1300 participants among the 2500 and validated on the remaining 1200 participants. The model was finally constructed using all the included participants and validated using a bootstrap method as proposed by Steyerberg, who has shown that this approach is better than splitting the dataset into a training sample and a test sample [33].
2.5.2. Statistical Analysis
The description of the population was carried out using the mean (±standard deviation, SD) or the median (range) for quantitative characteristics, and absolute and relative frequencies for qualitative characteristics.
A linear regression model was used to build the predictive model with the value of vitamin D measurement as the dependent variable (primary outcome). A first model was built and included the month of blood sampling modeled using a cyclic cubic spline in order to take into account the seasonality of the vitamin D measurement. The corrected R2 obtained using a bootstrap method was estimated to quantify the predictive ability of the model. The following step was to build bivariable models by adding separately each selected variable to the first model. For each bivariable model, the gain of predictive ability was quantified by the increase of the corrected R2 compared to the first model. The studied variables were selected a priori (before the statistical analysis and after questionnaire completion) so that they were fairly representative of the first five sections of the questionnaire and had a limited number of missing values. Variables of the sixth section corresponding to exposure to artificial ultraviolet radiation were not selected due to the very low number of concerned participants. The variables included in the multivariable model in addition to the month of blood sampling were those that increased significantly the model predictive ability according to the likelihood ratio test and were easy to collect by auto-questionnaire. The predictive ability of the final model was quantified by the corrected R2. The analysis was carried out on the complete dataset; no method of imputation was used to replace missing data.
The overall performance of the model for identifying participants with a vitamin D deficiency or a severe vitamin D deficiency was quantified by the area under the ROC curve (AUC). The sensitivity and specificity of the model for the diagnosis of vitamin D deficiency, and for the diagnosis of severe vitamin D deficiency, were estimated using a threshold corresponding to a value predicted by the model of 50 nmol/L (test positive when the predicted value ≤ 50 nmol/L).
All parameters were estimated and expressed with their 95% confidence interval (CI) using a bootstrap method. Analyses were performed using the software SAS® version 9.3, and R, version 3.4 for some graphs.
3. Results
3.1. Population Characteristics and Vitamin D Concentrations
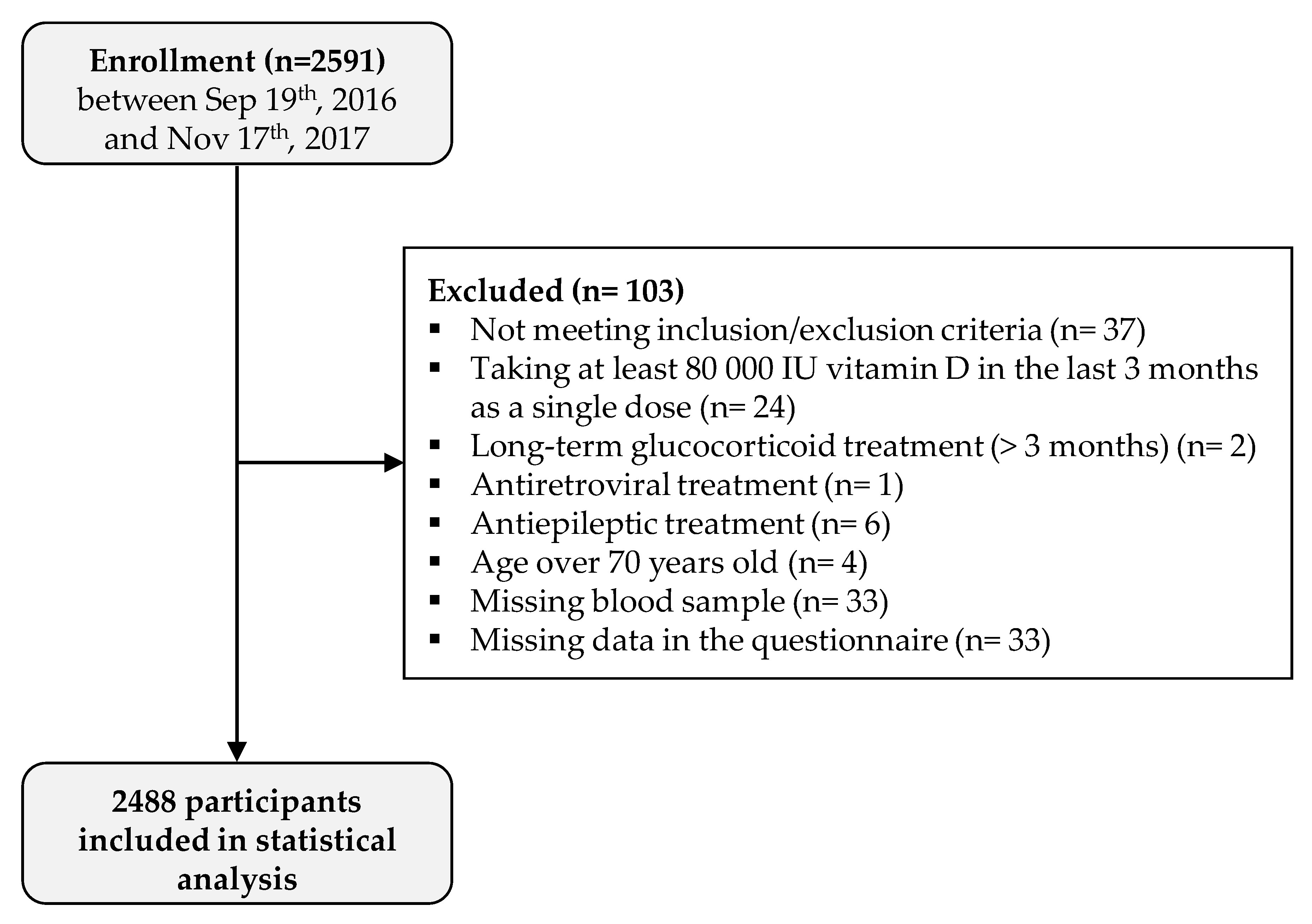
A total of 2591 participants were enrolled in the study. Serum 25(OH)D concentration was determined for 2558 individuals (33 missing blood samples), 33 (1.3%) did not fill out the questionnaire, and 37 (1.4%) were excluded because they met an exclusion criterion. Therefore, a total of 2488 participants were included in the analysis (Figure 1). Among the 2488 participants, 1513 (60.8%) were female, and information on body mass index (BMI) was available for 2473 participants: 674/2473 (27.3%) were considered as overweight (25 ≤ BMI < 30), and 418/2473 (16.9%) were considered as obese (BMI ≥ 30). Regarding skin phenotype, 1631/2475 (65.9%) participants had a light-colored skin (type I to III). A total of 525 (21.1%) participants took at least one vitamin D-supplemented product in the last 12 months, and 321 (12.9%) had had a vitamin D supplementation in the last 12 months (regular treatments with tablets or singles doses of at least 80,000 IU taken more than 3 months prior to study entry). A total of 461/2474 (18.6%) responders reported smoking, 1266 (50.9%) reported practicing sports, and 958 (38.5%) reported a “significant sun exposure” during holidays over the last 12 months (Table 1).
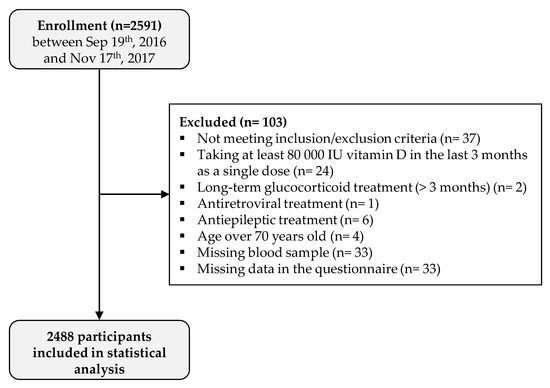
Figure 1.
Study flow chart.

Table 1.
Characteristics of the study population (n = 2488).
Serum 25(OH)D concentrations <50 nmol/L, and <25 nmol/L were found in 1080/2488 (43.4%) and 195/2488 (7.8%) participants, respectively. A sufficient vitamin D status (defined as serum 25(OH)D concentration ≥ 75 nmol/L) was found in 437/2448 (17.6%) participants.
3.2. Modeling of Seasonal Changes
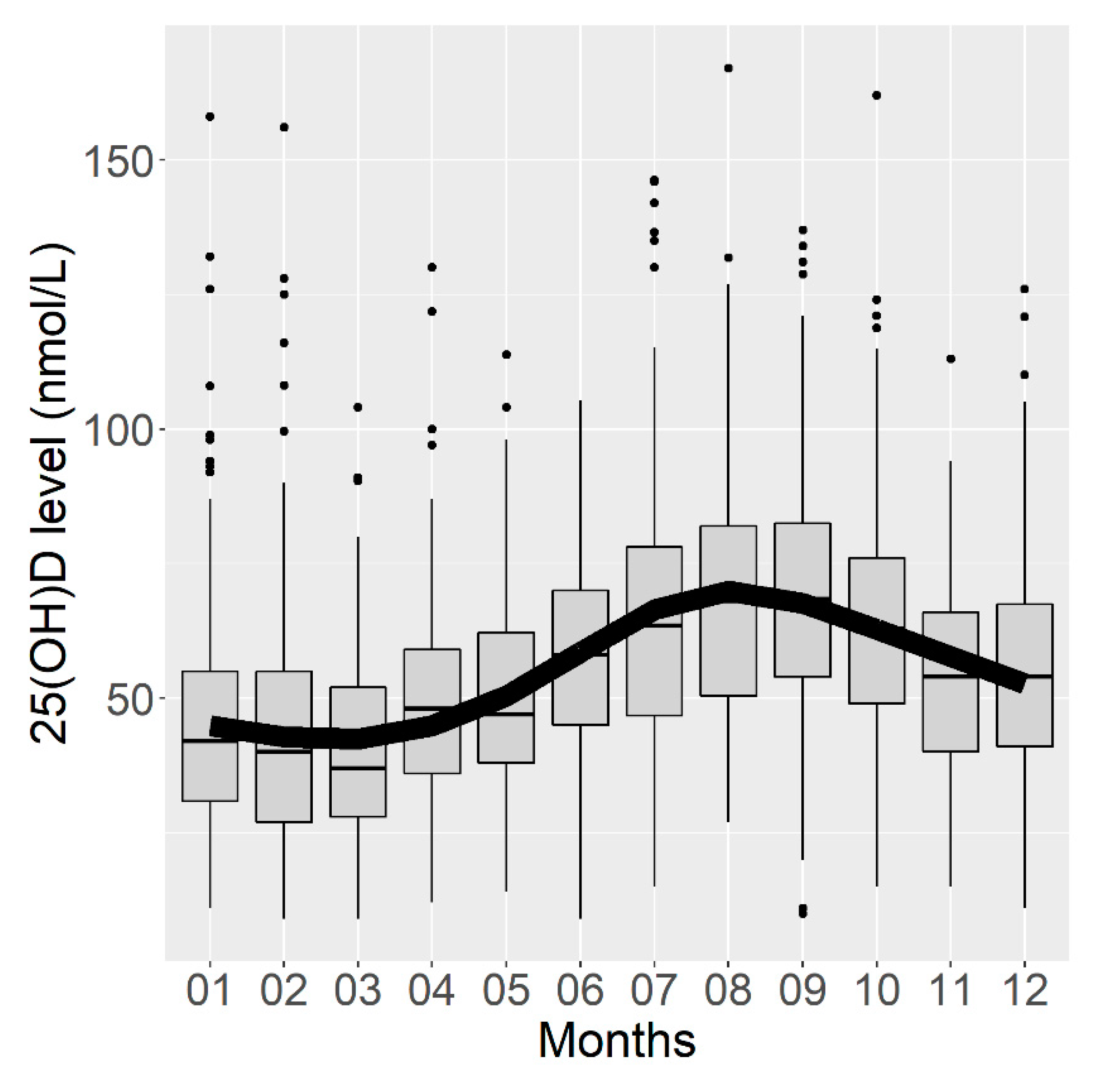
The analysis of serum 25(OH)D concentrations by month of blood sampling confirmed the seasonality of the vitamin D concentration, which was the highest in August and September and the lowest concentration in February and March (Figure 2). The corrected R2 of this first model was estimated at 0.161 (95% CI (0.122; 0.192)), meaning that 16.1% of the variance in the vitamin D concentration could be explained by the month of blood sampling.
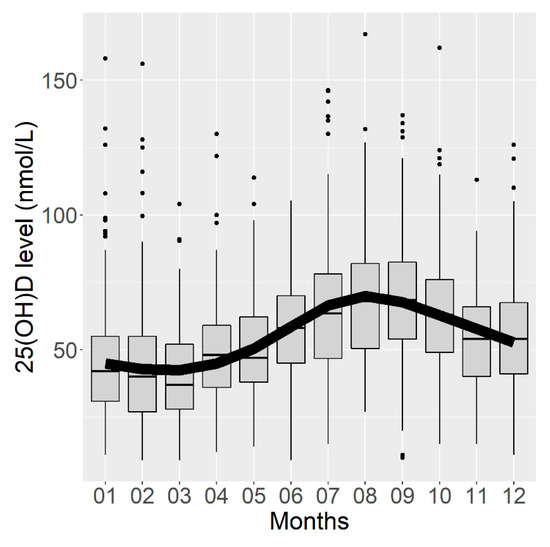
Figure 2.
Box plots representing serum vitamin D concentrations according to the month of blood sampling and curve of vitamin D concentrations predicted by a linear regression model using a cyclic cubic spline.
3.3. Prediction Model
The proportion of the explained variance in the vitamin D concentration increased from 0.1% to 4.2% depending on which variable was included in the bivariable model; this increase was maximal when the variable “Significant exposure during holidays over the last 12 months” was included in the bivariable model (+4.2%, corrected R2 = 0.203, 95% CI (0.163; 0.243); Table 2). All variables were significantly associated with the vitamin D concentration except “Intake of at least one vitamin D supplemented product in the last 12 months”.

Table 2.
Corrected R2 of the bivariable models adjusted to the month of the blood sampling.
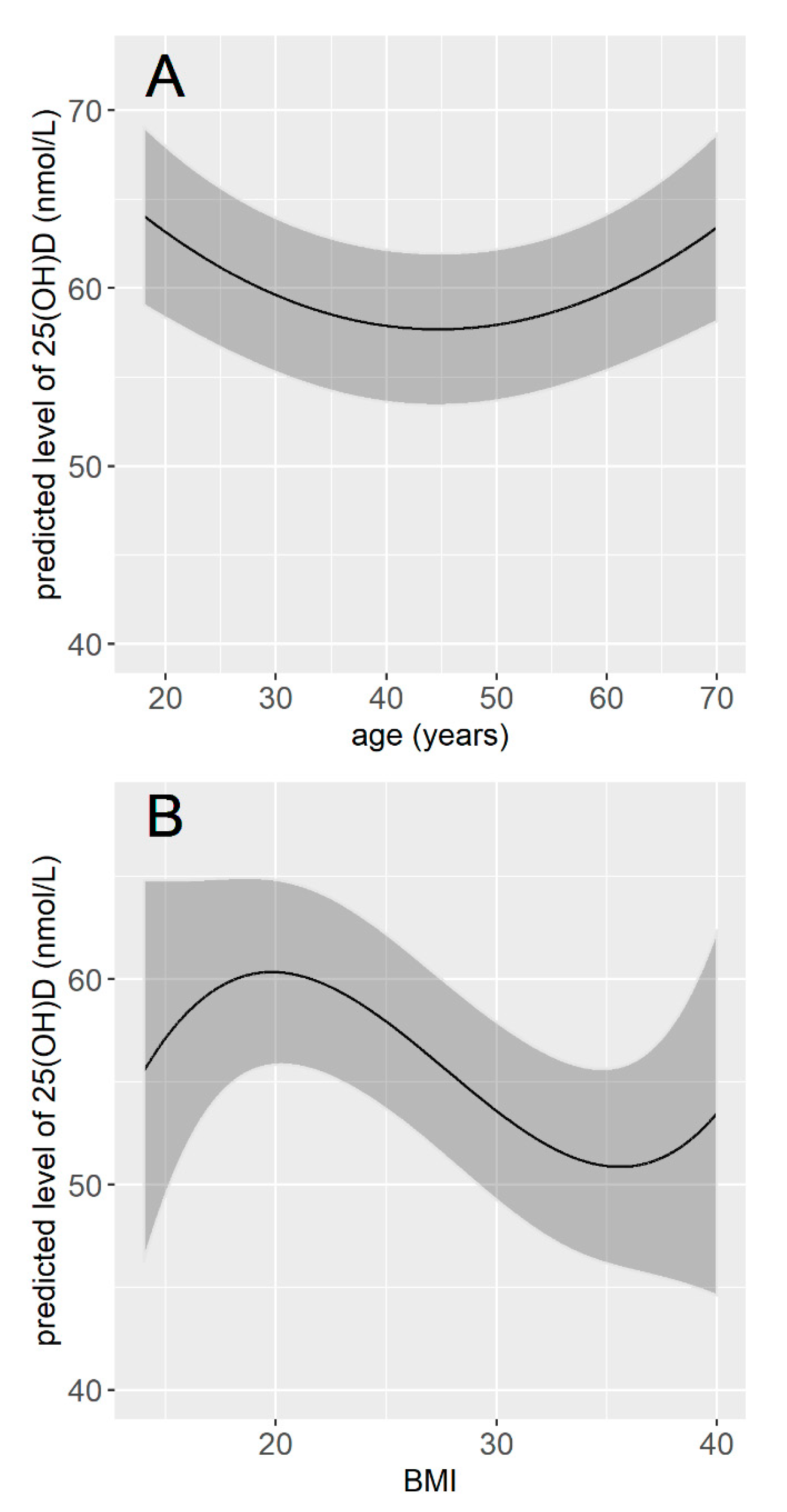
The multivariable linear regression model included 12 variables, which were the 11 variables retained from the bivariate analyses plus the seasonality. The intercept for each month showed that participants whose serum was sampled in March had vitamin D values on average 27 nmol/L lower than those whose serum was sampled in August. Regarding other variables, being a man, current smoker, unemployed, having a dark skin phototype, no vitamin D supplementation in the last 12 months, no significant exposure during holidays over the last 12 months, a lower cumulative sun exposure over the last week, no sporting activities (either outdoor or indoor), and living in the North were significantly associated with a lower vitamin concentration. The vitamin D concentration was also significantly associated with age and BMI, but these associations were not linear (Table 3). The vitamin D concentration tended to decrease with age until 40–50 years and to be higher for older age classes. It tended to be maximal for BMI values around 20 and lower for values below and above 20 (Figure 3). Participants with dark skin (type V and VI) had serum vitamin D concentration 15.37 nmol/L lower than participants with light-colored skin (type I to III), and participants with tanned skin (type IV) had serum vitamin D concentration 1.95 nmol/L lower than participants with light-colored skin (type I to III). Smokers had vitamin D concentration on average 3.75 nmol/L lower than non-smokers, and unemployed participants had vitamin D concentration 2.02 nmol/L lower than participants who were either employed or retired. Participants who had no significant sun exposure during holidays over the preceding 12 months had vitamin D concentration 8.01 nmol/L lower than participants who had, and participants who practiced any sport had vitamin D concentration on average 3.89 nmol/L higher than those who did not (Table 3). Overall, the final model explained 28% of the total variance in vitamin D concentration (0.282, 95% CI (0.238; 0.324)).

Table 3.
Multivariable regression model with 25(OH)D concentration as dependent variable (n = 2368).
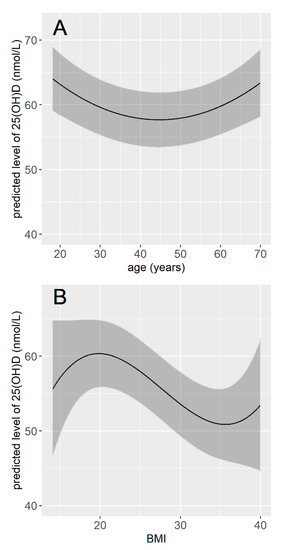
Figure 3.
Predicted concentration of vitamin D according to age (A) and according to BMI (B). The concentrations of vitamin D were predicted for the month of January, for a male, with light-colored skin, employment, living in the North of France, non-smoker, with vitamin D supplementation in the last 12 months, a significant sun exposure over the last 12 months, an average exposure time of 15 h during the week preceding the blood sample, no sporting activity, and for a BMI of 25 (A), or an age of 50 years old (B).
3.4. Model Performance
The AUC of the final model was estimated at 0.77 (95% CI (0.75; 0.80)) for identifying participants with a vitamin D deficiency and at 0.82 (95% CI (0.78; 0.85)) for identifying participants with severe vitamin D deficiency. The model identified severe vitamin D deficiency with a sensitivity of 77.9% (95% CI (69.1; 85.7)), a specificity of 68.3% (95% CI (64.8; 71.9)), a positive likelihood ratio (LR+) of 2.5, and a negative likelihood ratio (LR−) of 0.3. It identified vitamin D deficiency with a sensitivity of 56.7% (95% CI (52.0; 61.8)), a specificity of 81.0% (95% CI (77.2; 84.8)), an LR+ of 3.0, and an LR− of 0.5 (Table 4).

Table 4.
Performance of the model to predict vitamin D deficiency and severe vitamin D deficiency with a threshold of 50 nmol/L.
4. Discussion
In the present study, barely one-fifth of the study population had sufficient vitamin D concentration, most of them had vitamin D deficiency, and almost one-tenth of the population had severe vitamin D deficiency. These results are in line with those of the last French national public health survey ESTEBAN, which was conducted between 2014 and 2015 [34].
Using data collected specifically for this purpose with a comprehensive approach taking into account most types of factors that influence serum vitamin D concentration, and using detailed information regarding sun exposure, we developed a predictive model for vitamin D concentration. The linear regression analysis identified 12 variables as independent and statistically significant predictors for vitamin D concentration, i.e., month of blood sampling, age, sex, BMI, skin phototype, employment status, smoking status, latitude, vitamin D supplementation in the last 12 months, significant exposure during holidays over the last 12 months, sun exposure in the last week, and sport practice. These predictors had various association strengths with the vitamin D concentration: the month of blood sampling was the strongest predictor, illustrating the seasonality of the vitamin D concentration, the lowest concentrations were observed at the end of winter in March, and the highest concentrations were observed in August, which is consistent with a 2008 study by Holick et al. [35]. In most published predictive models, seasonality was taken into account using four modalities corresponding to the four seasons [10,12,17,18,19]. In our model, we accounted for the cyclic shape of the seasonality, which allowed a more accurate adjustment of the regression model for the month of blood sampling.
Skin phototype was the second most strongly associated factor in the multivariable model, especially dark skin (types V and VI according to the Fitzpatrick classification), which was associated with lower serum vitamin D concentration than light-colored skin (types I to III). Surprisingly, this factor has rarely been included in previously published models [19,36], although the physiological association between skin phototype and vitamin D synthesis has been demonstrated for a long time [37]. Although sun exposure is an important significant contributor to the predictive ability of the vitamin D status models, individual sun exposure behavior had been precisely measured in only about half of the published models so far [38]. In addition, individual sun exposure has been measured in various ways regarding intensity, chronology, and surface exposed. With these limitations, these models have suggested that the lengths of time spent under the sunlight, outdoor, or tanning in the past 12 months were indeed significant contributors to the predictive ability [10,15,16,17,19,36]. To find the best measure of individual sun exposure, a section was integrated herein with detailed and various questions about sun exposure habits, actual exposure in the preceding year, and recent exposure in the preceding week. The sun exposure measures that were both the best predictors of serum 25(OH)D concentration and the easiest to collect were “significant exposure during holidays over the last 12 months” (defined as having exposed one’s bust during at least one of the holiday periods over the last 12 months) and “sun exposure last week”, and this was particularly obvious after adjustment for latitude of the place of residence and skin phototype. Moreover, the latitude of the place of residence was a significant predictor, independently of sun exposure, as participants residing in the South of France had serum vitamin D values 7.55 nmol/L higher than participants residing in the North of France. Thus, in countries with a large variation in latitude, it is extremely important to take this factor into account.
Old and oldest individuals (aged 65 years and more) are at a higher risk of vitamin D deficiency, and studies conducted on elderly patients have found that older age was associated with a higher prevalence of hypovitaminosis D [10,12,36]. However, most studies conducted in younger individuals have either found no association [15,18,19] or even a reverse association [17], and a non-linear association was found herein. Sex, as aging, has not been consistently associated with vitamin D concentration in the literature. As previously found in the study by Bolek-Berquist et al. [16], male sex was a significant predictor of lower serum 25(OH)D concentration herein. Female sex has also been found as a significant predictor of hypovitaminosis D in previous studies [12,17,19], while in most others [15,34,36,39,40], no difference in 25(OH)D concentrations between sexes was found. The association between elevated BMI and lower vitamin D concentration found herein was consistent with the results of all previous reports that have included this variable in their models [38]. Nevertheless, the magnitude of the effect of these three socio-demographic variables (age, sex, and BMI) on vitamin D concentrations seems limited in a general adult population. Regarding lifestyle factors, the smoking status and sport practice were significant predictors of vitamin D concentration, which is in agreement with previous models that have included them [38].
The set of predictors included in our final model explained about one-third of the total variability in vitamin D concentration. The unexplained remaining variability can be attributed to a variety of factors such as usual memory biases and errors regarding lifetime exposure to risk factors, variability in serum 25(OH)D measurements, or other unknown or unmeasurable factors such as genetic factors [41,42,43]. Nevertheless, the performance of our model was comparable to that of previously published models regarding AUCs [10,12,17,36,39], R2 [10,12,18,36,44], and sensitivity and specificity [16,17,19]. Furthermore, it has the advantage of relying on parameters that can be easily and rapidly obtained in routine care. Another strength is that we explored a large number of detailed information on sun exposure and kept the items that were both easier to collect (and easier for patients to answer) and mostly correlated with the outcome.
From a pragmatic and preventive point of view, we chose the threshold of predicted value of 50 nmol/L for two reasons. The first one is that vitamin D concentration determination should be prescribed as relevantly as possible i.e., when there is a high probability of deficiency. The second one is that it is important not to miss patients with severe vitamin D deficiency and thus to choose a threshold that maximizes the sensitivity of the model. Had we chosen a threshold of 25 nmol/L for instance, the model would certainly have had better specificity but would have had lower sensitivity, and a greater number of severe deficiency cases would have been undetected. Using this threshold of 50 nmol/L could allow almost 80% of severe deficiency cases and almost 60% of deficiency cases to be detected, and only 20% of tested positive patients would not actually be deficient.
The strengths of the present study included the testing of a large cohort of individuals of both sexes, over a wide range of age, over an inclusion period of more than one year, and using a uniform method for the measurement of serum vitamin D concentration. Performing an ad hoc study using a specific questionnaire allowed a more reliable and precise collection and measurement of predictive factors. Contrary to most published models [38], we reported the contribution of individual risk factors in the predictive ability of our model. Individual sun exposure was measured through a number of various questions, making it possible to select the measure that was the easiest to collect among those with equivalent predictive abilities. Finally, as the analytical performance of vitamin D assays is highly variable [45], the use of automated Diasorin Liaison XL assay herein, which is the most frequently used immunoassay and considered as highly reliable, is a strength. In addition to its good analytical performance, it shows a good overall correlation with the chromatographic LC-MS/MS method, the reference for measurement of circulating vitamin D, with a mean bias criterion <3% compared to the chromatographic method [45].
We have to mention some limitations of the present study. First, the assessment of potential risk factors for vitamin D deficiency was based on self-reported information. Nevertheless, this way of collecting data seems the most suitable for the objective of building a screening tool that can be easily used in clinical practice to identify individuals at high risk of deficiency and those at very low risk of deficiency in order to better target the assays. Second, we cannot ensure that our sample was representative of the population as it was based on outpatients seen at the hospital who may be different from the general population. Nevertheless, the vitamin D measurements obtained herein were consistent with those from the last French national public health survey ESTEBAN [34], which suggests that our results may be extrapolated to the French population. Third, the model is sensitive to seasonality and latitude, which means that the present results may be extrapolated to neighboring European countries of the same latitude but should be adapted before being used in countries with very different latitude.
5. Conclusions
In conclusion, the present study enabled the development, in a general adult population, of a predictive model for vitamin D concentration that allows the identification of individuals with severe vitamin D deficiency (serum 25(OH)D concentration <25 nmol/L) with a sensitivity of 78%. This model may not replace proper vitamin D concentration determination, as it weakly increases or decreases the post-test probability, as documented by the LR+ and LR−. Further research is needed to find the most appropriate way of using this model in the decision-making process of test and/or vitamin D supplementation prescription. The feasibility and the external validity of this model in primary care settings will have to be tested before developing a score to classify patients and measuring its impact in real life.
Author Contributions
Conceptualization, M.V., B.M., J.F., J.H., P.C., M.R., and A.-M.S.; Data curation, M.-A.C.; Formal analysis, B.R. and M.R.; Funding acquisition, J.H., P.C., M.R., and A.-M.S.; Investigation, M.-A.C., T.T., S.M.-G., B.C., V.B., R.C., M.-H.L.P., M.-C.C., and J.-B.F.; Methodology, M.V., B.M., J.F., J.H., P.C., M.R., and A.-M.S.; Project administration, M.V., B.M., and P.R.; Supervision, A.-M.S.; Writing—original draft, M.V., B.M., B.R., M.R., and A.-M.S.; Writing—review and editing, P.R., T.T., S.M.-G., B.C., V.B., R.C., M.-H.L.P., M.-C.C., J.-B.F., J.H., and P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a public funding by “Direction Générale de l’offre de Soins”, grant Programme Hospitalier de Recherche Clinique interrégional (PHRCI) 2014 (Identifier number: PHRC-14-14-005).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee “CPP SUD-EST II” (IRB number 11263, 21 October 2015) and by the Commission Nationale de l’Informatique et des Libertés (CNIL, French commission on data protection).
Informed Consent Statement
Eligible patients received oral and written information and gave oral consent before being enrolled in the study.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request pending application and approval.
Acknowledgments
We thank all participants for their contribution to the SCOPYD study. We are grateful to all physicians who helped us with the recruitment of participants, and to nurses, research assistants, and students for their work and assistance: ACHIOU-ABEKHOUKH Sabiha, AMRANE Fatiha, BAY Caroline, BENBETKA Samia, BONIOL Mathieu, BOTTA Yolande, BOURGEAY Richard, BUSTAMENTE-CENCI Lindsay, CANALE Paulette, CARTELIER Sandrine, CHAUSSENDE Gabriela, COUTISSON Céline, de la POIX de FRÉMINVILLE Armelle, DEGUILHAUME Marie-Pierre, DENEVE Geneviève, DUMAS Mauricette, DURIF Julie, DUVERMY Annick, ELSENSOHN Béatrice, FAN Angélique, FOESSER Dominique, GARDON Karine, GOURNAY Alexandre, HATCHADOURIAN Sandrine, JUILLARD Sandrine, LOCRELLE Hervé, LONDICHE Brigitte, LOPES Jacqueline, LUCIANI Jean-François, LUYA Isabelle, MEULIN Géraldine, MORIER Marie-Françoise, PIQUET Véronique, PLANCKAERT Catherine, POUSSIN Sylvie, ROCHETTE Lara, TRÉHET-MANDEL Nadège, TRUC Cyrille, YATIMI Rachida. We thank Hélène Boyer (Direction de la recherche clinique et de l’innovation, Hospices Civils de Lyon) for her help in manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Risk Factors for Vitamin D Deficiency Included in the Questionnaire

Table A1.
Risk Factors for Vitamin D Deficiency Included in the Questionnaire.
Table A1.
Risk Factors for Vitamin D Deficiency Included in the Questionnaire.
| Section | Items |
|---|---|
| Socio-demographic data | Country of birth Education level Residential location Type and level of employment |
| Clinical data | Weight Size Skin type For women: number of pregnancies, age at first pregnancy, year of birth of last child, menopausal status Smoking status Physical activities (type, number of hours) Chronic muscle, joint, or bone pain with no known cause (presence, level of intensity) |
| Sun exposure | Usual sun exposure during working hours: frequency and number of hours spent for outdoor work and outdoor lunch. Usual sun exposure during leisure time: number of hours spend outdoors during the week and the weekend, according to the season Sun exposure last week: number of hours Holiday sun exposure in the last 12 months: vacation spots, time spent outdoors |
| Treatments | Vitamin D intake in drops, ampoules, or tablets in the last 12 months: dates and dosage Dietary supplements intake over the last 12 months Taking diuretics, oral contraceptives, menopausal hormone therapy, or other treatments |
| Dietary intake | Intake of fish, eggs, and dairy products Intake of vitamin D supplemented foods |
| Exposure to artificial ultraviolet radiation | Frequency over the last 12 months |
References
- Holick, M.F. The Vitamin D Deficiency Pandemic: Approaches for Diagnosis, Treatment and Prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Sattar, N.; Welsh, P.; Panarelli, M.; Forouhi, N.G. Increasing Requests for Vitamin D Measurement: Costly, Confusing, and without Credibility. Lancet Lond. Engl. 2012, 379, 95–96. [Google Scholar] [CrossRef]
- Bilinski, K.; Boyages, S. Evidence of Overtesting for Vitamin D in Australia: An Analysis of 4.5 Years of Medicare Benefits Schedule (MBS) Data. BMJ Open 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Caillet, P.; Goyer-Joos, A.; Viprey, M.; Schott, A.-M. Increase of Vitamin D Assays Prescriptions and Associated Factors: A Population-Based Cohort Study. Sci. Rep. 2017, 7, 10361. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Jolly, K.; MacArthur, C.; Manaseki-Holland, S.; Gittoes, N.; Hewison, M.; Scragg, R.; Nirantharakumar, K. Trends in the Incidence of Testing for Vitamin D Deficiency in Primary Care in the UK: A Retrospective Analysis of The Health Improvement Network (THIN), 2005–2015. BMJ Open 2019, 9, e028355. [Google Scholar] [CrossRef]
- Huber, C.A.; Nagler, M.; Rosemann, T.; Blozik, E.; Näpflin, M.; Markun, S. Trends in Micronutrient Laboratory Testing in Switzerland: A 7-Year Retrospective Analysis of Healthcare Claims Data. Int. J. Gen. Med. 2020, 13, 1341–1348. [Google Scholar] [CrossRef]
- Millen, A.E.; Wactawski-Wende, J.; Pettinger, M.; Melamed, M.L.; Tylavsky, F.A.; Liu, S.; Robbins, J.; LaCroix, A.Z.; LeBoff, M.S.; Jackson, R.D. Predictors of Serum 25-Hydroxyvitamin D Concentrations among Postmenopausal Women: The Women’s Health Initiative Calcium plus Vitamin D Clinical Trial. Am. J. Clin. Nutr. 2010, 91, 1324–1335. [Google Scholar] [CrossRef]
- Hacker-Thompson, A.; Schloetter, M.; Sellmeyer, D.E. Validation of a Dietary Vitamin D Questionnaire Using Multiple Diet Records and the Block 98 Health Habits and History Questionnaire in Healthy Postmenopausal Women in Northern California. J. Acad. Nutr. Diet. 2012, 112, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Nabak, A.C.; Johnson, R.E.; Keuler, N.S.; Hansen, K.E. Can a Questionnaire Predict Vitamin D Status in Postmenopausal Women? Public Health Nutr. 2014, 17, 739–746. [Google Scholar] [CrossRef][Green Version]
- Merlijn, T.; Swart, K.M.A.; Lips, P.; Heymans, M.W.; Sohl, E.; Van Schoor, N.M.; Netelenbos, C.J.; Elders, P.J.M. Prediction of Insufficient Serum Vitamin D Status in Older Women: A Validated Model. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2018, 29, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Peiris, A.N.; Bailey, B.A.; Guha, B.N.; Copeland, R.; Manning, T. Can a Model Predictive of Vitamin D Status Be Developed from Common Laboratory Tests and Demographic Parameters? South. Med. J. 2011, 104, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Sohl, E.; Heymans, M.W.; de Jongh, R.T.; den Heijer, M.; Visser, M.; Merlijn, T.; Lips, P.; van Schoor, N.M. Prediction of Vitamin D Deficiency by Simple Patient Characteristics. Am. J. Clin. Nutr. 2014, 99, 1089–1095. [Google Scholar] [CrossRef]
- Annweiler, C.; Riou, J.; Alessandri, A.; Gicquel, D.; Henni, S.; Féart, C.; Kabeshova, A. Clinical Identification of Geriatric Patients with Hypovitaminosis D: The “Vitamin D Status Predictor for Geriatrics” Study. Nutrients 2017, 9, 658. [Google Scholar] [CrossRef] [PubMed]
- Bjørn Jensen, C.; Thorne-Lyman, A.L.; Vadgård Hansen, L.; Strøm, M.; Odgaard Nielsen, N.; Cohen, A.; Olsen, S.F. Development and Validation of a Vitamin D Status Prediction Model in Danish Pregnant Women: A Study of the Danish National Birth Cohort. PLoS ONE 2013, 8, e53059. [Google Scholar] [CrossRef]
- Vignali, E.; Macchia, E.; Cetani, F.; Reggiardo, G.; Cianferotti, L.; Saponaro, F.; Marcocci, C. Development of an Algorithm to Predict Serum Vitamin D Levels Using a Simple Questionnaire Based on Sunlight Exposure. Endocrine 2017, 55, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Bolek-Berquist, J.; Elliott, M.E.; Gangnon, R.E.; Gemar, D.; Engelke, J.; Lawrence, S.J.; Hansen, K.E. Use of a Questionnaire to Assess Vitamin D Status in Young Adults. Public Health Nutr. 2009, 12, 236–243. [Google Scholar] [CrossRef]
- Kuwabara, A.; Tsugawa, N.; Mizuno, K.; Ogasawara, H.; Watanabe, Y.; Tanaka, K. A Simple Questionnaire for the Prediction of Vitamin D Deficiency in Japanese Adults (Vitaimn D Deficiency Questionnaire for Japanese: VDDQ-J). J. Bone Miner. Metab. 2019, 37, 854–863. [Google Scholar] [CrossRef]
- Bertrand, K.A.; Giovannucci, E.; Liu, Y.; Malspeis, S.; Eliassen, A.H.; Wu, K.; Holmes, M.D.; Laden, F.; Feskanich, D. Determinants of Plasma 25-Hydroxyvitamin D and Development of Prediction Models in Three US Cohorts. Br. J. Nutr. 2012, 108, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Deschasaux, M.; Souberbielle, J.-C.; Andreeva, V.A.; Sutton, A.; Charnaux, N.; Kesse-Guyot, E.; Latino-Martel, P.; Druesne-Pecollo, N.; Szabo de Edelenyi, F.; Galan, P.; et al. Quick and Easy Screening for Vitamin D Insufficiency in Adults: A Scoring System to Be Implemented in Daily Clinical Practice. Medicine 2016, 95, e2783. [Google Scholar] [CrossRef]
- Hanwell, H.E.C.; Vieth, R.; Cole, D.E.C.; Scillitani, A.; Modoni, S.; Frusciante, V.; Ritrovato, G.; Chiodini, I.; Minisola, S.; Carnevale, V. Sun Exposure Questionnaire Predicts Circulating 25-Hydroxyvitamin D Concentrations in Caucasian Hospital Workers in Southern Italy. J. Steroid Biochem. Mol. Biol. 2010, 121, 334–337. [Google Scholar] [CrossRef]
- Sham, L.; Yeh, E.A.; Magalhaes, S.; Parra, E.J.; Gozdzik, A.; Banwell, B.; Hanwell, H.E. Evaluation of Fall Sun Exposure Score in Predicting Vitamin D Status in Young Canadian Adults, and the Influence of Ancestry. J. Photochem. Photobiol. B 2015, 145, 25–29. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef] [PubMed]
- Malabanan, A.; Veronikis, I.E.; Holick, M.F. Redefining Vitamin D Insufficiency. Lancet Lond. Engl. 1998, 351, 805–806. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- International Association of Oil & Gas Producers Coordinate Conversions Ans Transformations Including Formulas 2020. Available online: https://drive.tiny.cloud/1/4m326iu12oa8re9cjiadxonharclteqb4mumfxj71zsttwkx/62018e48-9da4-43e7-b598-1202cd96ec9f (accessed on 17 November 2020).
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Haskell, W.; Snell, P.; Van Camp, S.P. Task Force 8: Classification of Sports. J. Am. Coll. Cardiol. 2005, 45, 1364–1367. [Google Scholar] [CrossRef]
- Chan, J.; Jaceldo-Siegl, K.; Fraser, G.E. Determinants of Serum 25 Hydroxyvitamin D Levels in a Nationwide Cohort of Blacks and Non-Hispanic Whites. Cancer Causes Control CCC 2010, 21, 501–511. [Google Scholar] [CrossRef]
- Guo, S.; Lucas, R.M.; Ponsonby, A.-L. Ausimmune Investigator Group A Novel Approach for Prediction of Vitamin d Status Using Support Vector Regression. PLoS ONE 2013, 8, e79970. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.P.; Barcikowski, R.S. The PEAR Method for Sample Sizes in Multiple Linear Regression. Mult. Linear Regres. Viewp. 2012, 38, 1–16. [Google Scholar]
- Algina, J.; Keselman, H. Cross-Validation Sample Sizes. Appl. Psychol. Meas. 2000, 24, 173–179. [Google Scholar] [CrossRef]
- Steyeberg, E. Clinical Prediction Models. A Practical Approach to Development, Validation and Updating. Edition Springer, New York, 2009; Springer: New York, NY, USA, 2009. [Google Scholar]
- Équipe de surveillance et d’épidémiologie nutritionnelle (Esen). Chapitre Dosages Biologiques: Vitamines et Minéraux. In Etude de Sante sur L’environnement, la Biosurveillance, L’activité Physique et la Nutrition (Esteban), 2014–2016. Volet Nutrition.; Santé publique France: Saint-Maurice, France, 2019; p. 61. [Google Scholar]
- Holick, M.F.; Chen, T.C. Vitamin D Deficiency: A Worldwide Problem with Health Consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef]
- Tran, B.; Armstrong, B.K.; McGeechan, K.; Ebeling, P.R.; English, D.R.; Kimlin, M.G.; Lucas, R.; van der Pols, J.C.; Venn, A.; Gebski, V.; et al. Predicting Vitamin D Deficiency in Older Australian Adults. Clin. Endocrinol. (Oxf.) 2013, 79, 631–640. [Google Scholar] [CrossRef]
- Clemens, T.L.; Adams, J.S.; Henderson, S.L.; Holick, M.F. Increased Skin Pigment Reduces the Capacity of Skin to Synthesise Vitamin D3. Lancet Lond. Engl. 1982, 1, 74–76. [Google Scholar] [CrossRef]
- Naureen, G.; Sanders, K.M.; Busija, L.; Scott, D.; Lim, K.; Talevski, J.; Connaughton, C.; Brennan-Olsen, S.L. Prediction Models and Questionnaires Developed to Predict Vitamin D Status in Adults: A Systematic Review. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2020, 31, 2287–2302. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Kabeshova, A.; Legeay, M.; Fantino, B.; Beauchet, O. Derivation and Validation of a Clinical Diagnostic Tool for the Identification of Older Community-Dwellers with Hypovitaminosis D. J. Am. Med. Dir. Assoc. 2015, 16, 536.e8–536.e19. [Google Scholar] [CrossRef]
- Rucker, D.; Allan, J.A.; Fick, G.H.; Hanley, D.A. Vitamin D Insufficiency in a Population of Healthy Western Canadians. CMAJ Can. Med. Assoc. J. J. Assoc. Med Can. 2002, 166, 1517–1524. [Google Scholar]
- Engelman, C.D.; Fingerlin, T.E.; Langefeld, C.D.; Hicks, P.J.; Rich, S.S.; Wagenknecht, L.E.; Bowden, D.W.; Norris, J.M. Genetic and Environmental Determinants of 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D Levels in Hispanic and African Americans. J. Clin. Endocrinol. Metab. 2008, 93, 3381–3388. [Google Scholar] [CrossRef]
- Shea, M.K.; Benjamin, E.J.; Dupuis, J.; Massaro, J.M.; Jacques, P.F.; D’Agostino, R.B.; Ordovas, J.M.; O’Donnell, C.J.; Dawson-Hughes, B.; Vasan, R.S.; et al. Genetic and Non-Genetic Correlates of Vitamins K and D. Eur. J. Clin. Nutr. 2009, 63, 458–464. [Google Scholar] [CrossRef]
- Touvier, M.; Deschasaux, M.; Montourcy, M.; Sutton, A.; Charnaux, N.; Kesse-Guyot, E.; Assmann, K.E.; Fezeu, L.; Latino-Martel, P.; Druesne-Pecollo, N.; et al. Determinants of Vitamin D Status in Caucasian Adults: Influence of Sun Exposure, Dietary Intake, Sociodemographic, Lifestyle, Anthropometric, and Genetic Factors. J. Investig. Dermatol. 2015, 135, 378–388. [Google Scholar] [CrossRef]
- Lee, A.; Samy, W.; Chiu, C.H.; Chan, S.K.C.; Gin, T.; Chui, P.T. Determinants of Serum 25-Hydroxyvitamin D Concentrations and a Screening Test for Moderate-to-Severe Hypovitaminosis D in Chinese Patients Undergoing Total Joint Arthroplasty. J. Arthroplasty 2016, 31, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Cavalier, E.; Bhattoa, H.P.; Pérez-López, F.R.; López-Baena, M.T.; Pérez-Roncero, G.R.; Chedraui, P.; Annweiler, C.; Della Casa, S.; Zelzer, S.; et al. Vitamin D Testing: Advantages and Limits of the Current Assays. Eur. J. Clin. Nutr. 2020, 74, 231–247. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).