Phycobiliproteins Ameliorate Gonadal Toxicity in Male Mice Treated with Cyclophosphamide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Spirulina
2.2. PBP Extraction and Determination
2.3. Animals
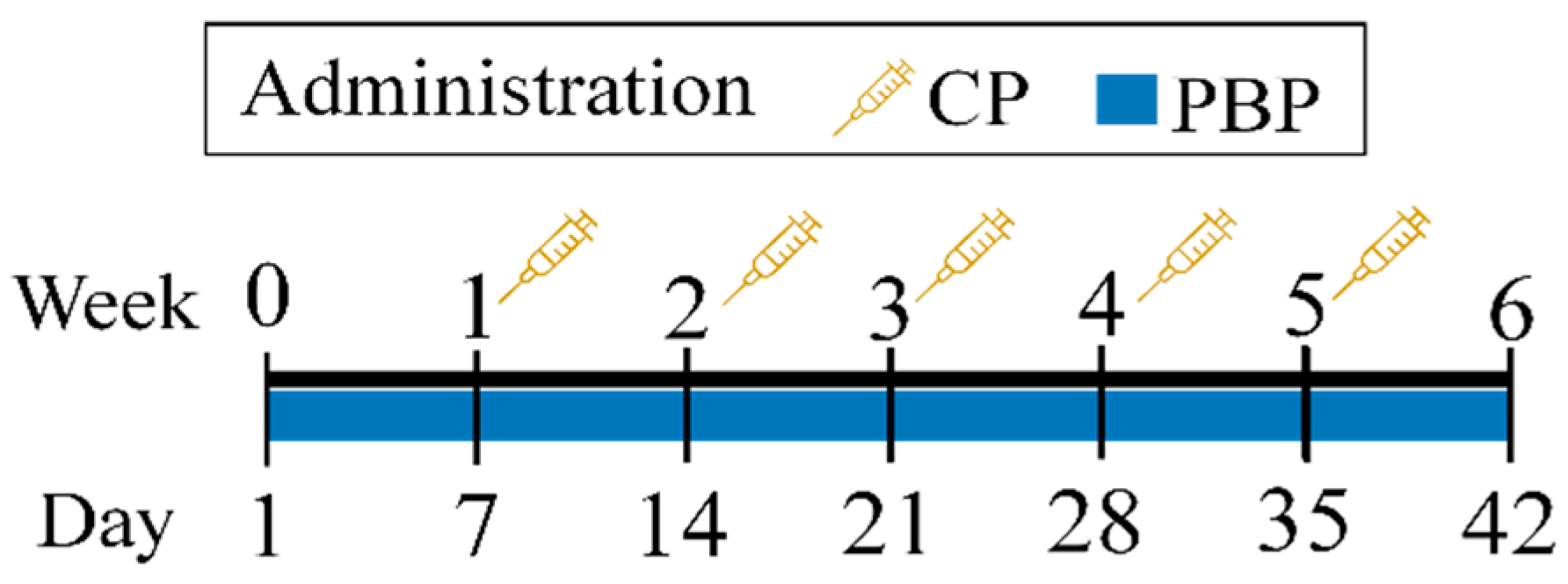
2.4. Experimental Design
2.5. Serum Testosterone, Body Weight, and Relative Testicular Weight
2.6. Lipoperoxidation and SOD and GPX Activity in the Testes
2.7. Testicular Histology
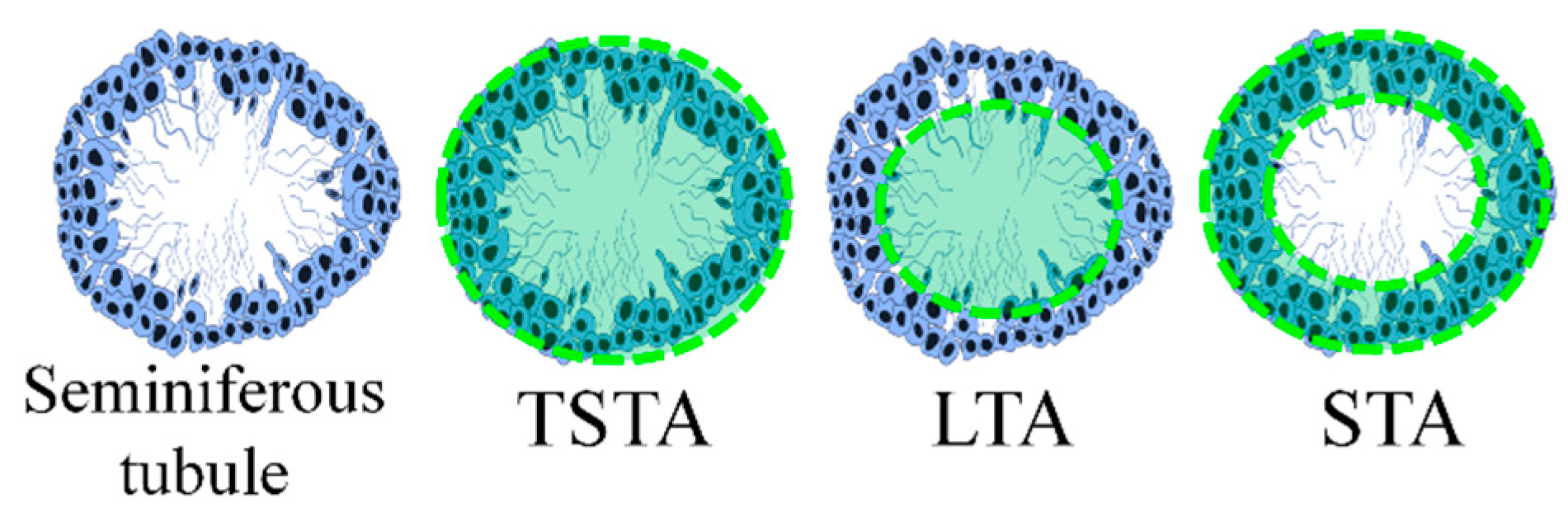
2.8. Histomorphometry
2.9. Sperm Parameter Assessment
2.10. Statistical Analysis
3. Results
3.1. PBP Determination
3.2. Serum Testosterone, Body Weight, and Relative Testicular Weight
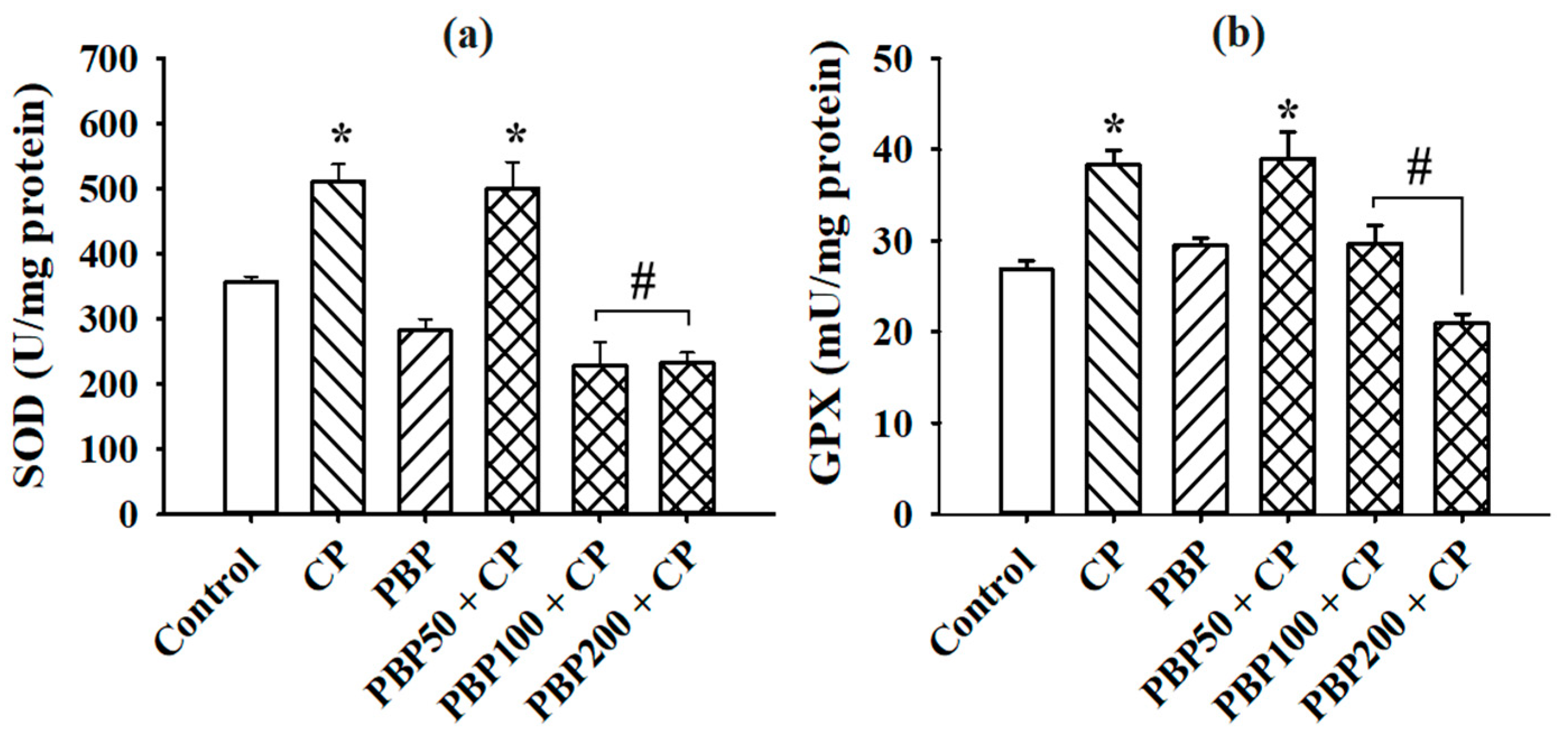
3.3. Lipoperoxidation and SOD and GPX Activity in the Testes
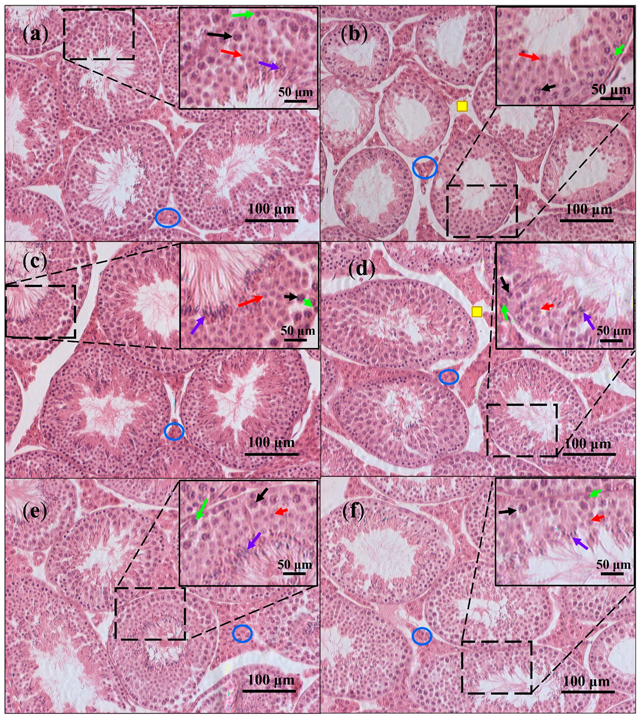
3.4. Testicular Histology
3.5. Histomorphometry
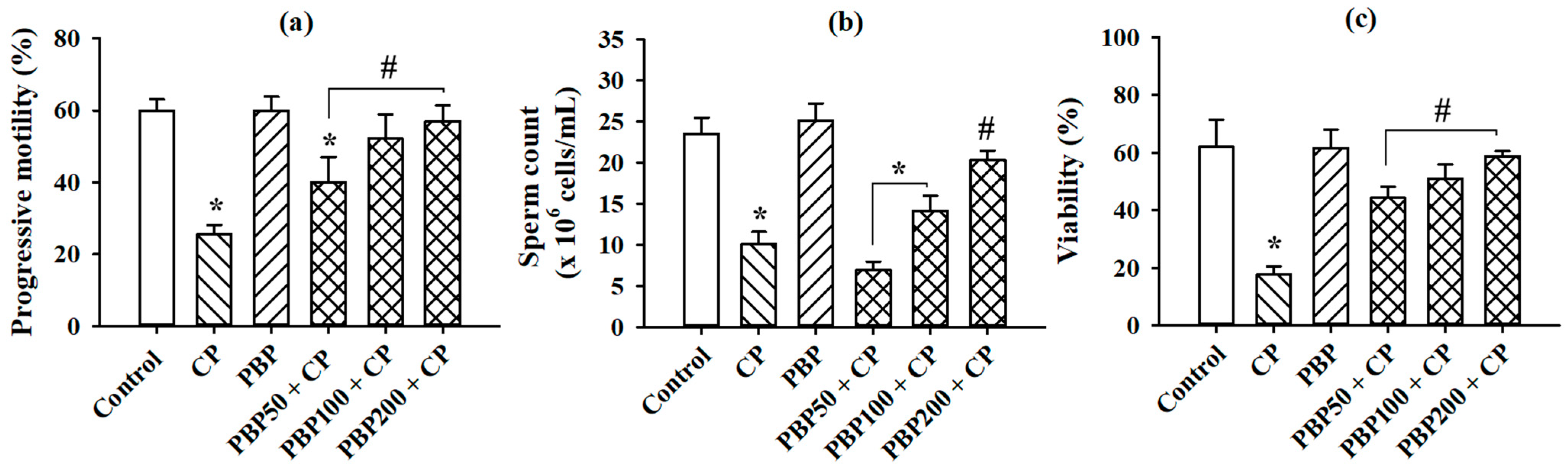
3.6. Sperm Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Jalali, A.S.; Hasanzadeh, S.; Malekinejad, H. Achillea millefolium inflorescence aqueous extract ameliorates cyclophosphamide-induced toxicity in rat testis: Stereological evidences. Chin. J. Nat. Med. 2012, 10, 247–254. [Google Scholar] [CrossRef]
- Potnuri, A.G.; Allakonda, L.; Lahkar, M. Crocin attenuates cyclophosphamide induced testicular toxicity by preserving glutathione redox system. Biomed. Pharmacother. 2018, 101, 174–180. [Google Scholar] [CrossRef]
- Ghobadi, E.; Moloudizargari, M.; Asghari, M.H.; Abdollahi, M. The mechanisms of cyclophosphamide-induced testicular toxicity and the protective agents. Expert Opin. Drug Metab. Toxicol. 2017, 13, 525–536. [Google Scholar] [CrossRef]
- Wetzels, J. Cyclophosphamide-induced gonadal toxicity: A treatment dilemma in patients with lupus nephritis? Neth. J. Med. 2004, 62, 347–352. [Google Scholar]
- Drobnis, E.Z.; Nangia, A.K. Immunosuppressants and Male Reproduction. Adv. Exp. Med. Biol. 2017, 1034, 179–210. [Google Scholar] [CrossRef]
- Hamzeh, M.; Hosseinimehr, S.J.; Karimpour, A.; Mohammadi, H.R.; Khalatbary, A.R.; Talebpour Amiri, F. Cerium Oxide Nanoparticles Protect Cyclophosphamide-Induced Testicular Toxicity in Mice. Int. J. Prev. Med. 2019, 10, 5. [Google Scholar] [CrossRef]
- Lu, W.P.; Mei, X.T.; Wang, Y.; Zheng, Y.P.; Xue, Y.F.; Xu, D.H. Zn(II)-curcumin protects against oxidative stress, deleterious changes in sperm parameters and histological alterations in a male mouse model of cyclophosphamide-induced reproductive damage. Environ. Toxicol. Pharmacol. 2015, 39, 515–524. [Google Scholar] [CrossRef]
- Crisol, L.; Matorras, R.; Aspichueta, F.; Expósito, A.; Hernández, M.L.; Ruiz-Larrea, M.B.; Mendoza, R.; Ruiz-Sanz, J.I. Glutathione peroxidase activity in seminal plasma and its relationship to classical sperm parameters and in vitro fertilization-intracytoplasmic sperm injection outcome. Fertil. Steril. 2012, 97, 852–857.e1. [Google Scholar] [CrossRef]
- Yan, L.; Liu, J.; Wu, S.; Zhang, S.; Ji, G.; Gu, A. Seminal superoxide dismutase activity and its relationship with semen quality and SOD gene polymorphism. J. Assist. Reprod. Genet. 2014, 31, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Li, X.L.; Lin, T.; He, D.W.; Wei, G.H.; Liu, J.H.; Li, L.S. The cyclophosphamide metabolite, acrolein, induces cytoskeletal changes and oxidative stress in Sertoli cells. Mol. Biol. Rep. 2012, 39, 493–500. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, I.C.; Lim, J.H.; Moon, C.; Bae, C.S.; Kim, S.H.; Shin, D.H.; Park, S.C.; Kim, H.C.; Kim, J.C. Protective effects of pine bark extract on developmental toxicity of cyclophosphamide in rats. Food Chem. Toxicol. 2012, 50, 109–115. [Google Scholar] [CrossRef]
- Watcho, P.; Mpeck, I.R.; Deeh Defo, P.B.; Wankeu-Nya, M.; Ngadjui, E.; Bonsou Fozin, G.R.; Kamtchouing, P.; Kamanyi, A. Cyclophosphamide-induced reproductive toxicity: Beneficial effects of Helichrysum odoratissimum (Asteraceae) in male Wistar rats. J. Integr. Med. 2019, 17, 366–373. [Google Scholar] [CrossRef]
- Özatik, F.Y.; Özatik, O.; Tekşen, Y.; Yiğitaslan, S.; Ari, N.S. Protective and therapeutic effect of Hydrogen sulfide on hemorrhagic cystitis and testis dysfunction induced with Cyclophosphamide. Turkish J. Med. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mulder, R.L.; Font-Gonzalez, A.; Green, D.M.; Loeffen, E.A.H.; Hudson, M.M.; Loonen, J.; Yu, R.; Ginsberg, J.P.; Mitchell, R.T.; Byrne, J.; et al. Fertility preservation for male patients with childhood, adolescent, and young adult cancer: Recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021, 22, e57–e67. [Google Scholar] [CrossRef]
- Adewoyin, M.; Ibrahim, M.; Roszaman, R.; Isa, M.L.M.; Alewi, N.A.M.; Rafa, A.A.A.; Anuar, M.N.N. Male Infertility: The Effect of Natural Antioxidants and Phytocompounds on Seminal Oxidative Stress. Diseases 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wu, Y.; Wang, G.; Jia, T.; Zhang, Y. Purification and bioactivities of phycocyanin. Crit. Rev. Food Sci. Nutr. 2017, 57, 3840–3849. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef] [PubMed]
- Righini, H.; Francioso, O.; Di Foggia, M.; Quintana, A.M.; Roberti, R. Preliminary Study on the Activity of Phycobiliproteins against Botrytis cinerea. Mar. Drugs 2020, 18, 600. [Google Scholar] [CrossRef]
- Vazquez-Sanchez, J.; Ramon-Gallegos, E.; Mojica-Villegas, A.; Madrigal-Bujaidar, E.; Perez-Pasten-Borja, R.; Chamorro-Cevallos, G. Spirulina maxima and its protein extract protect against hydroxyurea-teratogenic insult in mice. Food Chem. Toxicol. 2009, 47, 2785–2789. [Google Scholar] [CrossRef]
- Guzman-Gomez, O.; Garcia-Rodriguez, R.V.; Quevedo-Corona, L.; Perez-Pasten-Borja, R.; Rivero-Ramirez, N.L.; Rios-Castro, E.; Perez-Gutierrez, S.; Perez-Ramos, J.; Chamorro-Cevallos, G.A. Amelioration of Ethanol-Induced Gastric Ulcers in Rats Pretreated with Phycobiliproteins of Arthrospira (Spirulina) Maxima. Nutrients 2018, 10, 763. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Sánchez, R.; Ortiz-Butrón, R.; Blas-Valdivia, V.; Hernández-García, A.; Cano-Europa, E. Phycobiliproteins or C-phycocyanin of Arthrospira (Spirulina) maxima protect against HgCl2-caused oxidative stress and renal damage. Food Chem. 2012, 135, 2359–2365. [Google Scholar] [CrossRef]
- Castro-Garcia, S.Z.; Chamorro-Cevallos, G.; Quevedo-Corona, L.; McCarty, M.F.; Bobadilla-Lugo, R.A. Beneficial effects of phycobiliproteins from Spirulina maxima in a preeclampsia model. Life Sci. 2018, 211, 17–24. [Google Scholar] [CrossRef]
- Maurya, S.S.; Maurya, J.N.; Pandey, V.D. Factors regulating phycobiliprotein production in cyanobacteria. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 764–771. [Google Scholar]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, N.; Chiou, T.J.; Tzeng, W.F.; Chu, S.T. Cyclophosphamide treatment causes impairment of sperm and its fertilizing ability in mice. Toxicology 2006, 222, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Fleischer, S., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 1978; Volume 52, pp. 302–310. ISBN 0076-6879. [Google Scholar]
- Layton, C.; Bancroft, J.D.; Suvarna, S.K. 4—Fixation of tissues. In Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 40–63. ISBN 978-0-7020-6887-4. [Google Scholar]
- Bancroft, J.D.; Layton, C. 10—The hematoxylins and eosin. In Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 126–138. ISBN 978-0-7020-6887-4. [Google Scholar]
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. Available online: https://apps.who.int/iris/handle/10665/44261 (accessed on 18 September 2017).
- Tripathi, D.N.; Jena, G.B. Astaxanthin inhibits cytotoxic and genotoxic effects of cyclophosphamide in mice germ cells. Toxicology 2008, 248, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Mahecha, A.; Hales, B.F.; Robaire, B. Chronic Cyclophosphamide Treatment Alters the Expression of Stress Response Genes in Rat Male Germ Cells. Biol. Reprod. 2002, 66, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Gajjar, R.; Miller, S.D.; Meyers, K.E.; Ginsberg, J.P. Fertility preservation in patients receiving cyclophosphamide therapy for renal disease. Pediatr. Nephrol. 2015, 30, 1099–1106. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Su, H.-N.; Pu, Y.; Chen, J.; Liu, L.-N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular structure, production, applications, and prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef]
- Walter, A.; De Carvalho, J.; Thomaz-Soccol, V.; Faria, A.; Ghiggi, V.; Soccol, C. Study of Phycocyanin Production from Spirulina platensis under Different Light Spectra. Braz. Arch. Biol. Technol. 2011, 54, 675–682. [Google Scholar] [CrossRef]
- Chen, X.; Wu, M.; Yang, Q.; Wang, S. Preparation, characterization of food grade phycobiliproteins from Porphyra haitanensis and the application in liposome-meat system. Lwt 2017, 77, 468–474. [Google Scholar] [CrossRef]
- Marchetti, F.; Aardema, M.; Beevers, C.; van Benthem, J.; Douglas, G.R.; Godschalk, R.; Yauk, C.L.; Young, R.; Williams, A. Simulation of mouse and rat spermatogenesis to inform genotoxicity testing using OECD test guideline 488. Mutat. Res. Toxicol. Environ. Mutagen. 2018, 832–833, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, A.; Syed, M.A.; Najmi, A.K.; Ali, J.; Haque, S.E. Ameliorative effect of nerolidol on cyclophosphamide-induced gonadal toxicity in Swiss Albino mice: Biochemical-, histological- and immunohistochemical-based evidences. Andrologia 2020, 52, e13535. [Google Scholar] [CrossRef]
- Jewkes, B.C.; Gomella, M.G.; Lowry, E.T.; Benner, J.A.; Delay, E.R. Cyclophosphamide-Induced Disruptions to Appetitive Qualities and Detection Thresholds of NaCl: Comparison of Single-Dose and Dose Fractionation Effects. Chem. Senses 2018, 43, 399–410. [Google Scholar] [CrossRef]
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, 1211. [Google Scholar] [CrossRef] [Green Version]
- Yalcin, S.; Gumus, M.; Oksuzoglu, B.; Ozdemir, F.; Evrensel, T.; Sarioglu, A.A.; Sahin, B.; Mandel, N.M.; Goker, E. Nutritional Aspect of Cancer Care in Medical Oncology Patients. Clin. Ther. 2019, 41, 2382–2396. [Google Scholar] [CrossRef]
- Chandra, R.; Parra, R.; Iqbal, H.M.N. Phycobiliproteins: A Novel Green Tool from Marine Origin Blue-Green Algae and Red Algae. Protein Pept. Lett. 2017, 24, 118–125. [Google Scholar] [CrossRef]
- Mladenov, M.; Gokik, M.; Hadzi-Petrushev, N.; Gjorgoski, I.; Jankulovski, N. The relationship between antioxidant enzymes and lipid peroxidation in senescent rat erythrocytes. Physiol. Res. 2015, 64, 891–896. [Google Scholar] [CrossRef]
- Aliciguzel, Y.; Ozen, I.; Aslan, M.; Karayalcin, U. Activities of xanthine oxidoreductase and antioxidant enzymes in different tissues of diabetic rats. J. Lab. Clin. Med. 2003, 142, 172–177. [Google Scholar] [CrossRef]
- Ceribasi, A.O.; Turk, G.; Sonmez, M.; Sakin, F.; Atessahin, A. Toxic effect of cyclophosphamide on sperm morphology, testicular histology and blood oxidant-antioxidant balance, and protective roles of lycopene and ellagic acid. Basic Clin. Pharmacol. Toxicol. 2010, 107, 730–736. [Google Scholar] [CrossRef] [Green Version]
- Pavin, N.; Izaguirry, A.; Soares, M.; Spiazzi, C.; Mendez, A.; Gallas, L.; Brum, D.; Cibin, F. Tribulus terrestris Protects against Male Reproductive Damage Induced by Cyclophosphamide in Mice. Oxid. Med. Cell. Longev. 2018, 2018, 5758191. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Sun, Z.; Jiang, Y.; Chen, F.; Wang, M. Acrolein scavengers: Reactivity, mechanism and impact on health. Mol. Nutr. Food Res. 2011, 55, 1375–1390. [Google Scholar] [CrossRef]
- Drumond, A.L.; Weng, C.C.; Wang, G.; Chiarini-Garcia, H.; Eras-Garcia, L.; Meistrich, M.L. Effects of multiple doses of cyclophosphamide on mouse testes: Accessing the germ cells lost, and the functional damage of stem cells. Reprod. Toxicol. 2011, 32, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.P.; Yang, X.M.; Duan, Z.H.; Shang, J.H.; Luo, P.; Xiao, W.; Zhang, D.Y.; Liu, H.Z. Squid ink polysaccharide prevents autophagy and oxidative stress affected by cyclophosphamide in Leydig cells of mice: A pilot study. Iran. J. Basic Med. Sci. 2017, 20, 1194–1199. [Google Scholar] [CrossRef]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 2014, 30, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Wang, L.; Chen, H.; Huang, Y.; Yang, P.; Ahmed, N.; Wang, T.; Liu, Y.; Chen, Q. Molecular and Cellular Mechanisms of Apoptosis during Dissociated Spermatogenesis. Front. Physiol. 2017, 8, 188. [Google Scholar] [CrossRef] [Green Version]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Mens Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Musset, B.; Clark, R.A.; DeCoursey, T.E.; Petheo, G.L.; Geiszt, M.; Chen, Y.; Cornell, J.E.; Eddy, C.A.; Brzyski, R.G.; El Jamali, A. NOX5 in human spermatozoa: Expression, function, and regulation. J. Biol. Chem. 2012, 287, 9376–9388. [Google Scholar] [CrossRef] [Green Version]
- Villaverde, A.I.S.B.; Netherton, J.; Baker, M.A. From Past to Present: The Link between Reactive Oxygen Species in Sperm and Male Infertility. Antioxidants 2019, 8, 616. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Yu, Y.; Haigh, S.; Johnson, J.; Lucas, R.; Stepp, D.W.; Fulton, D.J.R. Regulation of NADPH oxidase 5 by protein kinase C isoforms. PLoS ONE 2014, 9, e88405. [Google Scholar] [CrossRef]
- Higashi, T.; Mai, Y.; Mazaki, Y. Protein kinase C-dependent cell damage by unsaturated carbonyl compounds in vascular cells. J. Biosci. Bioeng. 2018, 126, 527–532. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; McCarty, M.F.; O’Keefe, J.H. Antioxidant bilirubin works in multiple ways to reduce risk for obesity and its health complications. Open Heart 2018, 5, e000914. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Inoguchi, T.; Sasaki, S.; Maeda, Y.; McCarty, M.F.; Fujii, M.; Ikeda, N.; Kobayashi, K.; Sonoda, N.; Takayanagi, R. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R110–R120. [Google Scholar] [CrossRef] [Green Version]
- Al-Dhabi, N.A.; Valan Arasu, M. Quantification of Phytochemicals from Commercial Spirulina Products and Their Antioxidant Activities. Evid. Based Complement. Altern. Med. 2016, 2016, 7631864. [Google Scholar] [CrossRef] [Green Version]
- Chaiklahan, R.; Chirasuwan, N.; Triratana, P.; Loha, V.; Tia, S.; Bunnag, B. Polysaccharide extraction from Spirulina sp. and its antioxidant capacity. Int. J. Biol. Macromol. 2013, 58, 73–78. [Google Scholar] [CrossRef] [PubMed]



| PBP | Concentration (mg/mL) | Purity Index | Extraction Yield (mg/g, wb SP) |
|---|---|---|---|
| C-PC | 2.23 ± 0.02 | 0.7 ± 0.02 | 8.93 ± 0.44 |
| APC | 0.98 ± 0.14 | 0.3 ± 0.02 | 3.91 ± 0.56 |
| PE | 0.02 ± 0.001 | 0.005 ± 0.001 | 0.08 ± 0.004 |
| Group | TSTA (µm2) | LTA (µm2) | STA (µm2) |
|---|---|---|---|
| Control | 50,082 ± 945.4 | 10,771 ± 342.4 | 39,311 ± 755.1 |
| CP | 24,764 ± 511.3 a | 7763 ± 251.0 a | 17,001 ± 396.9 a |
| PBP | 56,481 ± 1535 ab | 8575 ± 600.2 a | 47,906 ± 1249 ab |
| PBP50 + CP | 35,576 ± 1247 b | 7452 ± 600.4 a | 28,124 ± 996.4 ab |
| PBP100 + CP | 38,683 ± 1237 b | 8209 ± 407.6 a | 30,474 ± 1085 ab |
| PBP200 + CP | 40,320 ± 1898 b | 9217 ± 659.9 a | 31,102 ± 1855 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briseño-Bugarín, J.; Hernández-Ochoa, I.; Araujo-Padilla, X.; Mojica-Villegas, M.A.; Montaño-González, R.I.; Gutiérrez-Salmeán, G.; Chamorro-Cevallos, G. Phycobiliproteins Ameliorate Gonadal Toxicity in Male Mice Treated with Cyclophosphamide. Nutrients 2021, 13, 2616. https://doi.org/10.3390/nu13082616
Briseño-Bugarín J, Hernández-Ochoa I, Araujo-Padilla X, Mojica-Villegas MA, Montaño-González RI, Gutiérrez-Salmeán G, Chamorro-Cevallos G. Phycobiliproteins Ameliorate Gonadal Toxicity in Male Mice Treated with Cyclophosphamide. Nutrients. 2021; 13(8):2616. https://doi.org/10.3390/nu13082616
Chicago/Turabian StyleBriseño-Bugarín, Jorge, Isabel Hernández-Ochoa, Xelha Araujo-Padilla, María Angélica Mojica-Villegas, Ricardo Iván Montaño-González, Gabriela Gutiérrez-Salmeán, and Germán Chamorro-Cevallos. 2021. "Phycobiliproteins Ameliorate Gonadal Toxicity in Male Mice Treated with Cyclophosphamide" Nutrients 13, no. 8: 2616. https://doi.org/10.3390/nu13082616
APA StyleBriseño-Bugarín, J., Hernández-Ochoa, I., Araujo-Padilla, X., Mojica-Villegas, M. A., Montaño-González, R. I., Gutiérrez-Salmeán, G., & Chamorro-Cevallos, G. (2021). Phycobiliproteins Ameliorate Gonadal Toxicity in Male Mice Treated with Cyclophosphamide. Nutrients, 13(8), 2616. https://doi.org/10.3390/nu13082616






