Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease
Abstract
1. Introduction
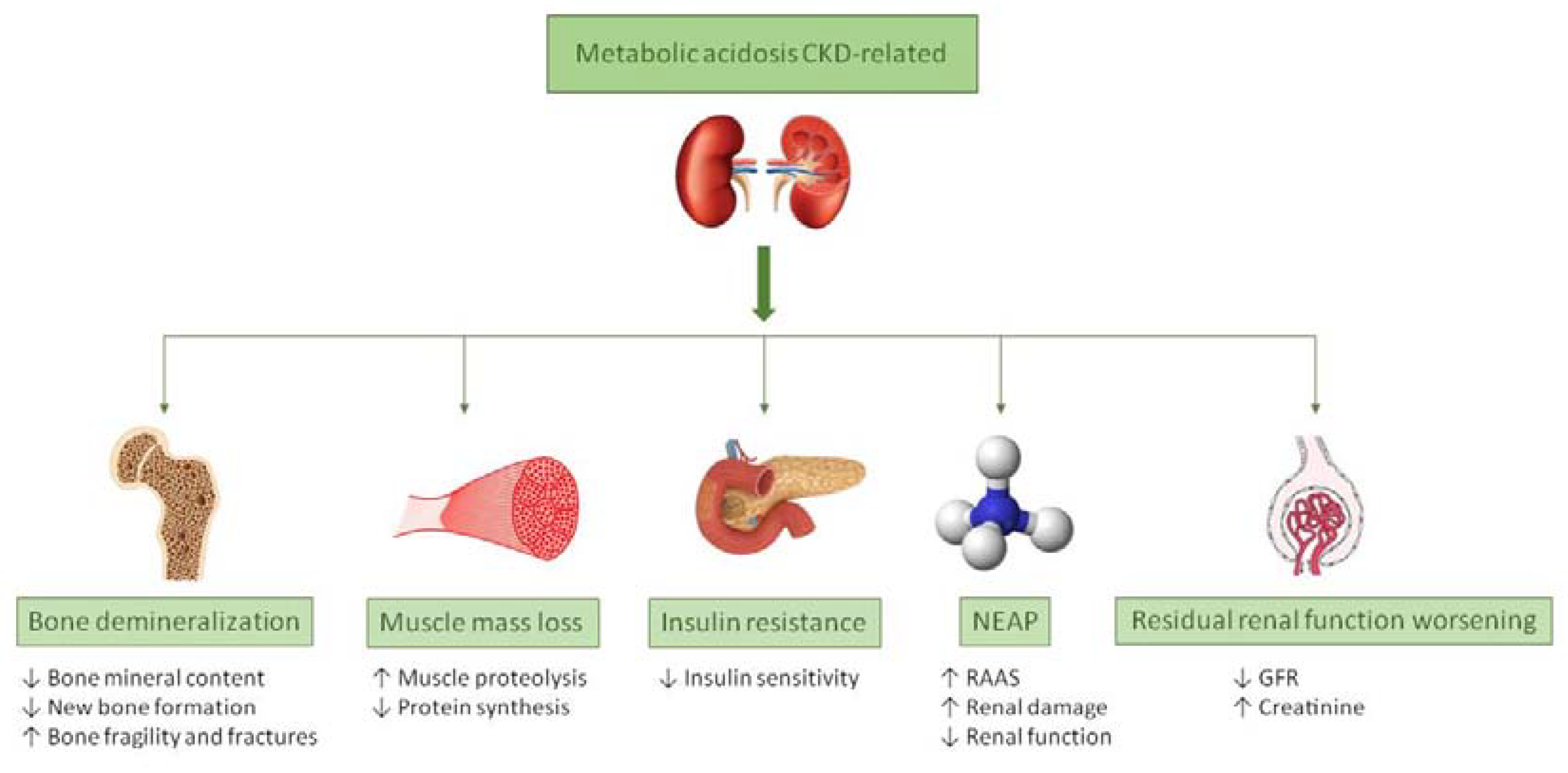
2. Metabolic Acidosis
2.1. Metabolic Acidosis and Bone Demineralization
2.2. Metabolic Acidosis and Muscle Mass Wasting
2.3. Metabolic Acidosis and Insulin Resistance
2.4. Metabolic Acidosis and NEAP
2.5. Metabolic Acidosis- Induced Kidney Injury
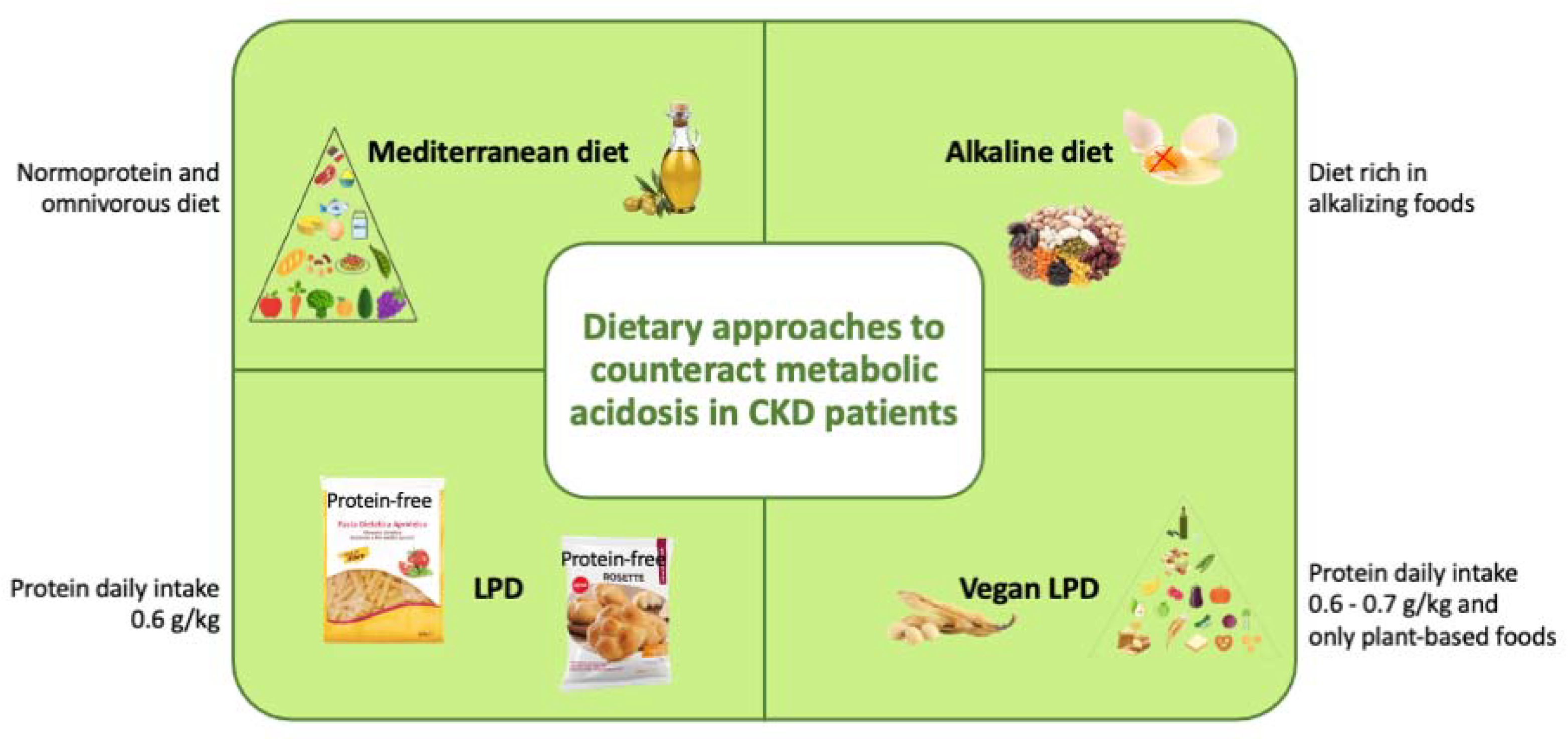
3. Dietary Approaches to Counteract Metabolic Acidosis in CKD
3.1. Mediterranean Diet
3.2. Alkaline Diet
3.3. Low-Protein Diet
3.4. Vegan Low-Protein Diet
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alkaline diet |
| b.w. | Body weigh |
| BCM | Body cell mass |
| CKD | Chronic kidney disease |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| DXA | Dual x-ray absorptiometry |
| ECM | Extracellular mass |
| ESRD | End stage renal disease |
| EVOO | Extra virgin olive oil |
| FGF23 | Fibroblast growth factor 23 |
| GFR | Glomerular filtration rate |
| IGF-1 | Insulin-like growth factor 1 |
| IMD | Italian Mediterranean diet |
| IMOD | Italian Mediterranean organic diet |
| K/DOQI | Kidney-disease outcome quality initiative |
| KDIGO | Kidney disease: improving global outcomes |
| LDL | Low-density lipoprotein |
| LPD | Low-protein diet |
| MD | Mediterranean diet |
| MPCs | Minor polar compounds |
| MUFA | Monounsaturated fatty acid |
| NCDs | Non-communicable diseases |
| NEAP | Net endogenous acid production |
| NOX | NADPH oxidase |
| PEW | Protein-energy wasting |
| PRAL | Potential renal acid load |
| PTH | Parathyroid hormone |
| PUFA | Polyunsaturated fatty acid |
| RAAS | Renin–angiotensin-aldosterone system |
| RDA | Recommended daily allowance |
| ROS | Reactive oxygen species |
| RRT | Renal replacement therapy |
| SCFAs | Short-chain fatty acids |
| SFA | Saturated fatty acid |
| SGLT2 | Sodium/glucose cotransporter 2 |
| TNF | Tumor necrosis factor |
| VLPD | Very low-protein diet |
References
- Cherno, M. Feuerbach’s “Man is what he eats”: A Rectification. J. Hist. Ideas 1963, 24, 397–406. [Google Scholar] [CrossRef]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic kidney disease diagnosis and management: A review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef]
- Melamed, M.L.; Raphael, K.L. Metabolic Acidosis in CKD: A Review of Recent Findings. Kidney Med. 2021, 3, 267–277. [Google Scholar] [CrossRef]
- Noce, A.; Canale, M.P.; Capria, A.; Rovella, V.; Tesauro, M.; Splendiani, G.; Annicchiarico-Petruzzelli, M.; Manzuoli, M.; Simonetti, G.; Di Daniele, N. Coronary artery calcifications predict long term cardiovascular events in non diabetic Caucasian hemodialysis patients. Aging 2015, 7, 269–279. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Di Lauro, M.; Urciuoli, S.; Pietroboni Zaitseva, A.; Wilson Jones, G.; Di Daniele, N.; Romani, A. Cardiovascular protection of nephropathic male patients by oral food supplements. Cardiovasc. Ther. 2020, 2020, 1807941. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Noce, A.; Di Giovamberardino, G.; De Stefano, A.; Calla, C.; Zenobi, R.; Dessi, M.; Di Daniele, N. Homocysteine, cysteine, folate and vitamin B(1)(2) status in type 2 diabetic patients with chronic kidney disease. J. Nephrol. 2015, 28, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Rovella, V.; Marrone, G.; Cattani, G.; Zingaretti, V.; Limongi, D.; D’Agostini, C.; Sorge, R.; Casasco, M.; Di Daniele, N.; et al. Hemodialysis biomarkers: Total advanced glycation end products (AGEs) against oxidized human serum albumin (HSAox). Acta Diabetol. 2019, 56, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Di Daniele, F.; Ottaviani, E.; Wilson Jones, G.; Bernini, R.; Romani, A.; Rovella, V. Impact of gut microbiota composition on onset and progression of chronic non-communicable diseases. Nutrients 2019, 11, 1073. [Google Scholar] [CrossRef] [PubMed]
- Annalisa, N.; Alessio, T.; Claudette, T.D.; Erald, V.; Antonino de, L.; Nicola, D.D. Gut microbioma population: An indicator really sensible to any change in age, diet, metabolic syndrome, and life-style. Mediat. Inflamm. 2014, 2014, 901308. [Google Scholar] [CrossRef] [PubMed]
- Dessi, M.; Noce, A.; Dawood, K.F.; Galli, F.; Taccone-Gallucci, M.; Fabrini, R.; Bocedi, A.; Massoud, R.; Fucci, G.; Pastore, A.; et al. Erythrocyte glutathione transferase: A potential new biomarker in chronic kidney diseases which correlates with plasma homocysteine. Amino Acids 2012, 43, 347–354. [Google Scholar] [CrossRef]
- Dessi, M.; Noce, A.; Agnoli, A.; De Angelis, S.; Fuiano, L.; Tozzo, C.; Taccone-Gallucci, M.; Fuiano, G.; Federici, G. The usefulness of the prognostic inflammatory and nutritional index (PINI) in a haemodialysis population. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 811–815. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.J. Chronic kidney disease. Nat. Rev. Dis. Primers 2017, 3, 17088. [Google Scholar] [CrossRef]
- Cupisti, A.; Gallieni, M.; Avesani, C.M.; D’Alessandro, C.; Carrero, J.J.; Piccoli, G.B. Medical nutritional therapy for patients with chronic kidney disease not on dialysis: The low protein diet as a medication. J. Clin. Med. 2020, 9, 3644. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Noce, A.; Vidiri, M.F.; Marrone, G.; Moriconi, E.; Bocedi, A.; Capria, A.; Rovella, V.; Ricci, G.; De Lorenzo, A.; Di Daniele, N. Is low-protein diet a possible risk factor of malnutrition in chronic kidney disease patients? Cell Death Discov. 2016, 2, 16026. [Google Scholar] [CrossRef] [PubMed]
- Di Daniele, N.; Di Renzo, L.; Noce, A.; Iacopino, L.; Ferraro, P.M.; Rizzo, M.; Sarlo, F.; Domino, E.; De Lorenzo, A. Effects of Italian Mediterranean organic diet vs. low-protein diet in nephropathic patients according to MTHFR genotypes. J. Nephrol. 2014, 27, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J.A.; Madias, N.E. Metabolic acidosis: Pathophysiology, diagnosis and management. Nat. Rev. Nephrol. 2010, 6, 274–285. [Google Scholar] [CrossRef]
- Siener, R. Dietary treatment of metabolic acidosis in chronic kidney disease. Nutrients 2018, 10, 512. [Google Scholar] [CrossRef]
- Wesson, D.E.; Nathan, T.; Rose, T.; Simoni, J.; Tran, R.M. Dietary protein induces endothelin-mediated kidney injury through enhanced intrinsic acid production. Kidney Int. 2007, 71, 210–217. [Google Scholar] [CrossRef]
- Vallet, M.; Metzger, M.; Haymann, J.P.; Flamant, M.; Gauci, C.; Thervet, E.; Boffa, J.J.; Vrtovsnik, F.; Froissart, M.; Stengel, B.; et al. Urinary ammonia and long-term outcomes in chronic kidney disease. Kidney Int. 2015, 88, 137–145. [Google Scholar] [CrossRef]
- Raphael, K.L. Metabolic acidosis and subclinical metabolic acidosis in CKD. J. Am. Soc. Nephrol. 2018, 29, 376. [Google Scholar] [CrossRef]
- Kopple, J.D.; Kalantar-Zadeh, K.; Mehrotra, R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int. 2005, 67, S21–S27. [Google Scholar] [CrossRef]
- Dubey, A.K.; Sahoo, J.; Vairappan, B.; Haridasan, S.; Parameswaran, S.; Priyamvada, P.S. Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: A randomized controlled trial. Nephrol. Dial. Transplant. 2020, 35, 121–129. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Ottaviani, E.; Guerriero, C.; Di Daniele, F.; Pietroboni Zaitseva, A.; Di Daniele, N. Uremic sarcopenia and its possible nutritional approach. Nutrients 2021, 13, 147. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Rovella, V.; Cusumano, A.; Di Daniele, N.; Casasco, M. Beneficial effects of physical activity on uremic sarcopenia. Med. Sport 2018, 71, 370–392. [Google Scholar] [CrossRef]
- Osuna-Padilla, I.A.; Leal-Escobar, G.; Garza-García, C.A.; Rodríguez-Castellanos, F.E. Dietary acid load: Mechanisms and evidence of its health repercussions. Nefrología 2019, 39, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Abramowitz, M.K. Advances in management of chronic metabolic acidosis in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [PubMed]
- Taccone-Gallucci, M.; Noce, A.; Bertucci, P.; Fabbri, C.; Manca-di-Villahermosa, S.; Della-Rovere, F.R.; De Francesco, M.; Lonzi, M.; Federici, G.; Scaccia, F.; et al. Chronic treatment with statins increases the availability of selenium in the antioxidant defence systems of hemodialysis patients. J. Trace Elem. Med. Biol. 2010, 24, 27–30. [Google Scholar] [CrossRef][Green Version]
- Di Renzo, L.; Noce, A.; De Angelis, S.; Miani, N.; Di Daniele, N.; Tozzo, C.; De Lorenzo, A. Anti-inflammatory effects of combined treatment with acetyl salicylic acid and atorvastatin in haemodialysis patients affected by normal weight obese syndrome. Pharmacol. Res. 2008, 57, 93–99. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Noce, A.; Bocedi, A.; Campo, M.; Marrone, G.; Di Lauro, M.; Cattani, G.; Di Daniele, N.; Romani, A. A pilot study of a natural food supplement as new possible therapeutic approach in chronic kidney disease patients. Pharmaceuticals 2020, 13, 148. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Remer, T. Acid-base in renal failure: Influence of diet on acid-base balance. Semin. Dial. 2000, 13, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J.A.; Madias, N.E. Consequences and therapy of the metabolic acidosis of chronic kidney disease. Pediatr. Nephrol. 2011, 26, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Krieger, N.S.; Frick, K.K.; Bushinsky, D.A. Mechanism of acid-induced bone resorption. Curr. Opin. Nephrol. Hypertens. 2004, 13, 423–436. [Google Scholar] [CrossRef]
- Krieger, N.S.; Sessler, N.E.; Bushinsky, D.A. Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am. J. Physiol. 1992, 262, F442–F448. [Google Scholar] [CrossRef] [PubMed]
- Bushinsky, D.A. Metabolic alkalosis decreases bone calcium efflux by suppressing osteoclasts and stimulating osteoblasts. Am. J. Physiol. 1996, 271, F216–F222. [Google Scholar] [CrossRef] [PubMed]
- Disthabanchong, S.; Radinahamed, P.; Stitchantrakul, W.; Hongeng, S.; Rajatanavin, R. Chronic metabolic acidosis alters osteoblast differentiation from human mesenchymal stem cells. Kidney Int. 2007, 71, 201–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frick, K.K.; Jiang, L.; Bushinsky, D.A. Acute metabolic acidosis inhibits the induction of osteoblastic egr-1 and type 1 collagen. Am. J. Physiol. 1997, 272, C1450–C1456. [Google Scholar] [CrossRef]
- Krieger, N.S.; Parker, W.R.; Alexander, K.M.; Bushinsky, D.A. Prostaglandins regulate acid-induced cell-mediated bone resorption. Am. J. Physiol. Renal. Physiol. 2000, 279, F1077–F1082. [Google Scholar] [CrossRef]
- Gasser, J.A.; Hulter, H.N.; Imboden, P.; Krapf, R. Effect of chronic metabolic acidosis on bone density and bone architecture in vivo in rats. Am. J. Physiol. Renal. Physiol. 2014, 306, F517–F524. [Google Scholar] [CrossRef]
- Mitch, W.E.; Medina, R.; Grieber, S.; May, R.C.; England, B.K.; Price, S.R.; Bailey, J.L.; Goldberg, A.L. Metabolic acidosis stimulates muscle protein degradation by activating the adenosine triphosphate-dependent pathway involving ubiquitin and proteasomes. J. Clin. Investig. 1994, 93, 2127–2133. [Google Scholar] [CrossRef]
- Bailey, J.L.; Wang, X.; England, B.K.; Price, S.R.; Ding, X.; Mitch, W.E. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J. Clin. Investig. 1996, 97, 1447–1453. [Google Scholar] [CrossRef]
- May, R.C.; Kelly, R.A.; Mitch, W.E. Mechanisms for defects in muscle protein metabolism in rats with chronic uremia. Influence of metabolic acidosis. J. Clin. Investig. 1987, 79, 1099–1103. [Google Scholar] [CrossRef]
- Wang, X.H.; Mitch, W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat. Rev. Nephrol. 2014, 10, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, Z.; Hu, J.; Du, J.; Mitch, W.E. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 2006, 147, 4160–4168. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Adverse effects of the metabolic acidosis of chronic kidney disease. Adv. Chronic Kidney Dis. 2017, 24, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Souto, G.; Donapetry, C.; Calvino, J.; Adeva, M.M. Metabolic acidosis-induced insulin resistance and cardiovascular risk. Metab. Syndr. Relat. Disord. 2011, 9, 247–253. [Google Scholar] [CrossRef]
- Farwell, W.R.; Taylor, E.N. Serum bicarbonate, anion gap and insulin resistance in the national health and nutrition examination survey. Diabet. Med. 2008, 25, 798–804. [Google Scholar] [CrossRef]
- Maalouf, N.M.; Cameron, M.A.; Moe, O.W.; Adams-Huet, B.; Sakhaee, K. Low urine pH: A novel feature of the metabolic syndrome. Clin. J. Am. Soc. Nephrol. 2007, 2, 883–888. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Beckles, A.D. Glucose intolerance following chronic metabolic acidosis in man. Am. J. Physiol. 1979, 236, E328–E334. [Google Scholar] [CrossRef]
- Reaich, D.; Graham, K.A.; Channon, S.M.; Hetherington, C.; Scrimgeour, C.M.; Wilkinson, R.; Goodship, T.H. Insulin-mediated changes in PD and glucose uptake after correction of acidosis in humans with CRF. Am. J. Physiol. 1995, 268, E121–E126. [Google Scholar] [CrossRef] [PubMed]
- Bellasi, A.; UBI Study Investigators; Di Micco, L.; Santoro, D.; Marzocco, S.; De Simone, E.; Cozzolino, M.; Di Lullo, L.; Guastaferro, P.; Di Iorio, B. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol. 2016, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Appel, L.J.; Astor, B.C.; Miller, E.R.; Beddhu, S.; Woodward, M.; Parekh, R.S.; Anderson, C.A. Estimated net endogenous acid production and serum bicarbonate in African Americans with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Henger, A.; Tutt, P.; Riesen, W.F.; Hulter, H.N.; Krapf, R. Acid-base and endocrine effects of aldosterone and angiotensin II inhibition in metabolic acidosis in human patients. J. Lab. Clin. Med. 2000, 136, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Appel, L.J.; Astor, B.C.; Miller, E.R.; Beddhu, S.; Woodward, M.; Parekh, R.S.; Anderson, C.A. Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int. 2012, 82, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, G.; Perico, N.; Macia, M.; Ruggenenti, P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int. 2005, 68, S57–S65. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.Y.; Chen, H.C.; Tsai, Y.C.; Yang, Y.K.; Lee, C.T. Activation of intrarenal renin-angiotensin system during metabolic acidosis. Am. J. Nephrol. 2011, 34, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Phisitkul, S.; Hacker, C.; Simoni, J.; Tran, R.M.; Wesson, D.E. Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney Int. 2008, 73, 192–199. [Google Scholar] [CrossRef]
- Wesson, D.E.; Simoni, J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int. 2010, 78, 1128–1135. [Google Scholar] [CrossRef]
- Kohan, D.E. Endothelin, hypertension and chronic kidney disease: New insights. Curr. Opin. Nephrol. Hypertens. 2010, 19, 134–139. [Google Scholar] [CrossRef]
- Banerjee, T.; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance team; Crews, D.C.; Wesson, D.E.; Tilea, A.; Saran, R.; Burrows, N.R.; E Williams, D.; Powe, N.R. Dietary acid load and chronic kidney disease among adults in the United States. BMC Nephrol. 2014, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E.; Buysse, J.M.; Bushinsky, D.A. Mechanisms of metabolic acidosis-induced kidney injury in chronic kidney disease. J. Am. Soc. Nephrol. 2020, 31, 469–482. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Retarding progression of chronic kidney disease: Use of modalities that counter acid retention. Curr. Opin. Nephrol. Hypertens. 2018, 27, 94–101. [Google Scholar] [CrossRef]
- Kobori, H.; Navar, L.G. Urinary angiotensinogen as a novel biomarker of intrarenal renin-angiotensin system in chronic kidney disease. Int. Rev. Thromb. 2011, 6, 108–116. [Google Scholar]
- Kohan, D.E.; Inscho, E.W.; Wesson, D.; Pollock, D.M. Physiology of endothelin and the kidney. Compr. Physiol. 2011, 1, 883–919. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Simoni, J.; Wesson, D.E. Endothelin-induced increased aldosterone activity mediates augmented distal nephron acidification as a result of dietary protein. J. Am. Soc. Nephrol. 2005, 16, 1929–1935. [Google Scholar] [CrossRef]
- Couto, E.; Boffetta, P.; Lagiou, P.; Ferrari, P.; Buckland, G.; Overvad, K.; Dahm, C.C.; Tjonneland, A.; Olsen, A.; Clavel-Chapelon, F.; et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br. J. Cancer 2011, 104, 1493–1499. [Google Scholar] [CrossRef]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef]
- Passey, C. Reducing the dietary acid load: How a more alkaline diet benefits patients with chronic kidney disease. J. Ren. Nutr. 2017, 27, 151–160. [Google Scholar] [CrossRef]
- Yari, Z.; Mirmiran, P. Alkaline diet: A novel nutritional strategy in chronic kidney disease? Iran. J. Kidney Dis. 2018, 12, 204–208. [Google Scholar] [PubMed]
- Bellizzi, V.; Cupisti, A.; Locatelli, F.; Bolasco, P.; Brunori, G.; Cancarini, G.; Caria, S.; De Nicola, L.; Di Iorio, B.R.; Di Micco, L.; et al. Low-protein diets for chronic kidney disease patients: The Italian experience. BMC Nephrol. 2016, 17, 77. [Google Scholar] [CrossRef]
- Cupisti, A.; Brunori, G.; Di Iorio, B.R.; D’Alessandro, C.; Pasticci, F.; Cosola, C.; Bellizzi, V.; Bolasco, P.; Capitanini, A.; Fantuzzi, A.L.; et al. Nutritional treatment of advanced CKD: Twenty consensus statements. J. Nephrol. 2018, 31, 457–473. [Google Scholar] [CrossRef]
- Ko, G.J.; Obi, Y.; Tortorici, A.R.; Kalantar-Zadeh, K. Dietary protein intake and chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 77–85. [Google Scholar] [CrossRef]
- Di Daniele, N. The role of preventive nutrition in chronic non-communicable diseases. Nutrients 2019, 11, 1074. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean diet and cardiovascular health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean diet: A review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef] [PubMed]
- Bendall, C.L.; Mayr, H.L.; Opie, R.S.; Bes-Rastrollo, M.; Itsiopoulos, C.; Thomas, C.J. Central obesity and the Mediterranean diet: A systematic review of intervention trials. Crit. Rev. Food Sci. Nutr. 2018, 58, 3070–3084. [Google Scholar] [CrossRef]
- Miranda, A.; Gomez-Gaete, C.; Mennickent, S. Role of Mediterranean diet on the prevention of Alzheimer disease. Rev. Med. Chile 2017, 145, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and vascular effect of the Mediterranean diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Noce, A.; Bigioni, M.; Calabrese, V.; Della Rocca, D.G.; Di Daniele, N.; Tozzo, C.; Di Renzo, L. The effects of Italian Mediterranean organic diet (imod) on health status. Curr. Pharm. Des. 2010, 16, 814–824. [Google Scholar] [CrossRef]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean diet on human gut microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health benefits of the Mediterranean diet: Metabolic and molecular mechanisms. J. Gerontol. Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Campo, M.; Urciuoli, S.; Marrone, G.; Noce, A.; Bernini, R. An industrial and sustainable platform for the production of bioactive micronized powders and extracts enriched in polyphenols from Olea europaea L. and Vitis vinifera L. wastes. Front. Nutr. 2020, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Becerra, K.; Ramos-Lopez, O.; Barron-Cabrera, E.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez-Lopez, E.; Martinez, J.A. Fatty acids, epigenetic mechanisms and chronic diseases: A systematic review. Lipids Health Dis. 2019, 18, 178. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Di Daniele, F.; Di Lauro, M.; Pietroboni Zaitseva, A.; Wilson Jones, G.; De Lorenzo, A.; Di Daniele, N. Potential cardiovascular and metabolic beneficial effects of omega-3 PUFA in male obesity secondary hypogonadism syndrome. Nutrients 2020, 12, 2519. [Google Scholar] [CrossRef]
- Dessi, M.; Noce, A.; Bertucci, P.; Noce, G.; Rizza, S.; De Stefano, A.; Manca di Villahermosa, S.; Bernardini, S.; De Lorenzo, A.; Di Daniele, N. Plasma and erythrocyte membrane phospholipids and fatty acids in Italian general population and hemodialysis patients. Lipids Health Dis. 2014, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Bernini, R.; Noce, A.; Urciuoli, S.; Di Lauro, M.; Zaitseva, A.P.; Marrone, G.; Di Daniele, N. Potential beneficial effects of extra virgin olive oils characterized by high content in minor polar compounds in nephropathic patients: A pilot study. Molecules 2020, 25, 4757. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Urciuoli, S.; Di Daniele, F.; Di Lauro, M.; Pietroboni Zaitseva, A.; Di Daniele, N.; Romani, A. Usefulness of extra virgin olive oil minor polar compounds in the management of chronic kidney disease patients. Nutrients 2021, 13, 581. [Google Scholar] [CrossRef]
- Alberti-Fidanza, A.; Fidanza, F.; Chiuchiu, M.P.; Verducci, G.; Fruttini, D. Dietary studies on two rural italian population groups of the seven countries study. Trend of food and nutrient intake from 1960 to 1991. Eur. J. Clin. Nutr. 1999, 53, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, J.; Frassetto, L.A.; Katzinger, J. Diet-induced acidosis: Is it real and clinically relevant? Br. J. Nutr. 2010, 103, 1185–1194. [Google Scholar] [CrossRef]
- Carnauba, R.A.; Baptistella, A.B.; Paschoal, V.; Hubscher, G.H. Diet-induced low-grade metabolic acidosis and clinical outcomes: A review. Nutrients 2017, 9, 538. [Google Scholar] [CrossRef]
- DuBose, T.D., Jr. Regulation of potassium homeostasis in CKD. Adv. Chronic Kidney Dis. 2017, 24, 305–314. [Google Scholar] [CrossRef]
- Cupisti, A.; Kovesdy, C.P.; D’Alessandro, C.; Kalantar-Zadeh, K. Dietary approach to recurrent or chronic hyperkalaemia in patients with decreased kidney function. Nutrients 2018, 10, 261. [Google Scholar] [CrossRef]
- KDIGO. Chapter 3: Management of progression and complications of CKD. Kidney Int. 2013, 3, 73–90. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.K. The alkaline diet: Is there evidence that an alkaline pH diet benefits health? J. Environ. Public Health 2012, 2012, 727630. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Neto Angeloco, L.; Arces de Souza, G.C.; Almeida Romao, E.; Garcia Chiarello, P. Alkaline diet and metabolic acidosis: Practical approaches to the nutritional management of chronic kidney disease. J. Ren. Nutr. 2018, 28, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Huang, T. Systematic review of the association between dietary acid load, alkaline water and cancer. BMJ Open 2016, 6, e010438. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.; Foulkes, E.; Evans, M.; Ausman, L. Acid/alkaline ash diets: Time for assessment and change. J. Am. Diet. Assoc. 1985, 85, 841–845. [Google Scholar]
- D’Alessandro, C.; Piccoli, G.B.; Cupisti, A. The “phosphorus pyramid”: A visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol. 2015, 16, 9. [Google Scholar] [CrossRef]
- Mahajan, A.; Simoni, J.; Sheather, S.J.; Broglio, K.R.; Rajab, M.H.; Wesson, D.E. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010, 78, 303–309. [Google Scholar] [CrossRef]
- Ruster, C.; Wolf, G. Renin-angiotensin-aldosterone system and progression of renal disease. J. Am. Soc. Nephrol. 2006, 17, 2985–2991. [Google Scholar] [CrossRef]
- Wesson, D.E. Endogenous endothelins mediate increased acidification in remnant kidneys. J. Am. Soc. Nephrol. 2001, 12, 1826–1835. [Google Scholar] [CrossRef]
- Dhaun, N.; Goddard, J.; Webb, D.J. The endothelin system and its antagonism in chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 943–955. [Google Scholar] [CrossRef]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014, 25, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Cianciaruso, B.; Pota, A.; Pisani, A.; Torraca, S.; Annecchini, R.; Lombardi, P.; Capuano, A.; Nazzaro, P.; Bellizzi, V.; Sabbatini, M. Metabolic effects of two low protein diets in chronic kidney disease stage 4,5. A randomized controlled trial. Nephrol. Dial. Transplant. 2008, 23, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional management of chronic kidney disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the international society of renal nutrition and metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Haworth, S.; Brotherton, A.M. Correcting metabolic acidosis leads to an increase in dietary protein intake in patients with established chronic kidney disease. J. Hum. Nutr. Diet. 2011, 24, 285. [Google Scholar] [CrossRef]
- Williams, B.; Hattersley, J.; Layward, E.; Walls, J. Metabolic acidosis and skeletal muscle adaptation to low protein diets in chronic uremia. Kidney Int. 1991, 40, 779–786. [Google Scholar] [CrossRef][Green Version]
- Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2000, 35, S1–S140. [Google Scholar]
- Kopple, J.D.; Monteon, F.J.; Shaib, J.K. Effect of energy intake on nitrogen metabolism in nondialyzed patients with chronic renal failure. Kidney Int. 1986, 29, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Su, X.; Xu, B.; Qiao, X.; Wang, L. Effect of diet protein restriction on progression of chronic kidney disease: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0206134. [Google Scholar] [CrossRef]
- Li, Q.; Wen, F.; Wang, Y.; Li, S.; Lin, S.; Qi, C.; Chen, Z.; Qiu, X.; Zhang, Y.; Zhang, S.; et al. Diabetic kidney disease benefits from intensive low-protein diet: Updated systematic review and meta-analysis. Diabetes Ther. 2021, 12, 21–36. [Google Scholar] [CrossRef]
- Rhee, C.M.; Ahmadi, S.F.; Kovesdy, C.P.; Kalantar-Zadeh, K. Low-protein diet for conservative management of chronic kidney disease: A systematic review and meta-analysis of controlled trials. J. Cachexia Sarcopenia Muscle 2018, 9, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.; Hodson, E.M.; Fouque, D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst. Rev. 2020, 10, CD001892. [Google Scholar] [CrossRef]
- Raikou, V.D. Metabolic acidosis status and mortality in patients on the end stage of renal disease. J. Transl. Int. Med. 2016, 4, 170–177. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Di Micco, L.; Marzocco, S.; De Simone, E.; De Blasio, A.; Sirico, M.L.; Nardone, L.; UBI Study Group. Very low-protein diet (VLPD) reduces metabolic acidosis in subjects with chronic kidney disease: The “nutritional light signal” of the renal acid load. Nutrients 2017, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Patschan, D.; Patschan, S.; Ritter, O. Chronic metabolic acidosis in chronic kidney disease. Kidney Blood Press Res. 2020, 45, 812–822. [Google Scholar] [CrossRef]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-supplemented vegetarian very low-protein diet and CKD progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Gennari, F.J.; Hood, V.L.; Greene, T.; Wang, X.; Levey, A.S. Effect of dietary protein intake on serum total CO2 concentration in chronic kidney disease: Modification of diet in renal disease study findings. Clin. J. Am. Soc. Nephrol. 2006, 1, 52–57. [Google Scholar] [CrossRef]
- Barsotti, G.; Cupisti, A.; Ciardella, F.; Morelli, E.; Niosi, F.; Giovannetti, S. Compliance with protein restriction: Effects on metabolic acidosis and progression of renal failure in chronic uremics on supplemented diet. Contrib. Nephrol. 1990, 81, 42–49. [Google Scholar] [CrossRef]
- Lai, S.; Molfino, A.; Testorio, M.; Perrotta, A.M.; Currado, A.; Pintus, G.; Pietrucci, D.; Unida, V.; La Rocca, D.; Biocca, S.; et al. Effect of low-protein diet and inulin on microbiota and clinical parameters in patients with chronic kidney disease. Nutrients 2019, 11, 3006. [Google Scholar] [CrossRef]
- Marrone, G.; Guerriero, C.; Palazzetti, D.; Lido, P.; Marolla, A.; Daniele, F.D.; Noce, A. Vegan diet health benefits in metabolic syndrome. Nutrients 2021, 13, 817. [Google Scholar] [CrossRef]
- Kontessis, P.; Jones, S.; Dodds, R.; Trevisan, R.; Nosadini, R.; Fioretto, P.; Borsato, M.; Sacerdoti, D.; Viberti, G. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. 1990, 38, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Ertuglu, L.A.; Afsar, B.; Ozdogan, E.; Kucuksumer, Z.S.; Ortiz, A.; Covic, A.; Kuwabara, M.; Cherney, D.Z.I.; van Raalte, D.H.; et al. Renal hyperfiltration defined by high estimated glomerular filtration rate: A risk factor for cardiovascular disease and mortality. Diabetes Obes. Metab. 2019, 21, 2368–2383. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, R.R.; Zipser, R.D.; Slomowitz, L.A.; Kopple, J.D. Glucagon and prostaglandins are mediators of amino acid-induced rise in renal hemodynamics. Kidney Int. 1988, 33, 1147–1155. [Google Scholar] [CrossRef]
- Alvirdizadeh, S.; Yuzbashian, E.; Mirmiran, P.; Eghtesadi, S.; Azizi, F. A prospective study on total protein, plant protein and animal protein in relation to the risk of incident chronic kidney disease. BMC Nephrol. 2020, 21, 489. [Google Scholar] [CrossRef]
- Elliott, P.; Stamler, J.; Dyer, A.R.; Appel, L.; Dennis, B.; Kesteloot, H.; Ueshima, H.; Okayama, A.; Chan, Q.; Garside, D.B.; et al. Association between protein intake and blood pressure: The INTERMAP Study. Arch. Intern. Med. 2006, 166, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Vidgen, E.; Augustin, L.S.; van Erk, M.; Geelen, A.; Parker, T.; Faulkner, D.; Vuksan, V.; Josse, R.G.; et al. High-protein diets in hyperlipidemia: Effect of wheat gluten on serum lipids, uric acid, and renal function. Am. J. Clin. Nutr. 2001, 74, 57–63. [Google Scholar] [CrossRef]
- Mirmiran, P.; Yuzbashian, E.; Bahadoran, Z.; Asghari, G.; Azizi, F. Dietary acid-base load and risk of chronic kidney disease in adults: Tehran lipid and glucose study. Iran. J. Kidney Dis. 2016, 10, 119–125. [Google Scholar] [PubMed]
- Nath, K.A.; Hostetter, M.K.; Hostetter, T.H. Increased ammoniagenesis as a determinant of progressive renal injury. Am. J. Kidney Dis. 1991, 17, 654–657. [Google Scholar] [CrossRef]
- Nath, K.A.; Hostetter, M.K.; Hostetter, T.H. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J. Clin. Investig. 1985, 76, 667–675. [Google Scholar] [CrossRef]
- Phisitkul, S.; Khanna, A.; Simoni, J.; Broglio, K.; Sheather, S.; Rajab, M.H.; Wesson, D.E. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 2010, 77, 617–623. [Google Scholar] [CrossRef]
- Katholi, R.E.; Woods, W.T., Jr.; Taylor, G.J.; Deitrick, C.L.; Womack, K.A.; Katholi, C.R.; McCann, W.P. Oxygen free radicals and contrast nephropathy. Am. J. Kidney Dis. 1998, 32, 64–71. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noce, A.; Marrone, G.; Wilson Jones, G.; Di Lauro, M.; Pietroboni Zaitseva, A.; Ramadori, L.; Celotto, R.; Mitterhofer, A.P.; Di Daniele, N. Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease. Nutrients 2021, 13, 2534. https://doi.org/10.3390/nu13082534
Noce A, Marrone G, Wilson Jones G, Di Lauro M, Pietroboni Zaitseva A, Ramadori L, Celotto R, Mitterhofer AP, Di Daniele N. Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease. Nutrients. 2021; 13(8):2534. https://doi.org/10.3390/nu13082534
Chicago/Turabian StyleNoce, Annalisa, Giulia Marrone, Georgia Wilson Jones, Manuela Di Lauro, Anna Pietroboni Zaitseva, Linda Ramadori, Roberto Celotto, Anna Paola Mitterhofer, and Nicola Di Daniele. 2021. "Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease" Nutrients 13, no. 8: 2534. https://doi.org/10.3390/nu13082534
APA StyleNoce, A., Marrone, G., Wilson Jones, G., Di Lauro, M., Pietroboni Zaitseva, A., Ramadori, L., Celotto, R., Mitterhofer, A. P., & Di Daniele, N. (2021). Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease. Nutrients, 13(8), 2534. https://doi.org/10.3390/nu13082534





