A Comprehensive, Multidisciplinary, Personalized, Lifestyle Intervention Program Is Associated with Increased Leukocyte Telomere Length in Children and Adolescents with Overweight and Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Study Protocol
2.3.1. Initial Assessment
2.3.2. Intervention
2.3.3. Annual Follow-Up Visit
2.4. Anthropometric and Body Composition Parameters
2.5. Assays
2.6. Leucocyte Telomere Length
2.7. Statistical Analysis
3. Results
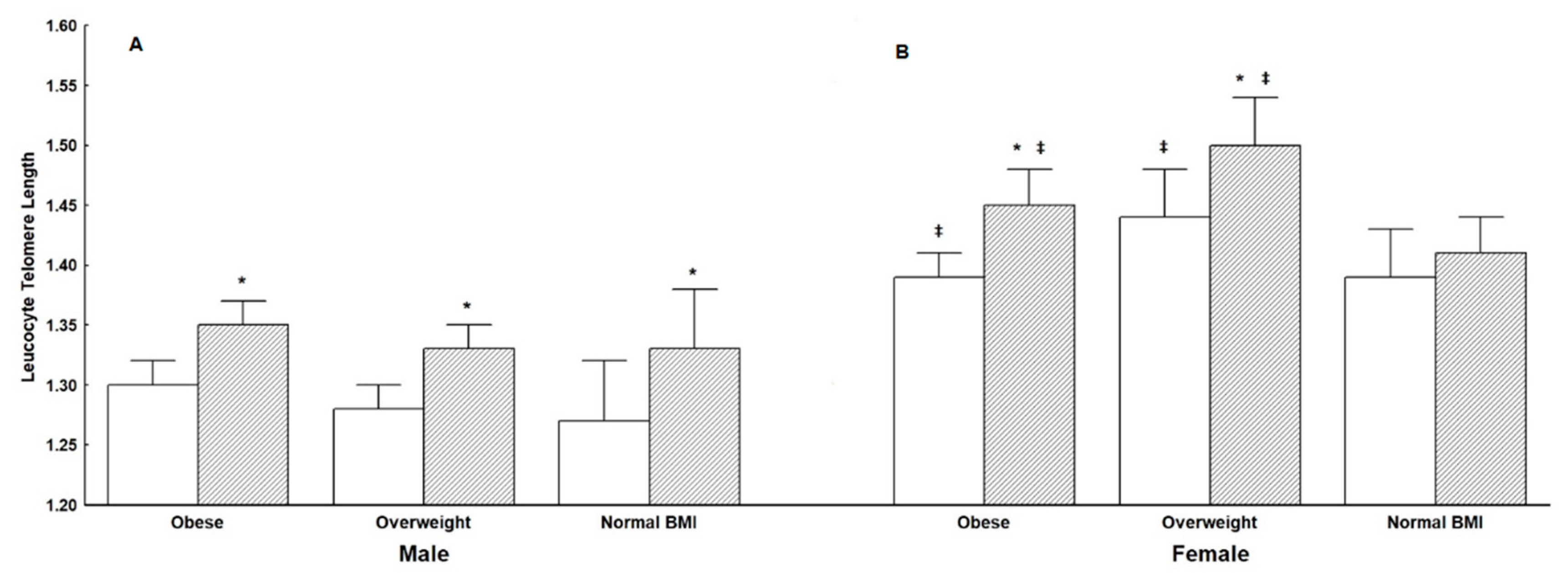
3.1. Leukocyte Telomere Length (LTL)
3.2. Predictors of Leukocyte Telomere Length (LTL) at Initial and Annual Assessment
3.3. Clinical Characteristics, Body Composition and Hematologic, Biochemical and Endocrinologic Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, W.; Zhang, Y.; Liu, D.; Songyang, Z.; Wan, M. Telomeres-structure, function, and regulation. Exp. Cell Res. 2013, 319, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Epel, E. How "reversible" is telomeric aging? Cancer Prev. Res. 2012, 5, 1163–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salpea, K.D.; Talmud, P.J.; Cooper, J.A.; Maubaret, C.G.; Stephens, J.W.; Abelak, K.; Humphries, S.E. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis 2010, 209, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviv, A.; Chen, W.; Gardner, J.P.; Kimura, M.; Brimacombe, M.; Cao, X.; Srinivasan, S.R.; Berenson, G.S. Leukocyte telomere dynamics: Longitudinal findings among young adults in the Bogalusa Heart Study. Am. J. Epidemiol. 2009, 169, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Nordfjall, K.; Eliasson, M.; Stegmayr, B.; Melander, O.; Nilsson, P.; Roos, G. Telomere length is associated with obesity parameters but with a gender difference. Obesity 2008, 16, 2682–2689. [Google Scholar] [CrossRef]
- Kumanyika, S.; Dietz, W.H. Solving Population-wide Obesity—Progress and Future Prospects. N. Engl. J. Med. 2020, 383, 2197–2200. [Google Scholar] [CrossRef]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Kassari, P.; Papaioannou, P.; Billiris, A.; Karanikas, H.; Eleftheriou, S.; Thireos, E.; Manios, Y.; Chrousos, G.P.; Charmandari, E. Electronic registry for the management of childhood obesity in Greece. Eur. J. Clin. Investig. 2018, 48, e12887. [Google Scholar] [CrossRef]
- Lobstein, T.; Jackson-Leach, R.; Moodie, M.L.; Hall, K.D.; Gortmaker, S.L.; Swinburn, B.A.; James, W.P.; Wang, Y.; McPherson, K. Child and adolescent obesity: Part of a bigger picture. Lancet 2015, 385, 2510–2520. [Google Scholar] [CrossRef] [Green Version]
- Tragomalou, A.; Moschonis, G.; Manios, Y.; Kassari, P.; Ioakimidis, I.; Diou, C.; Stefanopoulos, L.; Lekka, E.; Maglaveras, N.; Delopoulos, A.; et al. Novel e-Health Applications for the Management of Cardiometabolic Risk Factors in Children and Adolescents in Greece. Nutrients 2020, 12, 1380. [Google Scholar] [CrossRef]
- Tragomalou, A.; Moschonis, G.; Kassari, P.; Papageorgiou, I.; Genitsaridi, S.M.; Karampatsou, S.; Manios, Y.; Charmandari, E. A National e-Health Program for the Prevention and Management of Overweight and Obesity in Childhood and Adolescence in Greece. Nutrients 2020, 12, 2858. [Google Scholar] [CrossRef]
- Genitsaridi, S.M.; Giannios, C.; Karampatsou, S.; Papageorgiou, I.; Papadopoulos, G.; Farakla, I.; Koui, E.; Georgiou, A.; Romas, S.; Terzioglou, E.; et al. A Comprehensive Multidisciplinary Management Plan Is Effective in Reducing the Prevalence of Overweight and Obesity in Childhood and Adolescence. Horm. Res. Paediatr. 2020, 93, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Buxton, J.L.; Walters, R.G.; Visvikis-Siest, S.; Meyre, D.; Froguel, P.; Blakemore, A.I. Childhood obesity is associated with shorter leukocyte telomere length. J. Clin. Endocrinol. Metab. 2011, 96, 1500–1505. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Calzon, S.; Moleres, A.; Marcos, A.; Campoy, C.; Moreno, L.A.; Azcona-Sanjulian, M.C.; Martinez-Gonzalez, M.A.; Martinez, J.A.; Zalba, G.; Marti, A. Telomere length as a biomarker for adiposity changes after a multidisciplinary intervention in overweight/obese adolescents: The EVASYON study. PLoS ONE 2014, 9, e89828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjaer, T.W.; Faurholt-Jepsen, D.; Mehta, K.M.; Christensen, V.B.; Epel, E.; Lin, J.; Blackburn, E.; Wojcicki, J.M. Shorter preschool, leukocyte telomere length is associated with obesity at age 9 in Latino children. Clin. Obes. 2018, 8, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamprokostopoulou, A.; Moschonis, G.; Manios, Y.; Critselis, E.; Nicolaides, N.C.; Stefa, A.; Koniari, E.; Gagos, S.; Charmandari, E. Childhood obesity and leucocyte telomere length. Eur. J. Clin. Investig. 2019, 49, e13178. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, X.; Gutin, B.; Davis, C.L.; Keeton, D.; Thomas, J.; Stallmann-Jorgensen, I.; Mooken, G.; Bundy, V.; Snieder, H.; et al. Leukocyte telomere length in healthy Caucasian and African-American adolescents: Relationships with race, sex, adiposity, adipokines, and physical activity. J. Pediatr. 2011, 158, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Benetos, A.; Okuda, K.; Lajemi, M.; Kimura, M.; Thomas, F.; Skurnick, J.; Labat, C.; Bean, K.; Aviv, A. Telomere length as an indicator of biological aging: The gender effect and relation with pulse pressure and pulse wave velocity. Hypertension 2001, 37, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Gems, D. Evolution of sexually dimorphic longevity in humans. Aging 2014, 6, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Horn, L. Development of the 2010 US Dietary Guidelines Advisory Committee Report: Perspectives from a registered dietitian. J. Am. Diet. Assoc. 2010, 110, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.M.; Ingwersen, L.A.; Moshfegh, A.J. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: An observational validation study. J. Am. Diet. Assoc. 2004, 104, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Baxter, S.D.; Smith, A.F.; Nichols, M.D.; Guinn, C.H.; Hardin, J.W. Children’s dietary reporting accuracy over multiple 24-hour recalls varies by body mass index category. Nutr. Res. 2006, 26, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiotis, D.; Krikos, X.; Tsiftis, G.; Hatzisymeon, M.; Maniati-Christidi, M.; Dacou-Voutetaki, A. Body mass index and prevalence of obesity in subjects of Hellenic origin aged 0–18 years living in the Athens area. Ann. Clin. Pediatr. Univ. Athen. 2004, 51, 139–154. [Google Scholar]
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic. Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Pan, X.; Liu, L.; Liu, N. Telomere length maintenance, shortening, and lengthening. J. Cell Physiol. 2014, 229, 1323–1329. [Google Scholar] [CrossRef]
- Cawthon, R.M.; Smith, K.R.; O’Brien, E.; Sivatchenko, A.; Kerber, R.A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003, 361, 393–395. [Google Scholar] [CrossRef]
- Sun, Q.; Shi, L.; Prescott, J.; Chiuve, S.E.; Hu, F.B.; De Vivo, I.; Stampfer, M.J.; Franks, P.W.; Manson, J.E.; Rexrode, K.M. Healthy lifestyle and leukocyte telomere length in U.S. women. PLoS ONE 2012, 7, e38374. [Google Scholar] [CrossRef]
- Carulli, L.; Anzivino, C.; Baldelli, E.; Zenobii, M.F.; Rocchi, M.B.; Bertolotti, M. Telomere length elongation after weight loss intervention in obese adults. Mol. Genet. Metab. 2016, 118, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Katre, P.A.; Joshi, S.M.; Kumaran, K.; Bhat, D.S.; Lubree, H.G.; Memane, N.; Kinare, A.S.; Pandit, A.N.; Bhave, S.A.; et al. Higher glucose, insulin and insulin resistance (HOMA-IR) in childhood predict adverse cardiovascular risk in early adulthood: The Pune Children’s Study. Diabetologia 2015, 58, 1626–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedemann, C.; Heneghan, C.; Mahtani, K.; Thompson, M.; Perera, R.; Ward, A.M. Cardiovascular disease risk in healthy children and its association with body mass index: Systematic review and meta-analysis. BMJ 2012, 345, e4759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef]
- Speliotes, E.K.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; Meigs, J.B.; Sahani, D.V.; Hirschhorn, J.N.; O’Donnell, C.J.; Fox, C.S. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: The Framingham Heart Study. Hepatology 2010, 51, 1979–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Jousilahti, P.; Stehouwer, C.D.; Soderberg, S.; Onat, A.; Laatikainen, T.; Yudkin, J.S.; Dankner, R.; Morris, R.; Tuomilehto, J.; et al. Cardiovascular and all-cause mortality in relation to various anthropometric measures of obesity in Europeans. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Al-Attas, O.S.; Al-Daghri, N.; Bamakhramah, A.; Shaun Sabico, S.; McTernan, P.; Huang, T.T. Telomere length in relation to insulin resistance, inflammation and obesity among Arab youth. Acta Paediatr. 2010, 99, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Zannolli, R.; Mohn, A.; Buoni, S.; Pietrobelli, A.; Messina, M.; Chiarelli, F.; Miracco, C. Telomere length and obesity. Acta Paediatr. 2008, 97, 952–954. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef] [Green Version]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Bacopoulou, F.; Efthymiou, V.; Landis, G.; Rentoumis, A.; Chrousos, G.P. Waist circumference, waist-to-hip ratio and waist-to-height ratio reference percentiles for abdominal obesity among Greek adolescents. BMC Pediatr. 2015, 15, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, B.C.; Koh, G.C.; Chen, C.; Wong, M.T.; Fallows, S.J. Comparison of Body Mass Index (BMI), Body Adiposity Index (BAI), Waist Circumference (WC), Waist-To-Hip Ratio (WHR) and Waist-To-Height Ratio (WHtR) as predictors of cardiovascular disease risk factors in an adult population in Singapore. PLoS ONE 2015, 10, e0122985. [Google Scholar] [CrossRef]
- Maffeis, C.; Banzato, C.; Talamini, G. Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children. J. Pediatr. 2008, 152, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.H.; Park, S.; Park, M.J.; Yoo, E.G. Waist-to-Height Ratio as an Index for Cardiometabolic Risk in Adolescents: Results from the 1998-2008 KNHANES. Yonsei Med. J. 2016, 57, 658–663. [Google Scholar] [CrossRef]
- Paltoglou, G.; Schoina, M.; Valsamakis, G.; Salakos, N.; Avloniti, A.; Chatzinikolaou, A.; Margeli, A.; Skevaki, C.; Papagianni, M.; Kanaka-Gantenbein, C. Interrelations among the adipocytokines leptin and adiponectin, oxidative stress and aseptic inflammation markers in pre-and early-pubertal normal-weight and obese boys. Endocrine 2017, 55, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Wojcicki, J.M.; Medrano, R.; Lin, J.; Epel, E. Increased Cellular Aging by 3 Years of Age in Latino, Preschool Children Who Consume More Sugar-Sweetened Beverages: A Pilot Study. Child. Obes. 2018, 14, 149–157. [Google Scholar] [CrossRef]
- Groop, P.H.; Forsblom, C.; Thomas, M.C. Mechanisms of disease: Pathway-selective insulin resistance and microvascular complications of diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2005, 1, 100–110. [Google Scholar] [CrossRef]
- Paltoglou, G.; Avloniti, A.; Chatzinikolaou, A.; Stefanaki, C.; Papagianni, M.; Papassotiriou, I.; Fatouros, I.G.; Chrousos, G.P.; Kanaka-Gantenbein, C.; Mastorakos, G. In early pubertal boys, testosterone and LH are associated with improved anti-oxidation during an aerobic exercise bout. Endocrine 2019, 66, 370–380. [Google Scholar] [CrossRef]
- Revesz, D.; Milaneschi, Y.; Verhoeven, J.E.; Penninx, B.W. Telomere length as a marker of cellular aging is associated with prevalence and progression of metabolic syndrome. J. Clin. Endocrinol. Metab. 2014, 99, 4607–4615. [Google Scholar] [CrossRef] [Green Version]
- Duarte, L.C.; Speakman, J.R. Low resting metabolic rate is associated with greater lifespan because of a confounding effect of body fatness. Age 2014, 36, 9731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinehr, T. Obesity and thyroid function. Mol. Cell Endocrinol. 2010, 316, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Richards, J.B.; Gardner, J.P.; Swaminathan, R.; Kimura, M.; Xiaobin, L.; Aviv, A.; Spector, T.D. Telomere length in leukocytes correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporos. Int. 2007, 18, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Jeanclos, E.; Schork, N.J.; Kyvik, K.O.; Kimura, M.; Skurnick, J.H.; Aviv, A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension 2000, 36, 195–200. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.; Wong, J.Y.; Michaud, D.; Hunter, D.J.; De Vivo, I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol. Prev. Biomark. 2007, 16, 815–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuda, K.; Bardeguez, A.; Gardner, J.P.; Rodriguez, P.; Ganesh, V.; Kimura, M.; Skurnick, J.; Awad, G.; Aviv, A. Telomere length in the newborn. Pediatr. Res. 2002, 52, 377–381. [Google Scholar] [CrossRef]

| A. Anthropometry | Initial assessment | Annual assessment | Pbetween timepoints | ||||||
| Obese | Overweight | Normal BMI | Pwithin baseline | Obese | Overweight | Normal BMI | Pwithin follow-up | ||
| Age (years) | 10.04 ± 0.20 | 10.48 ± 0.20 | 9.65 ± 0.39 | NS | 11.04 ± 0.22 * | 11.52 ± 0.21 * | 10.74 ± 0.44 * | NS | 0.01/0.01/0.01 |
| Body weight (kg) | 62.54 ± 1.42 | 52.64 ± 1.21 # | 38.61 ± 1.58 +,# | 0.01 | 64.06 ± 1.56 * | 55.13 ± 1.35 *,# | 43.84 ± 1.72 *,+,# | 0.01 | 0.01/0.01/0.01 |
| Height (cm) | 144.47 ± 1.17 | 145.43 ± 1.08 | 138.03 ± 2.02 | NS | 149.77 ± 1.26 * | 151.37 ± 1.14 * | 147.06 ± 2.06 * | NS | 0.01/0.01/0.01 |
| BMI (kg/m2) | 28.62 ± 0.28 | 24.21 ± 0.21 # | 19.54 ± 0.35 +,# | 0.01 | 27.15 ± 0.32 * | 23.58 ± 0.27 *,# | 19.81 ± 0.38 +,# | 0.01 | 0.01/0.01/NS |
| BMI z-score | 3.22 ± 0.07 | 1.53 ± 0.02 # | 0.14 ± 0.10 +,# | 0.01 | 2.41 ± 0.07 * | 1.18 ± 0.06 *,# | 0.25 ± 0.08 +,# | 0.01 | 0.01/0.01/NS |
| SBP (mmHg) | 114.10 ± 0.96 | 110.76 ± 0.83 | 104.48 ± 1.32 + | 0.05 | 114.26 ± 1.06 | 109.64 ± 1.17 # | 106.65 ± 2.12 # | 0.01 | NS/NS/NS |
| DBP (mmHg) | 67.02 ± 0.78 | 65.75 ± 0.78 | 61.00 ± 1.17 +,# | 0.01 | 68.41 ± 0.84 | 65.54 ± 0.82 | 63.70 ± 1.58 # | 0.05 | NS/NS/NS |
| Waist (cm) | 86.73 ± 0.90 | 78.42 ± 0.80 # | 67.49 ± 1.31 +,# | 0.01 | 87.47 ± 1.24 | 80.05 ± 1.27 # | 70.57 ± 2.10 +,# | 0.01 | NS/NS/NS |
| Hip (cm) | 92.05 ± 1.07 | 86.60 ± 1.01 # | 74.72 ± 1.66 +,# | 0.05 | 94.56 ± 1.36 | 89.08 ± 1.24 # | 81.89 ± 2.07 *,+,# | 0.01 | NS/NS/0.01 |
| Waist to Hip ratio | 0.96 ± 0.01 | 0.91 ± 0.01 # | 0.92 ± 0.02 | 0.05 | 0.93 ± 0.01 | 0.90 ± 0.01 # | 0.86 ± 0.02 *,# | 0.01 | NS/NS/0.05 |
| Waist to Height ratio | 0.60 ± 0.01 | 0.54 ± 0.01 # | 0.49 ± 0.01 +,# | 0.01 | 0.58 ± 0.01 * | 0.53 ± 0.01 # | 0.48 ± 0.01 +,# | 0.01 | 0.01/NS/NS |
| B. Body composition | Initial assessment | Annual assessment | Pbetween timepoints | ||||||
| Obese | Overweight | Normal BMI | Pwithin baseline | Obese | Overweight | Normal BMI | Pwithin follow-up | ||
| Fat Percentage (%) | 37.44 ± 0.38 | 32.23 ± 0.34 # | 25.6 ± 0.74 +,# | 0.01 | 35.49 ± 0.42 * | 31.09 ± 0.39 *,# | 25.02 ± 0.75 +,# | 0.01 | 0.01/0.01/NS |
| Fat Mass (kg) | 24.15 ± 0.69 | 17.17 ± 0.51 # | 10.37 ± 0.58 +,# | 0.01 | 23.76 ± 0.70 | 17.76 ± 0.57 # | 10.89 ± 0.60 +,# | 0.01 | NS/NS/NS |
| Muscle Mass (kg) | 36.77 ± 0.79 | 33.63 ± 0.73 | 27.55 ± 1.09 +,# | 0.01 | 39.48 ± 0.83 * | 36.80 ± 0.75 *,# | 29.94 ± 1.10 *,+,# | 0.01 | 0.01/0.01/0.01 |
| Bone mass (kg) | 1.99 ± 0.04 | 1.85 ± 0.04 | 1.54 ± 0.06 +,# | 0.01 | 2.13 ± 0.04 * | 2.00 ± 0.04 *,# | 1.66 ± 0.06 *,+,# | 0.01 | 0.01/0.01/0.01 |
| Fat Free Mass (kg) | 38.76 ± 0.83 | 35.47 ± 0.77 | 29.09 ± 1.15 +,# | 0.01 | 41.64 ± 0.88 * | 38.80 ± 0.79 *,# | 31.61 ± 1.15 *,+,# | 0.01 | 0.01/0.01/0.01 |
| Total Body Water (kg) | 28.38 ± 0.61 | 25.97 ± 0.56 | 21.29 ± 0.84 +,# | 0.01 | 30.46 ± 0.64 * | 28.40 ± 0.58 *,# | 23.15 ± 0.84 *,+,# | 0.01 | 0.01/0.01/0.01 |
| BMR (kJ) | 6491.92 ± 90.37 | 6027.06 ± 83.21 # | 5287.79 ± 102.68 +,# | 0.01 | 6693.43 ± 95.96 * | 6281.82 ± 84.99 *,# | 5464.08 ± 105.64 *,+,# | 0.01 | 0.01/0.01/0.01 |
| C. Hematology | Initial assessment | Annual assessment | Pbetween timepoints | ||||||
| Obese | Overweight | Normal BMI | Pwithin baseline | Obese | Overweight | Normal BMI | Pwithin follow-up | ||
| WBC | 7.74 ± 0.13 | 7.07 ± 0.15 # | 7.12 ± 0.27 # | 0.05 | 7.54 ± 0.14 * | 7.16 ± 0.17 | 6.45 ± 0.26 *,+,# | 0.01 | 0.05/NS/0.05 |
| RBC | 5.02 ± 0.05 | 5.06 ± 0.06 | 4.93 ± 0.07 | NS | 5.20 ± 0.20 | 5.05 ± 0.04 | 4.99 ± 0.08 | NS | NS/NS/NS |
| Hb | 12.97 ± 0.14 | 13.08 ± 0.19 | 12.75 ± 0.12 | NS | 12.94 ± 0.08 | 13.19 ± 0.09 *,# | 12.93 ± 0.15 | 0.05 | NS/0.01/NS |
| Hct | 40.49 ± 0.23 | 40.81 ± 0.32 | 40.15 ± 0.35 | NS | 40.56 ± 0.22 | 40.96 ± 0.25 | 40.29 ± 0.41 | NS | NS/NS/NS |
| PLT | 303.93 ± 4.20 | 292.74 ± 5.06 # | 297.03 ± 7.61 | 0.05 | 288.30 ± 5.27 * | 290.29 ± 4.81 | 275.93 ± 6.45 * | NS | 0.01/NS/0.05 |
| ESR | 19.34 ± 0.92 | 18.35 ± 1.01 | 12.38 ± 1.25 +,# | 0.05 | 18.65 ± 0.94 | 15.93 ± 0.98 | 13.75 ± 1.53 # | 0.01 | NS/NS/NS |
| Ferritin | 61.71 ± 2.89 | 56.90 ± 2.28 | 51.07 ± 3.26 | NS | 56.45 ± 2.37 * | 52.85 ± 2.80 | 48.08 ± 4.62 * | NS | 0.05/NS/0.05 |
| Folic Acid | 11.75 ± 0.39 | 12.79 ± 0.84 | 13.60 ± 1.54 # | 0.05 | 9.40 ± 0.49 * | 9.78 ± 0.43 * | 12.87 ± 1.65 # | 0.05 | 0.01/0.01/NS |
| D. Biochemistry | Initial assessment | Annual assessment | Pbetween timepoints | ||||||
| Obese | Overweight | Normal BMI | Pwithin baseline | Obese | Overweight | Normal BMI | Pwithin follow-up | ||
| Urea | 28.84 ± 0.42 | 28.59 ± 0.46 | 28.30 ± 0.88 | NS | 28.59 ± 0.46 | 28.56 ± 0.55 | 26.71 ± 0.94 | NS | NS/NS/NS |
| Creatinine | 0.49 ± 0.01 | 0.50 ± 0.01 | 0.46 ± 0.02 | NS | 0.52 ± 0.01 * | 0.52 ± 0.01 * | 0.52 ± 0.02 * | NS | 0.01/0.01/0.01 |
| Uric Acid | 7.53 ± 1.01 | 5.22 ± 0.58 | 5.97 ± 1.95 | NS | 4.88 ± 0.08 | 5.05 ± 0.45 | 6.72 ± 1.80 | NS | NS/NS/NS |
| K | 4.44 ± 0.02 | 4.37 ± 0.03 | 4.37 ± 0.05 | NS | 4.39 ± 0.84 | 4.43 ± 0.03 | 4.45 ± 0.07 | NS | NS/NS/NS |
| Na | 137.07 ± 1.33 | 138.60 ± 1.16 | 138.13 ± 2.27 | NS | 138.99 ± 1.00 | 139.04 ± 1.07 | 133.02 ± 4.60 | NS | NS/NS/NS |
| Cl | 103.76 ± 0.68 | 102.76 ± 0.55 | 104.66 ± 1.69 | NS | 101.03 ± 1.55 | 100.47 ± 2.39 | 107.00 ± 3.53 | NS | NS/NS/NS |
| AST | 23.88 ± 0.42 | 23.07 ± 0.45 | 23.33 ± 0.49 | NS | 22.05 ± 0.43 * | 22.33 ± 0.52 * | 24.19 ± 1.02 # | 0.05 | 0.01/0.05/NS |
| ALT | 22.36 ± 0.85 | 19.35 ± 0.71 # | 15.78 ± 0.71 +,# | 0.05 | 19.52 ± 0.60 * | 18.62 ± 0.72 | 17.52 ± 1.06 | NS | 0.01/NS/NS |
| γ-GT | 14.97 ± 0.42 | 13.19 ± 0.34 # | 11.08 ± 0.38 # | 0.01 | 13.66 ± 0.35 * | 13.54 ± 0.45 | 11.21 ± 0.43 +,# | 0.05 | 0.01/NS/NS |
| Albumin | 4.70 ± 0.03 | 4.72 ± 0.03 | 4.67 ± 0.07 | NS | 4.58 ± 0.02 * | 4.64 ± 0.03 | 4.68 ± 0.08 | NS | 0.01/NS/NS |
| ALP | 233.35 ± 5.49 | 231.02 ± 6.09 | 230.13 ± 10.15 | NS | 218.56 ± 5.67 * | 220.84 ± 7.09 * | 224.71 ± 14.56 | NS | 0.01/0.05/NS |
| PO4 | 4.77 ± 0.06 | 4.68 ± 0.04 | 5.39 ± 0.72 | NS | 4.94 ± 0.28 | 4.72 ± 0.04 | 4.63 ± 0.08 | NS | NS/NS/NS |
| Ca | 10.23 ± 0.38 | 9.83 ± 0.03 | 9.78 ± 0.04 | NS | 9.79 ± 0.02 * | 9.83 ± 0.03 | 9.74 ± 0.07 | NS | 0.01/NS/NS |
| E. CVD risk factors | Initial assessment | Annual assessment | Pbetween timepoints | ||||||
| Obese | Overweight | Normal BMI | Pwithin baseline | Obese | Overweight | Normal BMI | Pwithin follow-up | ||
| Cholesterol | 152.27 ± 2.40 | 154.90 ± 2.46 | 151.63 ± 4.60 | NS | 154.75 ± 2.20 | 155.45 ± 2.89 | 147.91 ± 6.33 | NS | NS/NS/NS |
| Triglycerides | 84.82 ± 2.68 | 69.74 ± 2.54 # | 69.40 ± 5.18 # | 0.05 | 82.74 ± 3.07 | 74.75 ± 3.40 * | 71.31 ± 5.97 | NS | NS/0.05/NS |
| HDL | 50.58 ± 0.87 | 53.46 ± 1.03 | 57.60 ± 1.68 # | 0.05 | 51.79 ± 0.96 | 55.81 ± 1.12 *,# | 59.48 ± 2.37 *,# | 0.01 | NS/0.01/0.05 |
| LDL | 89.57 ± 1.59 | 89.72 ± 1.95 | 82.95 ± 3.44 | NS | 88.63 ± 1.67 * | 87.02 ± 2.05 | 80.62 ± 2.90 | NS | 0.05/NS/NS |
| ApoA1 | 137.02 ± 1.66 | 139.80 ± 1.54 | 146.17 ± 3.66 | NS | 140.60 ± 1.69 | 143.59 ± 1.83 | 150.60 ± 3.32 # | 0.05 | NS/NS/NS |
| ApoB | 75.19 ± 1.20 | 72.34 ± 1.26 # | 68.03 ± 1.97 # | 0.01 | 72.61 ± 1.14 * | 70.05 ± 1.45 | 66.05 ± 2.11 # | 0.05 | 0.01/NS/NS |
| Lp(a) | 15.93 ± 1.47 | 15.27 ± 1.66 | 14.96 ± 2.66 | NS | 16.62 ± 1.80 | 14.38 ± 2.03 | 10.23 ± 1.92 | NS | NS/NS/NS |
| Glucose | 79.03 ± 0.57 | 78.35 ± 0.76 | 78.02 ± 1.00 | NS | 80.91 ± 0.47 * | 81.00 ± 0.64 * | 79.76 ± 0.99 | NS | 0.01/0.01/NS |
| HbA1c | 5.28 ± 0.02 | 5.22 ± 0.02 | 5.18 ± 0.03 | NS | 5.22 ± 0.02 * | 5.20 ± 0.02 | 5.19 ± 0.0 | NS | <0.01/NS/NS |
| HbA1 | 5.98 ± 0.03 | 5.91 ± 0.03 # | 5.93 ± 0.05 | 0.01 | 6.02 ± 0.03 * | 5.94 ± 0.03 * | 5.98 ± 0.05 * | NS | 0.05/0.01/0.05 |
| HOMA-IR | 3.62 ± 0.17 | 2.55 ± 0.12 # | 1.83 ± 0.16 # | 0.05 | 3.47 ± 0.17 | 2.91 ± 0.14 # | 1.98 ± 0.16 +,# | 0.01 | NS/NS/NS |
| QUICKI | 0.33 ± 0.01 | 0.34 ± 0.01 # | 0.37 ± 0.01 +,# | 0.01 | 0.33 ± 0.01 | 0.33 ± 0.01 * | 0.35 ± 0.01 *,+,# | 0.05 | NS/0.01/0.05 |
| F. Hormones | Initial assessment | Annual assessment | Pbetween timepoints | ||||||
| Obese | Overweight | Normal BMI | Pwithin baseline | Obese | Overweight | Normal BMI | Pwithin follow-up | ||
| TSH | 2.92 ± 0.09 | 2.93 ± 0.13 | 2.82 ± 0.20 | NS | 3.04 ± 0.12 | 2.94 ± 0.15 | 2.39 ± 0.17 +,# | 0.05 | NS/NS/NS |
| FT4 | 1.76 ± 0.55 | 1.13 ± 0.01 | 1.31 ± 0.16 | NS | 1.10 ± 0.01 | 1.10 ± 0.01 | 1.09 ± 0.03 | NS | NS/NS/NS |
| T3 | 148.68 ± 1.71 | 138.40 ± 2.15 # | 134.98 ± 3.59 # | 0.01 | 141.90 ± 2.23 * | 135.51 ± 2.61 *,# | 131.13 ± 4.00 # | 0.05 | 0.01/0.05/NS |
| AntiTG | 21.14 ± 0.66 | 22.86 ± 1.58 | 22.89 ± 1.95 | NS | 27.72 ± 5.56 | 26.51 ± 3.66 | 30.08 ± 7.54 | NS | NS/NS/NS |
| AntiTPO | 17.11 ± 4.13 | 30.23 ± 8.75 | 30.12 ± 16.22 | NS | 18.04 ± 5.11 | 32.10 ± 10.97 | 35.26 ± 22.98 | NS | NS/NS/NS |
| IGF1 | 304.23 ± 10.27 | 322.79 ± 14.29 | 269.96 ± 20.79 | NS | 387.42 ± 14.66 * | 394.87 ± 16.93 * | 336.14 ± 22.18 * | NS | 0.01/0.01/0.01 |
| IGFBP3 | 5.03 ± 0.07 | 5.11 ± 0.08 | 4.73 ± 0.15 | NS | 6.07 ± 0.68 | 5.35 ± 0.11 | 5.01 ± 0.15 | NS | NS/NS/NS |
| PRL | 11.29 ± 0.38 | 11.63 ± 0.51 | 11.73 ± 0.94 | NS | 12.51 ± 0.52 * | 12.47 ± 0.56 * | 12.53 ± 0.86 | NS | 0.01/0.01/NS |
| LH | 1.94 ± 0.19 | 2.92 ± 0.62 | 1.19 ± 0.22 | NS | 2.91 ± 0.26 * | 3.39 ± 0.40 | 2.74 ± 0.52 | NS | <0.01/NS/NS |
| FSH | 2.53 ± 0.12 | 2.86 ± 0.19 | 2.29 ± 0.22 | NS | 3.00 ± 0.15 * | 3.61 ± 0.26 *,# | 3.42 ± 0.37 * | 0.05 | 0.01/0.01/0.05 |
| ACTH | 34.35 ± 5.45 | 28.60 ± 1.81 | 22.14 ± 1.41 | NS | 29.67 ± 1.62 | 28.72 ± 1.82 | 25.20 ± 2.07 | NS | NS/NS/NS |
| Cortisol | 13.53 ± 0.40 | 14.17 ± 0.49 | 15.48 ± 2.31 +,# | 0.05 | 13.30 ± 0.44 | 13.41 ± 0.48 | 11.39 ± 0.60 * | NS | NS/NS/0.01 |
| PTH | 33.41 ± 1.88 | 33.22 ± 0.87 | 35.90 ± 0.90 | NS | 35.58 ± 2.06 | 34.55 ± 0.81 | 36.51 ± 0.91 | NS | NS/NS/NS |
| 25OHVitD | 24.29 ± 1.38 | 24.10 ± 0.73 | 25.74 ± 1.23 | NS | 23.81 ± 0.73 | 26.35 ± 2.32 | 28.23 ± 1.91 | NS | NS/NS/NS |
| Insulin | 18.24 ± 0.80 | 12.91 ± 0.57 # | 9.30 ± 0.76 +,# | 0.01 | 17.22 ± 0.82 | 14.48 ± 0.68 # | 9.81 ± 0.73 +,# | 0.01 | NS/NS/NS |
| SHBG | 43.18 ± 1.81 | 53.47 ± 2.49 # | 80.23 ± 5.79 +,# | 0.01 | 44.62 ± 2.09 | 52.87 ± 2.43 # | 73.41 ± 6.59 +,# | 0.01 | NS/NS/NS |
| G. LTL | Initial assessment | Annual assessment | Pbetween timepoints | ||||||
| Obese | Overweight | Normal BMI | Pwithin baseline | Obese | Overweight | Normal BMI | Pwithin follow-up | ||
| LTL | 1.35 ± 0.02 | 1.36 ± 0.02 | 1.33 ± 0.03 | NS | 1.41 ± 0.02 * | 1.42 ± 0.02 * | 1.38 ± 0.03 * | NS | 0.01/0.01/0.01 |
| A. anthropometry | Initial assessment | Annual assessment | Pbetween timepoints |
| Age (years) | 10.14 ± 0.13 | 11.23 ± 0,15 | <0.01 |
| Body weight (kg) | 55.99 ± 0.95 | 58.61 ± 1.03 * | <0.01 |
| Height (cm) | 143.95 ± 0.77 | 150.02 ± 0.82 * | <0.01 |
| BMI (kg/m2) | 25.91 ± 0.22 | 25.06 ± 0.24 * | <0.01 |
| BMI z-score | 2.25 ± 0.06 | 1.72 ± 0.06 * | <0.01 |
| SBP (mmHg) | 111.62 ± 0.62 | 112.05 ± 0.77 | NS |
| DBP (mmHg) | 65.75 ± 0.52 | 67.04 ± 0.58 | NS |
| Waist (cm) | 81.41 ± 0.65 | 83.37 ± 0.92 | NS |
| Hip (cm) | 87.95 ± 0.75 | 91.51 ± 0.93 | NS |
| Waist to Hip ratio | 0.94 ± 0.01 | 0.91 ± 0.01 | NS |
| Waist to Height ratio | 0.57 ± 0.01 | 0.55 ± 0.01 * | <0.01 |
| B. Body composition | Initial assessment | Annual assessment | Pbetween timepoints |
| Fat Percentage (%) | 34.09 ± 0.31 | 32.57 ± 0.32 * | <0.01 |
| Fat Mass (kg) | 19.92 ± 0.46 | 19.94 ± 0.47 | NS |
| Muscle Mass (kg) | 34.45 ± 0.52 | 37.23 ± 0.32 * | <0.01 |
| Bone mass (kg) | 1.88 ± 0.03 | 2.02 ± 0.03 * | <0.01 |
| Fat Free Mass (kg) | 36.33 ± 0.55 | 37.23 ± 0.54 * | <0.01 |
| Total Body Water (kg) | 26.60 ± 0.40 | 28.73 ± 0.42 * | <0.01 |
| BMR (kJ) | 6171.22 ± 59.48 | 6382.51 ± 62.21 | NS |
| C. Hematology | Initial assessment | Annual assessment | Pbetween timepoints |
| WBC | 7.43 ± 0.09 | 7.28 ± 0.10 * | <0.05 |
| RBC | 5.02 ± 0.03 | 5.12 ± 0.11 | NS |
| Hb | 12.88 ± 0.10 | 13.03 ± 0.06 * | <0.01 |
| Hct | 40.56 ± 0.17 | 40.67 ± 0.16 | NS |
| PLT | 299.21 ± 2.99 | 287.54 ± 3.37 * | <0.01 |
| ESR | 18.18 ± 0.63 | 17.14 ± 0.64 | NS |
| Ferritin | 58.66 ± 1.77 | 54.29 ± 1.69 * | <0.01 |
| Folic Acid | 13.17 ± 0.41 | 9.88 ± 0.36 * | <0.01 |
| D. Biochemistry | Initial assessment | Annual assessment | Pbetween timepoints |
| Urea | 28.69 ± 0.29 | 28.36 ± 0.33 | NS |
| Creatinine | 0.49 ± 0.01 | 0.52 ± 0.01 * | <0.01 |
| Uric Acid | 5.45 ± 0.62 | 5.15 ± 0.26 | NS |
| K | 4.41 ± 0.02 | 4.40 ± 0.05 | NS |
| Na | 137.73 ± 0.86 | 138.32 ± 0.84 | NS |
| Cl | 103.57 ± 0.47 | 101.53 ± 1.24 | NS |
| AST | 23.54 ± 0.28 | 22.39 ± 0.32 * | <0.01 |
| ALT | 20.50 ± 0.53 | 18.98 ± 0.43 * | <0.01 |
| γGT | 13.86 ± 0.26 | 13.34 ± 0.25 * | <0.01 |
| Albumin | 4.71 ± 0.02 | 4.61 ± 0.02 * | <0.01 |
| ALP | 232.13 ± 3.80 | 220.07 ± 4.25 * | <0.01 |
| PO4 | 4.82 ± 0.10 | 4.83 ± 0.15 | NS |
| Ca | 10.04 ± 0.20 | 9.80 ± 0.02 * | <0.01 |
| E. CVD risk factors | Initial assessment | Annual assessment | Pbetween timepoints |
| Cholesterol | 153.08 ± 1.64 | 154.22 ± 1.71 | NS |
| Triglycerides | 77.72 ± 1.82 | 78.64 ± 2.15 | NS |
| HDL | 52.46 ± 0.63 | 54.07 ± 0.71 * | <0.01 |
| LDL | 90.37 ± 1.16 | 87.15 ± 1.20 * | <0.01 |
| ApoA1 | 139.14 ± 1.13 | 142.80 ± 1.18 * | <0.05 |
| ApoB | 73.31 ± 0.81 | 70.98 ± 0.83 * | <0.01 |
| Lp(a) | 15.58 ± 1.02 | 15.12 ± 1.22 | NS |
| Glucose | 78.67 ± 0.42 | 80.81 ± 0.36 * | <0.01 |
| HbA1c | 5.25 ± 0.01 | 5.21 ± 0.01 * | <0.01 |
| HbA1 | 5.98 ± 0.02 | 5.99 ± 0.02 * | <0.01 |
| HOMA-IR | 3.02 ± 0.11 | 3.10 ± 0.10 | NS |
| QUICKI | 0.34 ± 0.01 | 0.33 ± 0.01 * | <0.01 |
| F. Hormones | Initial assessment | Annual assessment | Pbetween timepoints |
| TSH | 2.91 ± 0.07 | 2.93 ± 0.09 | NS |
| FT4 | 1.49 ± 0.29 | 1.61 ± 0.51 | NS |
| T3 | 143.38 ± 1.28 | 138.42 ± 1.58 * | <0.01 |
| AntiTG | 21.96 ± 0.69 | 27.57 ± 3.34 | NS |
| AntiTPO | 23.37 ± 4.29 | 24.98 ± 5.42 | NS |
| IGF1 | 306.18 ± 7.81 | 384.02 ± 10.17 * | <0.01 |
| IGBP3 | 5.02 ± 0.05 | 5.69 ± 0.37 | NS |
| PRL | 11.47 ± 0.29 | 12.50 ± 0.35 * | <0.01 |
| LH | 2.18 ± 0.24 | 3.06 ± 0.21 * | <0.01 |
| FSH | 2.62 ± 0.09 | 3.26 ± 0.13 * | <0.01 |
| ACTH | 30.79 ± 2.95 | 28.82 ± 1.10 | NS |
| Cortisol | 14.00 ± 0.40 | 13.11 ± 0.30 * | <0.01 |
| PTH | 34.64 ± 0,61 | 35.70 ± 0.61 | NS |
| 25OHVitD | 24.41 ± 0.78 | 25.24 ± 0.95 | NS |
| Insulin | 15.26 ± 0.50 | 15.40 ± 0.52 | NS |
| SHBG | 51.90 ± 1.62 | 50.97 ± 1.67 | NS |
| G. LTL | Initial assessment | Annual assessment | Pbetween timepoints |
| LTL | 1.35 ± 0.01 | 1.41 ± 0.01 * | <0.01 |
| Criterion A | YES (n = 193) | Pbetween timepoints | No (n = 214) | Pbetween timepoints | ||
| Initial assessment | Annual assessment | Initial assessment | Annual assessment | |||
| LTL | 1.35 ± 0.02 | 1.41 ± 0.02 * | <0.01 | 1.37 ± 0.02 | 1.43 ± 0.02 * | <0.01 |
| Criterion B | YES (n = 175) | Pbetween timepoints | No (n = 232) | Pbetween timepoints | ||
| Initial assessment | Annual assessment | Initial assessment | Annual assessment | |||
| LTL | 1.33 ± 0.02 | 1.40 ± 0.02 * | <0.01 | 1.38 ± 0.02 | 1.43 ± 0.02 * | <0.01 |
| LTL Initial Assessment | LTL Annual Assessment | Delta LTL | p | |
|---|---|---|---|---|
| Table 4A | ||||
| Waist circumference | β: −0.14 | β: −0.13 | - | <0.01/<0.05/NS |
| Table 4B | ||||
| BMR | β: −0.54 | β: −0.49 | - | <0.01/<0.01/NS |
| Bone mass | β: 0.43 | β: 0.13 | - | <0.05/<0.05/NS |
| Table 4C | ||||
| Waist circumference | β: −0.27 | - | - | <0.01/NS/NS |
| Waist to hip ratio | - | β: −0.15 | - | NS/<0.01/NS |
| Waist to height ratio | - | - | β: 0.41 | NS/NS/<0.05 |
| Table 4D | ||||
| Glucose | β: −0.39 | β: −0.19 | - | <0.01/0.01/NS |
| QUICKI | β: −0.3 | - | - | <0.05/NS/NS |
| Insulin | β: −1.1 | - | - | <0.05/NS/NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paltoglou, G.; Raftopoulou, C.; Nicolaides, N.C.; Genitsaridi, S.M.; Karampatsou, S.I.; Papadopoulou, M.; Kassari, P.; Charmandari, E. A Comprehensive, Multidisciplinary, Personalized, Lifestyle Intervention Program Is Associated with Increased Leukocyte Telomere Length in Children and Adolescents with Overweight and Obesity. Nutrients 2021, 13, 2682. https://doi.org/10.3390/nu13082682
Paltoglou G, Raftopoulou C, Nicolaides NC, Genitsaridi SM, Karampatsou SI, Papadopoulou M, Kassari P, Charmandari E. A Comprehensive, Multidisciplinary, Personalized, Lifestyle Intervention Program Is Associated with Increased Leukocyte Telomere Length in Children and Adolescents with Overweight and Obesity. Nutrients. 2021; 13(8):2682. https://doi.org/10.3390/nu13082682
Chicago/Turabian StylePaltoglou, George, Christina Raftopoulou, Nicolas C. Nicolaides, Sofia M. Genitsaridi, Sofia I. Karampatsou, Marina Papadopoulou, Penio Kassari, and Evangelia Charmandari. 2021. "A Comprehensive, Multidisciplinary, Personalized, Lifestyle Intervention Program Is Associated with Increased Leukocyte Telomere Length in Children and Adolescents with Overweight and Obesity" Nutrients 13, no. 8: 2682. https://doi.org/10.3390/nu13082682
APA StylePaltoglou, G., Raftopoulou, C., Nicolaides, N. C., Genitsaridi, S. M., Karampatsou, S. I., Papadopoulou, M., Kassari, P., & Charmandari, E. (2021). A Comprehensive, Multidisciplinary, Personalized, Lifestyle Intervention Program Is Associated with Increased Leukocyte Telomere Length in Children and Adolescents with Overweight and Obesity. Nutrients, 13(8), 2682. https://doi.org/10.3390/nu13082682







