Dietary Fat Chain Length, Saturation, and PUFA Source Acutely Affect Diet-Induced Thermogenesis but Not Satiety in Adults in a Randomized, Crossover Trial
Abstract
1. Introduction
2. Methods
2.1. Study Design and Intervention
2.2. Participants
2.3. Study Implementation
2.3.1. Whole Room Calorimetry
2.3.2. Pretest Procedures
2.3.3. Test Day Protocol
2.4. Meal Compositions
2.5. Fatty Acid Determination of Liquid Meals
2.6. Urine Collection and Analysis
2.7. Blood Collection
2.8. Data and Statistical Analyses
3. Results
3.1. Participant Characteristics
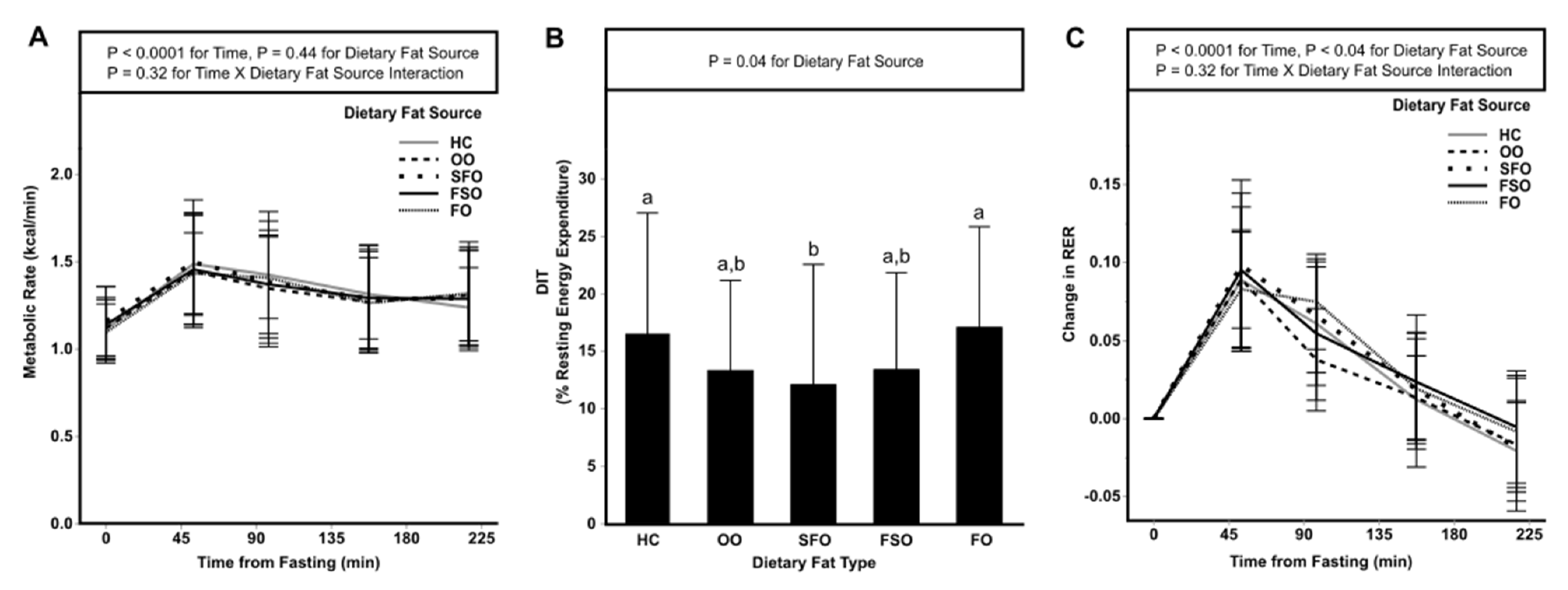
3.2. Postprandial Energy Expenditure
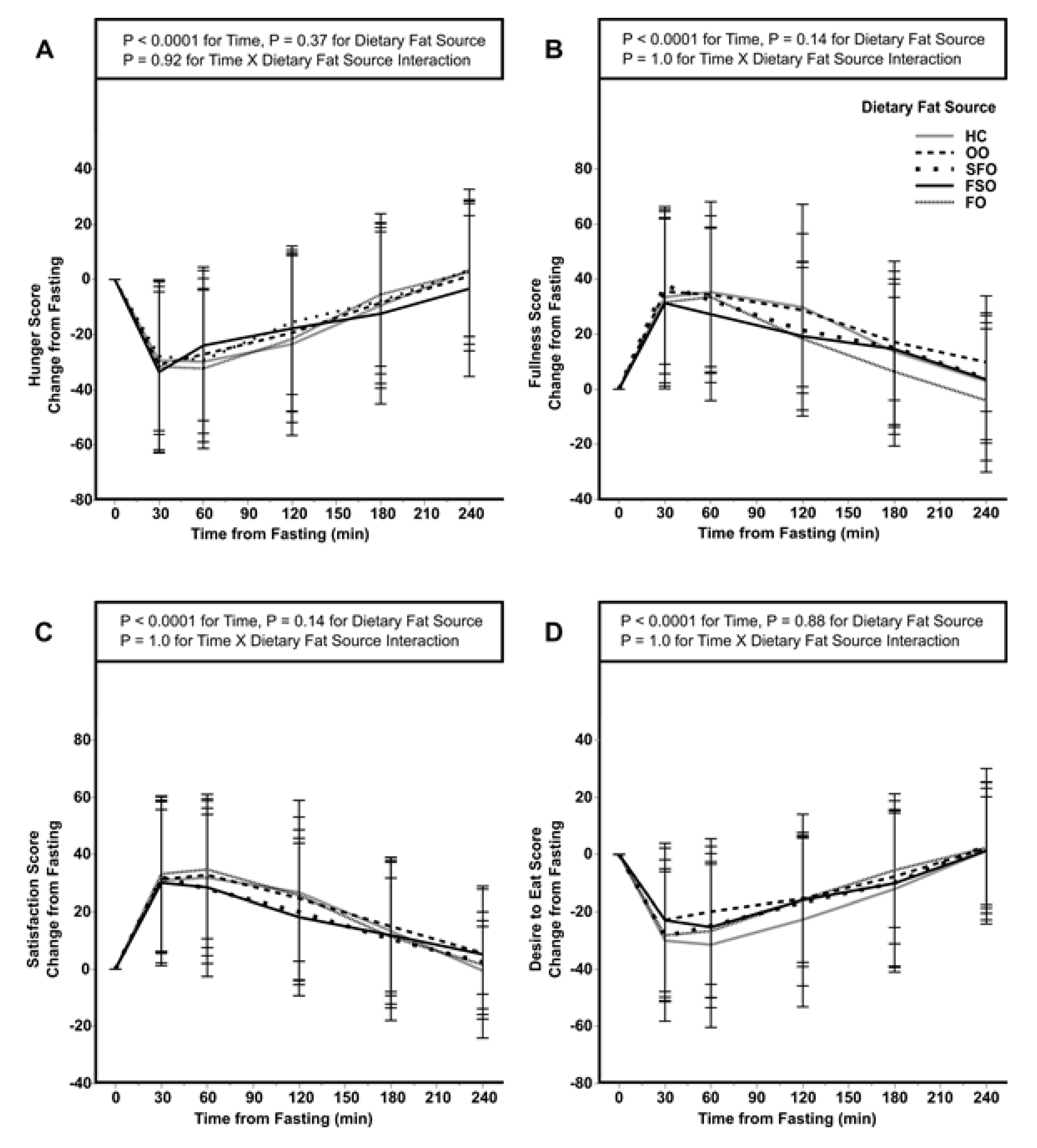
3.3. Subjective Measures of Satiety and Energy Intake of a Subsequent Meal
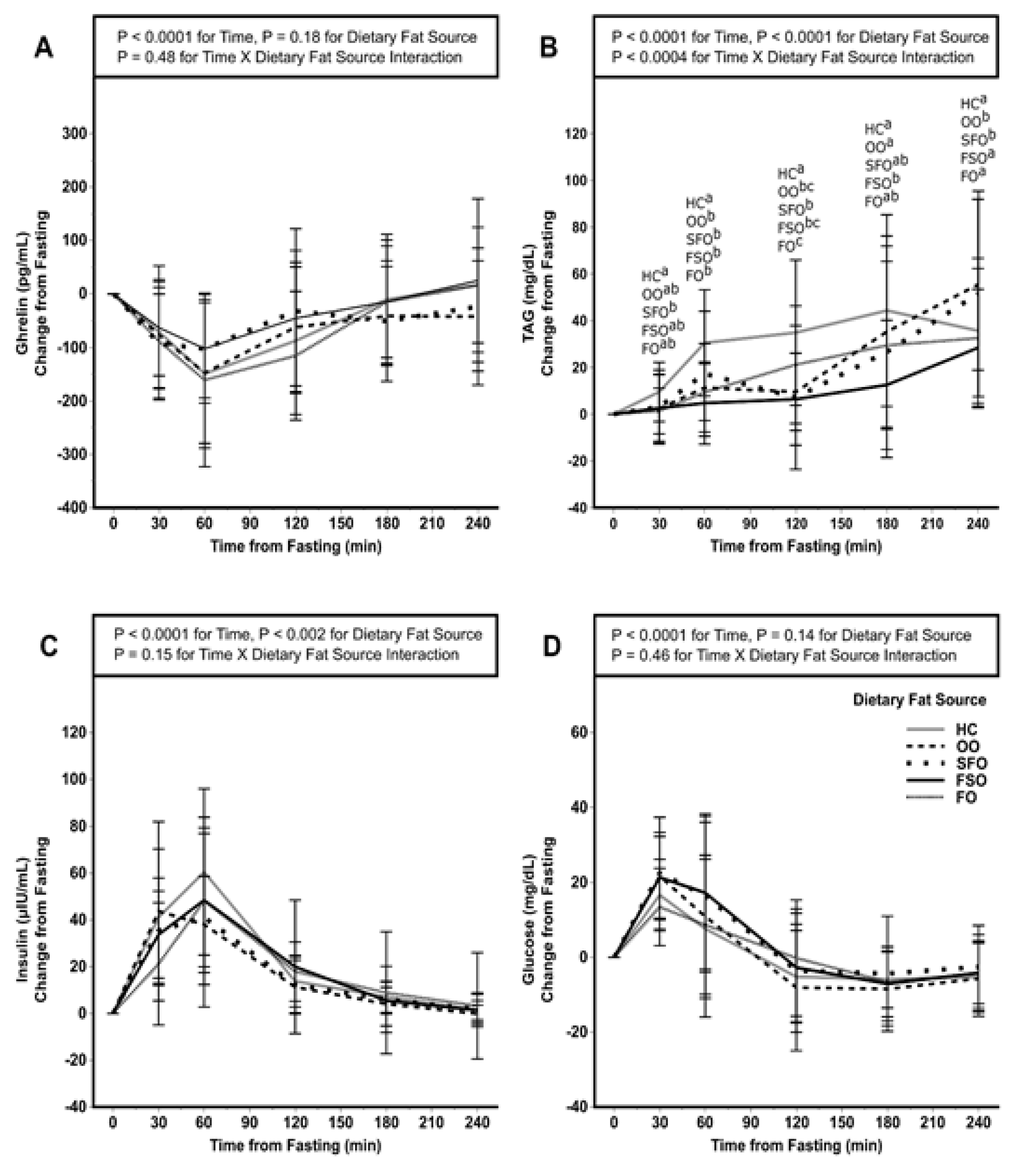
3.4. Postprandial Ghrelin, TAG, Insulin, and Glucose
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bray, G.A.; Paeratakul, S.; Popkin, B.M. Dietary fat and obesity: A review of animal, clinical and epidemiological studies. Physiol. Behav. 2004, 83, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Popkin, B.M. Dietary fat intake does affect obesity! Am. J. Clin. Nutr 1998, 68, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Rehm, C.D.; Rogers, G.; Ruan, M.; Wang, D.D.; Hu, F.B.; Mozaffarian, D.; Zhang, F.F.; Bhupathiraju, S.N. Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality Among US Adults, 1999–2016. JAMA 2019, 322, 1178–1187. [Google Scholar] [CrossRef]
- Wright, J.D.; Wang, C.Y.; Kennedy-Stephenson, J.; Ervin, R.B. Dietary intake of ten key nutrients for public health, United States, 1999–2000. Adv. Data 2003, 334, 1–4. [Google Scholar]
- Raatz, S.K.; Conrad, Z.; Johnson, L.K.; Picklo, M.J.; Jahns, L. Relationship of the Reported Intakes of Fat and Fatty Acids to Body Weight in US Adults. Nutrients 2017, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Visscher, T.L.; Seidell, J.C.; Menotti, A.; Blackburn, H.; Nissinen, A.; Feskens, E.J.; Kromhout, D. Underweight and overweight in relation to mortality among men aged 40–59 and 50–69 years: The Seven Countries Study. Am. J. Epidemiol. 2000, 151, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Hu, F.B. Dietary Fat and Risk of Cardiovascular Disease: Recent Controversies and Advances. Annu. Rev. Nutr. 2017, 37, 423–446. [Google Scholar] [CrossRef]
- Calcagno, M.; Kahleova, H.; Alwarith, J.; Burgess, N.N.; Flores, R.A.; Busta, M.L.; Barnard, N.D. The Thermic Effect of Food: A Review. J. Am. Coll. Nutr. 2019, 38, 547–551. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Jones, P.J. Physiological effects of medium-chain triglycerides: Potential agents in the prevention of obesity. J. Nutr. 2002, 132, 329–332. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Jones, P.J. Greater rise in fat oxidation with medium-chain triglyceride consumption relative to long-chain triglyceride is associated with lower initial body weight and greater loss of subcutaneous adipose tissue. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, H.C.; Kozimor, A.L.; Paton, C.M.; Cooper, J.A. Acute effect of dietary fatty acid composition on postprandial metabolism in women. Exp. Physiol. 2014, 99, 1182–1190. [Google Scholar] [CrossRef]
- Clevenger, H.C.; Stevenson, J.L.; Cooper, J.A. Metabolic responses to dietary fatty acids in obese women. Physiol. Behav. 2015, 139, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.; Jew, S.; AbuMweis, S. The effect of dietary oleic, linoleic, and linolenic acids on fat oxidation and energy expenditure in healthy men. Metabolism 2008, 57, 1198–1203. [Google Scholar] [CrossRef]
- Soares, M.; Cummings, S.; Mamo, J.; Kenrick, M.; Piers, L. The acute effects of olive oil v. cream on postprandial thermogenesis and substrate oxidation in postmenopausal women. Br. J. Nutr. 2004, 91, 245–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piers, L.S.; Walker, K.Z.; Stoney, R.M.; Soares, M.J.; O’Dea, K. The influence of the type of dietary fat on postprandial fat oxidation rates: Monounsaturated (olive oil) vs. saturated fat (cream). Int. J. Obes. Relat. Metab. Disord. 2002, 26, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Casas-Agustench, P.; López-Uriarte, P.; Bulló, M.; Ros, E.; Gómez-Flores, A.; Salas-Salvadó, J. Acute effects of three high-fat meals with different fat saturations on energy expenditure, substrate oxidation and satiety. Clin. Nutr. 2009, 28, 39. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Helt, B.; Raben, A.; Toubro, S.; Astrup, A. Effects of Different Dietary Fat Types on Postprandial Appetite and Energy Expenditure. Obes. Res. 2003, 11, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- DeLany, J.P.; Windhauser, M.M.; Champagne, C.M.; Bray, G.A. Differential oxidation of individual dietary fatty acids in humans. Am. J. Clin. Nutr. 2000, 72, 905–911. [Google Scholar] [CrossRef]
- French, S.J.; Conlon, C.A.; Mutuma, S.T.; Arnold, M.; Read, N.W.; Meijer, G.; Francis, J. The effects of intestinal infusion of long-chain fatty acids on food intake in humans. Gastroenterology 2000, 119, 943–948. [Google Scholar] [CrossRef]
- Maljaars, J.; Romeyn, E.A.; Haddeman, E.; Peters, H.P.; Masclee, A.A. Effect of fat saturation on satiety, hormone release, and food intake. Am. J. Clin. Nutr. 2009, 89, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Alfenas, R.C.; Mattes, R.D. Effect of fat sources on satiety. Obes. Res. 2003, 11, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Strik, C.M.; Lithander, F.E.; McGill, A.-T.; MacGibbon, A.K.; McArdle, B.H.; Poppitt, S.D. No evidence of differential effects of SFA, MUFA or PUFA on post-ingestive satiety and energy intake: A randomised trial of fatty acid saturation. Nutr. J. 2010, 9, 24. [Google Scholar] [CrossRef]
- Kozimor, A.; Chang, H.; Cooper, J.A. Effects of dietary fatty acid composition from a high fat meal on satiety. Appetite 2013, 69, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Casperson, S.L.; Conrad, Z.; Raatz, S.K.; Derner, J.; Roemmich, J.N.; Jahns, L.; Picklo, M.J. Impact of beef consumption on saturated fat intake in the United States adult population: Insights from modeling the influences of bovine genetics and nutrition. Meat Sci. 2020, 169, 108225. [Google Scholar] [CrossRef]
- Wang, B.-S.; Wang, X.-J.; Gong, L.-K. The construction of a Williams design and randomization in cross-over clinical trials using SAS. J. Stat. Softw. 2009, 29, 1–10. [Google Scholar] [CrossRef]
- Angelotti, A.; Cole, R.M.; Schnell, P.M.; Raatz, S.K.; Belury, M.A. Evaluation of a rapid assessment questionnaire using a biomarker for dietary intake of n-3 fatty acids. Lipids 2019, 54, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Casperson, S.L.; Hall, C.; Roemmich, J.N. Postprandial energy metabolism and substrate oxidation in response to the inclusion of a sugar- or non-nutritive sweetened beverage with meals differing in protein content. BMC Nutr. 2017, 3, 49. [Google Scholar] [CrossRef]
- Taylor-Piliae, R.E.; Norton, L.C.; Haskell, W.L.; Mahbouda, M.H.; Fair, J.M.; Iribarren, C.; Hlatky, M.A.; Go, A.S.; Fortmann, S.P. Validation of a new brief physical activity survey among men and women aged 60–69 years. Am. J. Epidemiol. 2006, 164, 598–606. [Google Scholar] [CrossRef] [PubMed]
- USDA. National Nutrient Database for Standard Reference, Release 27; U.S. Department of Agriculture, Agricultural Research Service: Beltsville, MD, USA, 2014. Available online: http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 1 May 2021).
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Raatz, S.K.; Johnson, L.K.; Bukowski, M.R. Enhanced Bioavailability of EPA From Emulsified Fish Oil Preparations Versus Capsular Triacylglycerol. Lipids 2016, 51, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.; Schoeller, D.A. Polyunsaturated: Saturated ratio of diet fat influences energy substrate utilization in the human. Metab. Clin. Exp. 1988, 37, 145–151. [Google Scholar] [CrossRef]
- Bergeron, N.; Havel, R.J. Influence of diets rich in saturated and omega-6 polyunsaturated fatty acids on the postprandial responses of apolipoproteins B-48, B-100, E, and lipids in triglyceride-rich lipoproteins. Arter. Thromb. Vasc. Biol. 1995, 15, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Demacker, P.N.; Reijnen, I.G.; Katan, M.B.; Stuyt, P.M.; Stalenhoef, A.F. Increased removal of remnants of triglyceride-rich lipoproteins on a diet rich in polyunsaturated fatty acids. Eur. J. Clin. Investig. 1991, 21, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.G.; Wolstencroft, E.J.; Bateman, P.A.; Yaqoob, P.; Williams, C.M. Greater enrichment of triacylglycerol-rich lipoproteins with apolipoproteins E and C-III after meals rich in saturated fatty acids than after meals rich in unsaturated fatty acids. Am. J. Clin. Nutr. 2005, 81, 25–34. [Google Scholar] [CrossRef]
- McCloy, U.; Ryan, M.A.; Pencharz, P.B.; Ross, R.J.; Cunnane, S.C. A comparison of the metabolism of eighteen-carbon 13C-unsaturated fatty acids in healthy women. J. Lipid Res. 2004, 45, 474–485. [Google Scholar] [CrossRef]
- MacIntosh, C.G.; Holt, S.H.; Brand-Miller, J.C. The degree of fat saturation does not alter glycemic, insulinemic or satiety responses to a starchy staple in healthy men. J. Nutr. 2003, 133, 2577–2580. [Google Scholar] [CrossRef]
- Lawton, C.L.; Delargy, H.J.; Brockman, J.; Smith, F.C.; Blundell, J.E. The degree of saturation of fatty acids influences post-ingestive satiety. Br. J. Nutr. 2000, 83, 473–482. [Google Scholar] [CrossRef]
- Stevenson, J.L.; Clevenger, H.C.; Cooper, J.A. Hunger and satiety responses to high-fat meals of varying fatty acid composition in women with obesity. Obesity 2015, 23, 1980–1986. [Google Scholar] [CrossRef]
- Stevenson, J.L.; Miller, M.K.; Skillman, H.E.; Paton, C.M.; Cooper, J.A. A PUFA-rich diet improves fat oxidation following saturated fat-rich meal. Eur. J. Nutr. 2017, 56, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Poppitt, S.D.; Strik, C.M.; MacGibbon, A.K.H.; McArdle, B.H.; Budgett, S.C.; McGill, A.T. Fatty acid chain length, postprandial satiety and food intake in lean men. Physiol. Behav. 2010, 101, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, R.; Maher, T.; Clegg, M.E. Coconut oil has less satiating properties than medium chain triglyceride oil. Physiol. Behav. 2017, 179, 422–426. [Google Scholar] [CrossRef]
- Michalski, M.C. Specific molecular and colloidal structures of milk fat affecting lipolysis, absorption and postprandial lipemia. Eur. J. Lipid Sci. Technol. 2009, 111, 413–431. [Google Scholar] [CrossRef]
- Jones, P.J.; Pencharz, P.B.; Clandinin, M.T. Whole body oxidation of dietary fatty acids: Implications for energy utilization. Am. J. Clin. Nutr. 1985, 42, 769–777. [Google Scholar] [CrossRef]
- Friedman, M.I.; Ramirez, I.; Bowden, C.R.; Tordoff, M.G. Fuel partitioning and food intake: Role for mitochondrial fatty acid transport. Am. J. Physiol. 1990, 258, R216–R221. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, S.; Sato, T.; Kangawa, K.; Nakazato, M. The homeostatic force of ghrelin. Cell Metab. 2018, 27, 786–804. [Google Scholar] [CrossRef]
- Feinle-Bisset, C.; Patterson, M.; Ghatei, M.A.; Bloom, S.R.; Horowitz, M. Fat digestion is required for suppression of ghrelin and stimulation of peptide YY and pancreatic polypeptide secretion by intraduodenal lipid. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E948–E953. [Google Scholar] [CrossRef]
- Feltrin, K.L.; Little, T.J.; Meyer, J.H.; Horowitz, M.; Smout, A.J.; Wishart, J.; Pilichiewicz, A.N.; Rades, T.; Chapman, I.M.; Feinle-Bisset, C. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am. J. Physiol. 2004, 287, R524–R533. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Mayrsohn, B.; O’Keeffe, M.; Kissileff, H.R.; Choudhury, A.R.; Laferrère, B. Impact of medium and long chain triglycerides consumption on appetite and food intake in overweight men. Eur. J. Clin. Nutr. 2014, 68, 1134–1140. [Google Scholar] [CrossRef]
- Poppitt, S.D.; Leahy, F.E.; Keogh, G.F.; Wang, Y.; Mulvey, T.B.; Stojkovic, M.; Chan, Y.K.; Choong, Y.S.; McArdle, B.H.; Cooper, G.J. Effect of high-fat meals and fatty acid saturation on postprandial levels of the hormones ghrelin and leptin in healthy men. Eur. J. Clin. Nutr. 2006, 60, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Tighe, A.P.; Sadovsky, R.; Davidson, M.H. Prescription omega-3 fatty acids and their lipid effects: Physiologic mechanisms of action and clinical implications. Expert Rev. Cardiovasc. Ther. 2008, 6, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Bulchandani, D. Why do omega-3 fatty acids lower serum triglycerides? Curr. Opin. Lipidol. 2006, 17, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Davis, P.A.; Schneeman, B.O. Interaction of fat availability and sex on postprandial satiety and cholecystokinin after mixed-food meals. Am. J. Clin. Nutr. 2004, 80, 1207–1214. [Google Scholar] [CrossRef]
- Quatela, A.; Callister, R.; Patterson, A.; MacDonald-Wicks, L. The Energy Content and Composition of Meals Consumed after an Overnight Fast and Their Effects on Diet Induced Thermogenesis: A Systematic Review, Meta-Analyses and Meta-Regressions. Nutrients 2016, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- Piers, L.; Walker, K.Z.; Stoney, R.M.; Soares, M.J.; O’Dea, K. Substitution of saturated with monounsaturated fat in a 4-week diet affects body weight and composition of overweight and obese men. Br. J. Nutr. 2003, 90, 717. [Google Scholar] [CrossRef]

| Test Meal | Amount (g) | Energy (kcal) | Protein (g) | Carbohydrate (g) | Fat (g) |
|---|---|---|---|---|---|
| Heavy Cream | |||||
| Frozen Strawberries | 100 | 35 | 0.4 | 9.1 | 0.1 |
| Orange Sherbet | 100 | 128 | 1.1 | 31.4 | 0.0 |
| Skim Milk | 100 | 34 | 3.4 | 5.0 | 0.1 |
| Heavy Cream | 86 | 287 | 1.8 | 2.4 | 30.0 |
| Total | 386 | 484 | 6.7 | 47.9 | 30.2 |
| Olive Oil | |||||
| Strawberries | 100 | 35 | 0.4 | 9.1 | 0.1 |
| Orange Sherbet | 100 | 128 | 1.1 | 31.4 | 0.0 |
| Skim Milk | 100 | 34 | 3.4 | 5.0 | 0.1 |
| Olive Oil | 30 | 265 | 0.0 | 0.0 | 30.0 |
| Total | 330 | 462 | 4.9 | 45.5 | 30.2 |
| Sunflower Oil | |||||
| Strawberries | 100 | 35 | 0.4 | 9.1 | 0.1 |
| Orange Sherbet | 100 | 128 | 1.1 | 31.4 | 0.0 |
| Skim Milk | 100 | 34 | 3.4 | 5.0 | 0.1 |
| Sunflower Oil | 30 | 259 | 0.0 | 0.0 | 29.4 |
| Total | 330 | 456 | 4.9 | 45.5 | 29.6 |
| Flaxseed Oil | |||||
| Strawberries | 100 | 35 | 0.4 | 9.1 | 0.1 |
| Orange Sherbet | 100 | 128 | 1.1 | 31.4 | 0.0 |
| Skim Milk | 100 | 34 | 3.4 | 5.0 | 0.1 |
| Flaxseed Oil | 30 | 270 | 0.0 | 0.0 | 30.0 |
| Total | 330 | 467 | 4.9 | 45.5 | 30.2 |
| Fish Oil | |||||
| Strawberries | 100 | 35 | 0.4 | 9.1 | 0.1 |
| Orange Sherbet | 100 | 128 | 1.1 | 31.4 | 0.0 |
| Skim Milk | 100 | 34 | 3.4 | 5.0 | 0.1 |
| CorOmega Omega3 Squeeze | 40 | 320 | 0.0 | 0.0 | 29.6 |
| Total | 340 | 517 | 4.9 | 45.5 | 29.8 |
| Age (years) | 25.7 ± 6.6 |
| Body fat (%) | 33.5 ± 8.2 |
| Body mass index (kg/m2) | 27.7 ± 3.8 |
| Fat free mass (kg) | 52.2 ± 10.1 |
| Endpoint | HC 2 | OO | SFO | FSO | FO | p 3 |
|---|---|---|---|---|---|---|
| EE (kcal) | 293 ± 55 | 286 ± 60 | 292 ± 64 | 289 ± 58 | 288 ± 49 | 0.27 |
| OXFAT (g) | 10.3 ± 12.2 | 7.9 ± 10 | 5.6 ± 14.2 | 7.3 ± 10.7 | 8.7 ± 12.1 | 0.31 |
| OXCHO (g) | 9.5 ± 6.5 | 6.5 ± 6.1 | 8.8 ± 8.5 | 9.4 ± 6.6 | 8.2 ± 6.8 | 0.31 |
| Endpoint | HC | OO | SFO | FSO | FO | p 3 |
|---|---|---|---|---|---|---|
| Ghrelin (pg/mL × h) | −249 ± 428 1 | −242 ± 344 | −201 ± 338 | −189 ± 389 | −290 ± 379 | 0.86 |
| TAG (mg/dL × h) | 126 ± 88 a,2 | 81 ± 62 ab | 75 ± 101 ab | 49 ± 69 b | 62 ± 77 b | <0.01 |
| Insulin (μIU/mL × h) | 78 ± 31 | 67 ± 31 | 70 ± 48 | 79 ± 51 | 64 ± 28 | 0.11 |
| Glucose (mg/dL × h) | −3 ± 26 | 1 ± 36 | 16 ± 33 | 14 ± 25 | 1 ± 40 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rust, B.M.; Raatz, S.K.; Casperson, S.L.; Duke, S.E.; Picklo, M.J. Dietary Fat Chain Length, Saturation, and PUFA Source Acutely Affect Diet-Induced Thermogenesis but Not Satiety in Adults in a Randomized, Crossover Trial. Nutrients 2021, 13, 2615. https://doi.org/10.3390/nu13082615
Rust BM, Raatz SK, Casperson SL, Duke SE, Picklo MJ. Dietary Fat Chain Length, Saturation, and PUFA Source Acutely Affect Diet-Induced Thermogenesis but Not Satiety in Adults in a Randomized, Crossover Trial. Nutrients. 2021; 13(8):2615. https://doi.org/10.3390/nu13082615
Chicago/Turabian StyleRust, Bret M., Susan K. Raatz, Shanon L. Casperson, Sara E. Duke, and Matthew J. Picklo. 2021. "Dietary Fat Chain Length, Saturation, and PUFA Source Acutely Affect Diet-Induced Thermogenesis but Not Satiety in Adults in a Randomized, Crossover Trial" Nutrients 13, no. 8: 2615. https://doi.org/10.3390/nu13082615
APA StyleRust, B. M., Raatz, S. K., Casperson, S. L., Duke, S. E., & Picklo, M. J. (2021). Dietary Fat Chain Length, Saturation, and PUFA Source Acutely Affect Diet-Induced Thermogenesis but Not Satiety in Adults in a Randomized, Crossover Trial. Nutrients, 13(8), 2615. https://doi.org/10.3390/nu13082615







