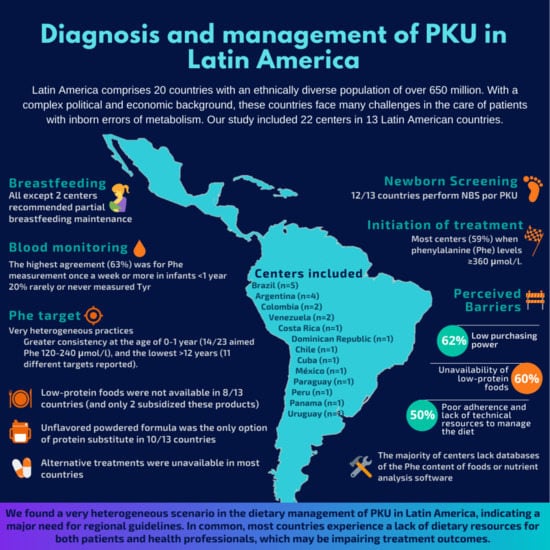
Current Practices and Challenges in the Diagnosis and Management of PKU in Latin America: A Multicenter Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Aspects
3. Results
3.1. Participants
3.2. Newborn Screening and Phenylalanine (Phe) Monitoring
3.3. Treatment Targets and Dietary Practices
3.4. Nutritional Resources
3.5. Alternative Treatments and Challenges
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.C.; Adams, J. Current status of newborn screening worldwide: 2015. Semin. Perinatol. 2015, 39, 171–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrajo, G.J.C. Newborn Screening for Phenylketonuria: Latin American Consensus Guidelines. J. Inborn Errors Metab. Screen. 2016, 4. [Google Scholar] [CrossRef]
- Borrajo, G.J.C. Newborn screening in Latin America: A brief overview of the state of the art. Am. J. Med. Genet. C Semin. Med. Genet. 2021. [Google Scholar] [CrossRef] [PubMed]
- Atun, R.; De Andrade, L.O.M.; Almeida, G.; Cotlear, D.; Dmytraczenko, T.; Frenz, P.; Garcia, P.; Gómez-Dantés, O.; Knaul, F.M.; Muntaner, C.; et al. Health-system reform and universal health coverage in Latin America. Lancet 2015, 28, 1230–1247. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development/The World Bank. Health at a Glance: Latin America and the Caribbean 2020; OECD: Paris, France, 2020. [Google Scholar]
- Rose, A.M.; Grosse, S.D.; Garcia, S.P.; Bach, J.; Kleyn, M.; Simon, N.J.E.; Prosser, L.A. The financial and time burden associated with phenylketonuria treatment in the United States. Mol. Genet. Metab. Rep. 2019, 21, 1–9. [Google Scholar] [CrossRef]
- Guest, J.F.; Bai, J.J.; Taylor, R.R.; Sladkevicius, E.; Lee, P.J.; Lachmann, R.H. Costs and outcomes over 36 years of patients with phenylketonuria who do and do not remain on a phenylalanine-restricted diet. J. Intellect. Disabil. Res. 2013, 57, 567–579. [Google Scholar] [CrossRef]
- Castro, G.; Hamilton, V.; Cornejo, V. Chilean Nutrition Management Protocol for Patients with Phenylketonuria. J. Inborn Errors Metab. Screen. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Chiesa, A.; Fraga, C.; Prieto, L.; Pardo, M.L. Modelo de atención de pacientes con fenilcetonuria (PKU) en Argentina. Acta Pediatr. Mex. 2012, 33, 308–310. [Google Scholar]
- García-Restrepo, N.; Hernández, G.J.; Londoño, M.L.; Muriel-Ramírez, R. Deficiencia de fenilalanina hidroxilasa: Espectro clínico y estado actual del diagnóstico en Colombia. Biosalud 2018, 17, 49–64. [Google Scholar]
- Vieira Neto, E.; Maia Filho, H.S.; Monteiro, C.B.; Carvalho, L.M.; Tonon, T.; Vanz, A.P.; Schwartz, I.V.D.; Ribeiro, M.G. Quality of life and adherence to treatment in early-treated Brazilian phenylketonuria pediatric patients. Braz. J. Med. Biol. Res. 2018, 51. [Google Scholar] [CrossRef]
- Lemes, A.; Queijo, C.; Zabala, C.; Queiruga, G. Modelo de atención de la Fenilcetonuria (PKU) en Uruguay. Acta Pediatr. Mex. 2012, 33, 311–314. [Google Scholar]
- Van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 1–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vockley, J.; Andersson, H.C.; Antshel, K.M.; Braverman, N.E.; Burton, B.K.; Frazier, D.M.; Mitchell, J.; Smith, W.E.; Thompson, B.H.; Berry, S.A. Phenylalanine hydroxylase deficiency: Diagnosis and management guideline. Genet. Med. 2014, 16, 188–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waisbren, S.E.; Noel, K.; Fahrbach, K.; Cella, C.; Frame, D.; Dorenbaum, A.; Levy, H. Phenylalanine blood levels and clinical outcomes in phenylketonuria: A systematic literature review and meta-analysis. Mol. Genet. Metab. 2007, 92, 63–70. [Google Scholar] [CrossRef]
- Singh, R.H.; Cunningham, A.C.; Mofidi, S.; Douglas, T.D.; Frazier, D.M.; Hook, D.G.; Jeffers, L.; McCune, H.; Moseley, K.D.; Ogata, B.; et al. Updated, web-based nutrition management guideline for PKU: An evidence and consensus based approach. Mol. Genet. Metab. 2016, 118, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Van Spronsen, F.J.; Van Rijn, M.; Bekhof, J.; Koch, R.; Smit, P.G.A. Phenylketonuria: Tyrosine supplementation in phenylalanine-restricted diets. Am. J. Clin. Nutr. 2001, 73, 153–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, L.; Burns, C.; Sailer-Hammons, M.; Kurtz, A.; Rohr, F. Multiclinic Observations on the Simplified Diet in PKU. J. Nutr. Metab. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.; Jacobs, P.; Fingerhut, R.; Torresani, T.; Thöny, B.; Blau, N.; Baumgartner, M.R.; Rohrbach, M. Positive effect of a simplified diet on blood phenylalanine control in different phenylketonuria variants, characterized by newborn BH 4 loading test and PAH analysis. Mol. Genet. Metab. 2012, 106, 264–268. [Google Scholar] [CrossRef]
- Banta-Wright, S.A.; Shelton, K.C.; Lowe, N.D.; Knafl, K.A.; Houck, G.M. Breast-feeding Success Among Infants with Phenylketonuria. J. Pediatr. Nurs. 2012, 27, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Kanufre, V.C.; Starling, A.L.P.; Leão, E.; Aguiar, M.J.B.; Santos, J.S.; Soares, R.D.L.; Silveira, A.M. Breastfeeding in the treatment of children with phenylketonuria. J. Pediatr. 2007, 83. [Google Scholar] [CrossRef]
- de Castro, M.J.; de Lamas, C.; Sánchez-Pintos, P.; González-Lamuño, D.; Couce, M.L. Bone status in patients with phenylketonuria: A systematic review. Nutrients 2020, 12, 2154. [Google Scholar] [CrossRef]
- Acosta, P.B. Recommendations for protein and energy intakes by patients with phenylketonuria. Eur. J. Pediatr. 1996, 155, S121–S124. [Google Scholar] [CrossRef]
- Greenfield, H.; Southgate, D.A.T. Food Composition Data; FAO: Rome, Italy, 1992. [Google Scholar]
- Cochrane, B.; Schwahn, B.; Galloway, P.; Robinson, P.; Gerasimidis, K. A questionnaire survey on the usage of low protein staple foods by people with phenylketonuria in Scotland. J. Hum. Nutr. Diet. 2014, 27, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Handoom, B.; Megdad, E.; Al-Qasabi, D.; Al Mesned, M.; Hawary, R.; Al-Nufiee, S.; Al-Hassnan, Z.; Alsayed, M.D.; Eldali, A. The effects of low protein products availability on growth parameters and metabolic control in selected amino acid metabolism disorders patients. Int. J. Pediatr. Adolesc. Med. 2018, 5, 60–68. [Google Scholar] [CrossRef] [PubMed]
- The World Bank GNI Per Capita, Atlas method (Current US$)—Latin America & Caribbean (Excluding High Income). Available online: https://data.worldbank.org/indicator/NY.GNP.PCAP.CD?locations=XJ (accessed on 18 January 2021).
- Pena, M.J.; De Almeida, M.F.; Van Dam, E.; Ahring, K.; Bélanger-Quintana, A.; Dokoupil, K.; Gokmen-Ozel, H.; Lammardo, A.M.; MacDonald, A.; Robert, M.; et al. Protein substitutes for phenylketonuria in Europe: Access and nutritional composition. Eur. J. Clin. Nutr. 2016, 70, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.; O’Driscoll, M.; MacDonald, A. Living with Phenylketonuria: Lessons from the PKU community. Mol. Genet. Metab. Rep. 2018, 17, 57–63. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.; Van Rijn, M.; Gokmen-Ozel, H.; Burgard, P. The reality of dietary compliance in the management of phenylketonuria. J. Inherit. Metab. Dis. 2010, 33, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Ashe, K.; Kelso, W.; Farrand, S.; Panetta, J.; Fazio, T.; De Jong, G.; Walterfang, M. Psychiatric and Cognitive Aspects of Phenylketonuria: The Limitations of Diet and Promise of New Treatments. Front. Psychiatry 2019, 10, 1–20. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poloni, S.; dos Santos, B.B.; Chiesa, A.; Specola, N.; Pereyra, M.; Saborío-Rocafort, M.; Salazar, M.F.; Leal-Witt, M.J.; Castro, G.; Peñaloza, F.; et al. Current Practices and Challenges in the Diagnosis and Management of PKU in Latin America: A Multicenter Survey. Nutrients 2021, 13, 2566. https://doi.org/10.3390/nu13082566
Poloni S, dos Santos BB, Chiesa A, Specola N, Pereyra M, Saborío-Rocafort M, Salazar MF, Leal-Witt MJ, Castro G, Peñaloza F, et al. Current Practices and Challenges in the Diagnosis and Management of PKU in Latin America: A Multicenter Survey. Nutrients. 2021; 13(8):2566. https://doi.org/10.3390/nu13082566
Chicago/Turabian StylePoloni, Soraia, Bruna Bento dos Santos, Ana Chiesa, Norma Specola, Marcela Pereyra, Manuel Saborío-Rocafort, María Florencia Salazar, María Jesús Leal-Witt, Gabriela Castro, Felipe Peñaloza, and et al. 2021. "Current Practices and Challenges in the Diagnosis and Management of PKU in Latin America: A Multicenter Survey" Nutrients 13, no. 8: 2566. https://doi.org/10.3390/nu13082566
APA StylePoloni, S., dos Santos, B. B., Chiesa, A., Specola, N., Pereyra, M., Saborío-Rocafort, M., Salazar, M. F., Leal-Witt, M. J., Castro, G., Peñaloza, F., Wong, S. P., Porras, R. B., Paranza, L. O., Sanabria, M. C., Amieva, M. V., Morales, M., Naranjo, A. R. C., Mahfoud, A., Colmenares, A. R., ... Cornejo, V. (2021). Current Practices and Challenges in the Diagnosis and Management of PKU in Latin America: A Multicenter Survey. Nutrients, 13(8), 2566. https://doi.org/10.3390/nu13082566