Effect of the Interaction between Dietary Patterns and the Gastric Microbiome on the Risk of Gastric Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. 16S rRNA Gene Sequencing
2.4. Statistical Analysis
3. Results
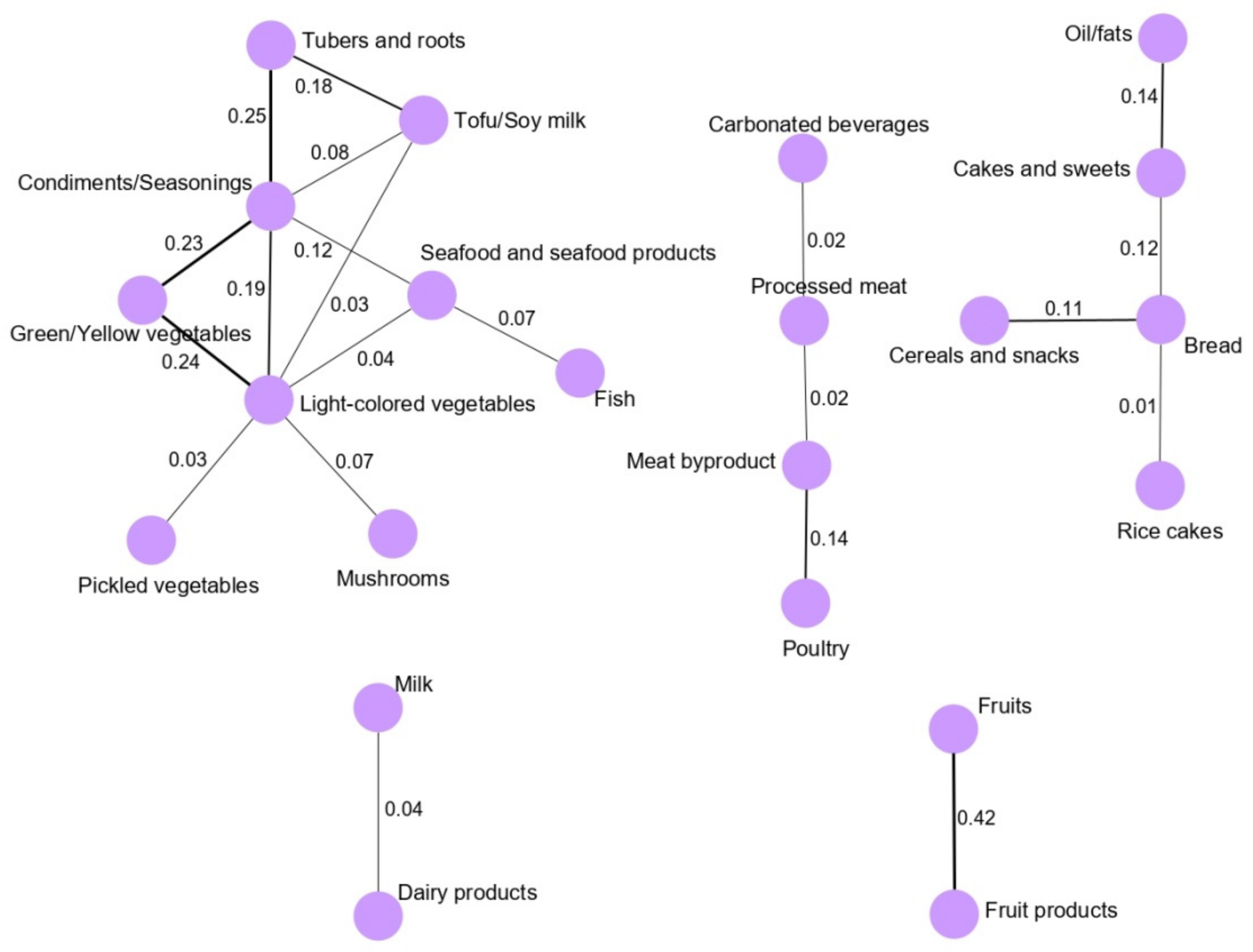
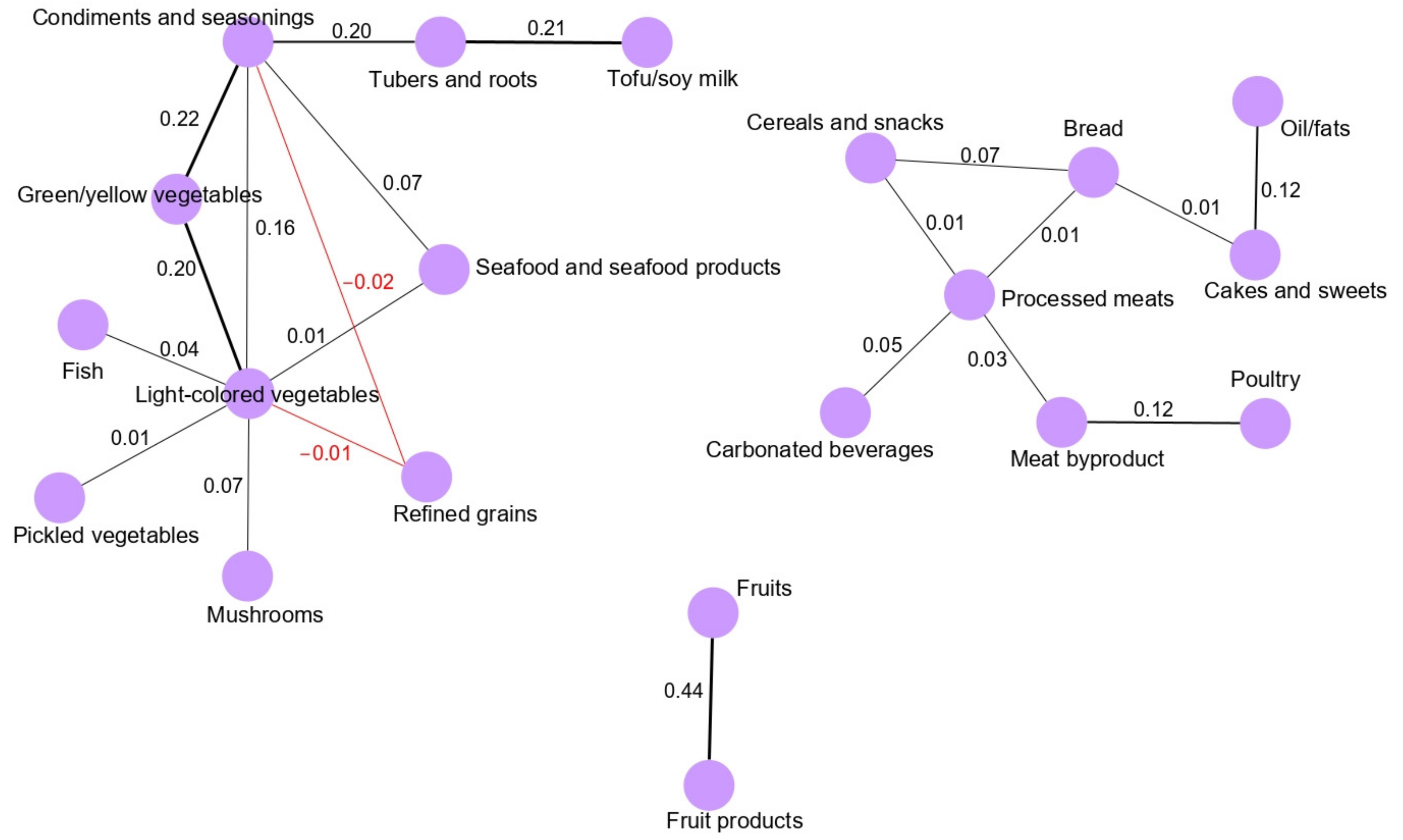
3.1. Dietary Pattern Networks Derived Using GGMs
3.2. Association between Dietary Patterns and GC Risk
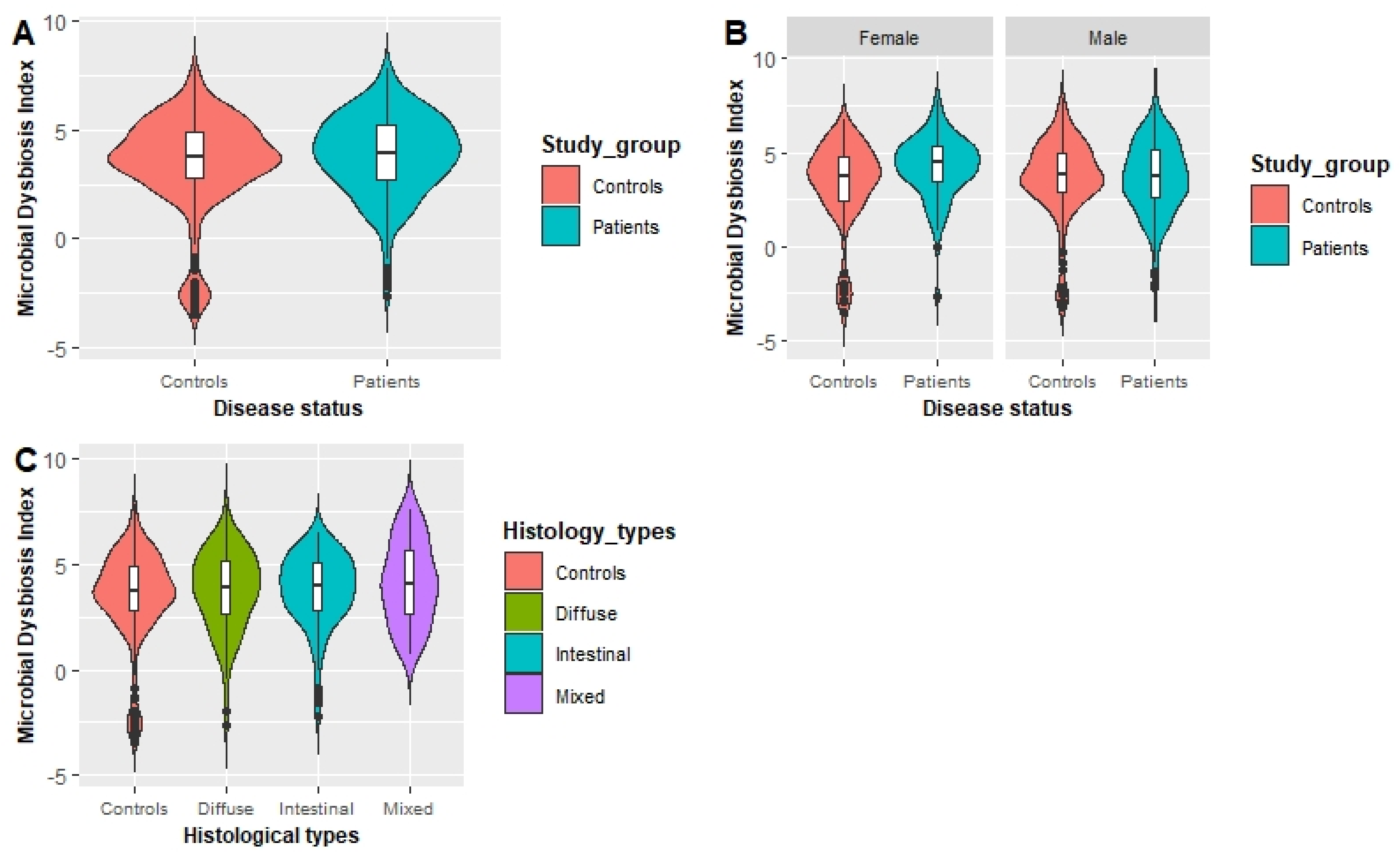
3.3. Association between MDI and the GC Risk
3.4. Effect of the Interaction between GGM-Derived Dietary Patterns and the Gastric Microbiome on the Risk of GC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- The Global Cancer Observatory. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/7-Stomach-fact-sheet.pdf (accessed on 20 July 2021).
- Hong, S.; Won, Y.J.; Lee, J.J.; Jung, K.W.; Kong, H.J.; Im, J.S.; Seo, H.G. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2018. Cancer Res. Treat. 2021, 53, 301–315. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.; Choi, I.J.; Kim, Y.-I.; Kim, J. Dietary patterns and gastric cancer risk in a Korean population: A case–control study. Eur. J. Nutr. 2020, 60, 389–397. [Google Scholar] [CrossRef]
- Gunathilake, M.; Lee, J.; Choi, I.J.; Kim, Y.-I.; Kim, J. Identification of Dietary Pattern Networks Associated with Gastric Cancer Using Gaussian Graphical Models: A Case-Control Study. Cancers 2020, 12, 1044. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nöthlings, U.; Boeing, H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef]
- De Stefani, E.; Correa, P.; Boffetta, P.; Deneo-Pellegrini, H.; Ronco, A.L.; Mendilaharsu, M. Dietary patterns and risk of gastric cancer: A case-control study in Uruguay. Gastric Cancer 2004, 7, 211–220. [Google Scholar] [CrossRef]
- Bastos, J.; Lunet, N.; Peleteiro, B.; Lopes, C.; Barros, H. Dietary patterns and gastric cancer in a Portuguese urban population. Int. J. Cancer 2010, 127, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Denova-Gutiérrez, E.; Hernández-Ramírez, R.U.; López-Carrillo, L. Dietary patterns and gastric cancer risk in Mexico. Nutr. Cancer 2014, 66, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Castelló, A.; de Larrea, N.F.; Martín, V.; Davila-Batista, V.; Boldo, E.; Guevara, M.; Moreno, V.; Castano-Vinyals, G.; Gómez-Acebo, I.; Fernandez-Tardon, G. High adherence to the Western, Prudent, and Mediterranean dietary patterns and risk of gastric adenocarcinoma: MCC-Spain study. Gastric Cancer 2018, 21, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Masaki, M.; Sugimori, H.; Nakamura, K.-I.; Tadera, M. Dietary patterns and stomach cancer among middle-aged male workers in Tokyo. Asian Pac. J. Cancer Prev. 2003, 4, 61–66. [Google Scholar]
- Kim, M.K.; Sasaki, S.; Sasazuki, S.; Tsugane, S. Prospective study of three major dietary patterns and risk of gastric cancer in Japan. Int. J. Cancer 2004, 110, 435–442. [Google Scholar] [CrossRef]
- Iqbal, K.; Buijsse, B.; Wirth, J.; Schulze, M.B.; Floegel, A.; Boeing, H. Gaussian graphical models identify networks of dietary intake in a German adult population. J. Nutr. 2016, 146, 646–652. [Google Scholar] [CrossRef]
- Iqbal, K.; Schwingshackl, L.; Floegel, A.; Schwedhelm, C.; Stelmach-Mardas, M.; Wittenbecher, C.; Galbete, C.; Knüppel, S.; Schulze, M.B.; Boeing, H. Gaussian graphical models identified food intake networks and risk of type 2 diabetes, CVD, and cancer in the EPIC-Potsdam study. Eur. J. Nutr. 2018, 58, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shao, L.; Liu, X.; Ji, F.; Mei, Y.; Cheng, Y.; Liu, F.; Yan, C.; Li, L.; Ling, Z. Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with gastric cancer. EBioMedicine 2019, 40, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Chen, B.; Pan, H.; Wang, D.; Liu, M.; Yang, Y.; Zou, M.; Yang, J.; Xiao, K.; Zhao, R. Detection of Microbial 16S rRNA Gene in the Serum of Patients With Gastric Cancer. Front. Oncol. 2019, 9, 608. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Vogtmann, E.; Liu, A.; Qin, J.; Chen, W.; Abnet, C.C.; Wei, W. Microbial characterization of esophageal squamous cell carcinoma and gastric cardia adenocarcinoma from a high-risk region of China. Cancer 2019, 125, 3993–4002. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, J.G.; Lee, A.R.; Eun, C.S.; Han, D.S. Network construction of gastric microbiome and organization of microbial modules associated with gastric carcinogenesis. Sci. Rep. 2019, 9, 12444. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef]
- Gunathilake, M.; Lee, J.; Choi, I.J.; Kim, Y.-I.; Yoon, J.; Sul, W.J.; Kim, J.F.; Kim, J. Alterations in gastric microbial communities are associated with risk of gastric cancer in a Korean population: A case-control study. Cancers 2020, 12, 2619. [Google Scholar] [CrossRef]
- Oriach, C.S.; Robertson, R.C.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Food for thought: The role of nutrition in the microbiota-gut–brain axis. Clin. Nutr. Exp. 2016, 6, 25–38. [Google Scholar] [CrossRef]
- Tindall, A.M.; Petersen, K.S.; Kris-Etherton, P.M. Dietary patterns affect the gut microbiome—The link to risk of cardiometabolic diseases. J. Nutr. 2018, 148, 1402–1407. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. Microbial modulation of cardiovascular disease. Nat. Rev. Microbiol. 2018, 16, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Shikany, J.M.; Demmer, R.T.; Johnson, A.J.; Fino, N.F.; Meyer, K.; Ensrud, K.E.; Lane, N.E.; Orwoll, E.S.; Kado, D.M.; Zmuda, J.M. Association of dietary patterns with the gut microbiota in older, community-dwelling men. Am. J. Clin. Nutr. 2019, 110, 1003–1014. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; la Storia, A.; Laghi, L.; Serrazanetti, D.I.; di Cagno, R.; Ferrocino, I.; Lazzi, C. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Shively, C.A.; Register, T.C.; Craft, S.; Yadav, H. Gut microbiome-Mediterranean diet interactions in improving host health. Research 2019, 8, 699. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, P.J.; Dowden, R.A.; Campbell, S.C. Role of dietary lipids in modulating inflammation through the gut microbiota. Nutrients 2019, 11, 117. [Google Scholar] [CrossRef]
- Woo, H.D.; Fernandez-Jimenez, N.; Ghantous, A.; Degli Esposti, D.; Cuenin, C.; Cahais, V.; Choi, I.J.; Kim, Y.I.; Kim, J.; Herceg, Z. Genome-wide profiling of normal gastric mucosa identifies Helicobacter pylori and cancer associated DNA methylome changes. Int. J. Cancer 2018, 143, 597–609. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.; Park, M.; Joo, Y.; Kim, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435. [Google Scholar] [CrossRef] [PubMed]
- Quantitative Insights Into Microbial Ecology. Available online: http://qiime.org/ (accessed on 20 July 2021).
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Schwager, E.; Weingart, G.; Bielski, C.; Huttenhower, C. CCREPE: Compositionality corrected by Permutation and Renormalization. Bioconductor 2014. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.683.4029&rep=rep1&type=pdf (accessed on 1 September 2020).
- AlShawaqfeh, M.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.; Steiner, J.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93, 136. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund International CUPD, Nutrition Physical Activity. Available online: http://www.Wcrf.Org/int/continuous-updateproject/cup-findings-reports/stomach-cancer (accessed on 20 July 2021).
- Hoang, B.V.; Lee, J.; Choi, I.J.; Kim, Y.-W.; Ryu, K.W.; Kim, J. Effect of dietary vitamin C on gastric cancer risk in the Korean population. World J. Gastroenterol. 2016, 22, 6257. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.; Choi, I.J.; Kim, Y.I.; Kwon, O.; Kim, H.; Kim, J. Dietary carotenoids intake and the risk of gastric cancer: A case-control study in Korea. Nutrients 2018, 10, 1031. [Google Scholar] [CrossRef] [PubMed]
- Bahmanyar, S.; Ye, W. Dietary patterns and risk of squamous-cell carcinoma and adenocarcinoma of the esophagus and adenocarcinoma of the gastric cardia: A population-based case-control study in Sweden. Nutr. Cancer 2006, 54, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yu, J. Effect of Helicobacter pylori infection on the composition of gastric microbiota in the development of gastric cancer. Gastrointest. Tumors 2015, 2, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Rodríguez, N.; Goh, K.-L.; Fock, K.M.; Mitchell, H.M.; Kaakoush, N.O. Dysbiosis of the microbiome in gastric carcinogenesis. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Chen, X.-H.; Wang, A.; Chu, A.-N.; Gong, Y.-H.; Yuan, Y. Mucosa-associated microbiota in gastric cancer tissues compared with non-cancer tissues. Front. Microbiol. 2019, 10, 1261. [Google Scholar] [CrossRef] [PubMed]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J.Y. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018, 67, 1024–1032. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Yan, H.; Terry, P.D.; Wang, J.-S.; Cheng, L.; Wu, W.-A.; Hu, S.-E. Interaction between dietary factors and Helicobacter pylori infection in noncardia gastric cancer: A population-based case-control study in China. J. Am. Coll. Nutr. 2012, 31, 375–384. [Google Scholar] [CrossRef]
- González, C.A.; López-Carrillo, L. Helicobacter pylori, nutrition and smoking interactions: Their impact in gastric carcinogenesis. Scand. J. Gastroenterol. 2010, 45, 6–14. [Google Scholar] [CrossRef]
- Epplein, M.; Nomura, A.M.; Hankin, J.H.; Blaser, M.J.; Perez-Perez, G.; Stemmermann, G.N.; Wilkens, L.R.; Kolonel, L.N. Association of Helicobacter pylori infection and diet on the risk of gastric cancer: A case–control study in Hawaii. Cancer Causes Control 2008, 19, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Machida-Montani, A.; Sasazuki, S.; Inoue, M.; Natsukawa, S.; Shaura, K.; Koizumi, Y.; Kasuga, Y.; Hanaoka, T.; Tsugane, S. Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer 2004, 7, 46–53. [Google Scholar] [CrossRef]
- Yanaka, A.; Fahey, J.W.; Fukumoto, A.; Nakayama, M.; Inoue, S.; Zhang, S.; Tauchi, M.; Suzuki, H.; Hyodo, Z.; Yamamoto, M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori–infected mice and humans. Cancer Prev. Res. 2009, 2, 353–360. [Google Scholar] [CrossRef]
- Tong, Y.; Gao, H.; Qi, Q.; Liu, X.; Li, J.; Gao, J.; Li, P.; Wang, Y.; Du, L.; Wang, C. High fat diet, gut microbiome and gastrointestinal cancer. Theranostics 2021, 11, 5889. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Matsuda, K.; Oishi, K. Probiotics: A Dietary Factor to Modulate the Gut Microbiome, Host Immune System, and Gut–Brain Interaction. Microorganisms 2020, 8, 1401. [Google Scholar] [CrossRef]
- Landskron, G.; de la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]
- Nasr, R.; Shamseddine, A.; Mukherji, D.; Nassar, F.; Temraz, S. The Crosstalk between microbiome and immune response in gastric cancer. Int. J. Mol. Sci. 2020, 21, 6586. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Wang, T.; Yu, G.-F.; Zhuang, D.-M.; Zhang, Z.; Zhang, H.-X.; Zhao, D.-P.; Yu, A.-L. Anti-proliferation effects of interferon-gamma on gastric cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 5513–5518. [Google Scholar] [CrossRef]
- Slattery, M.L. Defining dietary consumption: Is the sum greater than its parts? Am. J. Clin. Nutr. 2008, 88, 14–15. [Google Scholar] [CrossRef]
- Gorst-Rasmussen, A.; Dahm, C.C.; Dethlefsen, C.; Scheike, T.; Overvad, K. Respond to “Dietary Pattern Analysis”. Am. J. Epidemiol. 2011, 173, 1109–1110. [Google Scholar] [CrossRef]
- Assi, N.; Moskal, A.; Slimani, N.; Viallon, V.; Chajes, V.; Freisling, H.; Monni, S.; Knueppel, S.; Förster, J.; Weiderpass, E.; et al. A treelet transform analysis to relate nutrient patterns to the risk of hormonal receptor-defined breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2016, 19, 242–254. [Google Scholar] [CrossRef] [PubMed]

| All (n = 556) | Male (n = 353) | Female (n = 203) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Patients (n = 268) | Controls (n = 288) | p-Value ** | Patients (n = 172) | Controls (n = 181) | p-Value ** | Patients (n = 96) | Controls (n = 107) | p-Value ** |
| Age (year) | 53.68 ± 9.60 | 51.53 ± 7.21 | 0.003 | 54.69 ± 8.86 | 52.07 ± 6.46 | 0.002 | 51.86 ± 10.59 | 50.62 ± 8.29 | 0.355 |
| Sex [n (%)] | 0.745 | ||||||||
| Male | 172 (64.18) | 181 (62.85) | |||||||
| Female | 96 (35.82) | 107 (37.15) | |||||||
| Body mass index (kg/m2) [n (%)] | 23.91 ± 3.02 | 23.99 ± 3.11 | 0.747 | 24.30 ± 2.85 | 24.48 ± 3.04 | 0.573 | 23.21 ± 3.20 | 23.18 ± 3.07 | 0.939 |
| Smoking status [n (%)] | 0.006 | 0.006 | 0.243 | ||||||
| Current smoker | 78 (29.10) | 51 (17.71) | 75 (43.60) | 50 (27.62) | 3 (3.13) | 1 (0.93) | |||
| Ex-smoker | 80 (29.85) | 98 (34.03) | 74 (43.02) | 95 (52.49) | 6 (6.25) | 3 (2.80) | |||
| Nonsmoker | 109 (40.67) | 139 (48.26) | 23 (13.37) | 36 (19.89) | 86 (89.58) | 103 (96.26) | |||
| Missing | 1 (0.37) | 0 (0.00) | 1 (1.04) | 0 (0.00) | |||||
| Alcohol consumption [n (%)] | 0.559 | 0.618 | 0.860 | ||||||
| Current drinker | 163 (60.82) | 184 (63.89) | 123 (71.51) | 137 (75.69) | 40 (41.67) | 47 (43.93) | |||
| Ex-drinker | 26 (9.70) | 21 (7.29) | 21 (12.21) | 17 (9.39) | 5 (5.21) | 4 (3.74) | |||
| Nondrinker | 78 (29.10) | 83 (28.82) | 28 (16.28) | 27 (14.92) | 50 (52.08) | 56 (52.34) | |||
| Missing | 1 (0.37) | 0 (0.00) | 1 (1.04) | 0 (0.00) | |||||
| Family history of gastric cancer | 0.003 | 0.015 | 0.112 | ||||||
| Yes | 56 (20.90) | 34 (11.81) | 41 (23.84) | 25 (13.81) | 15 (15.63) | 9 (8.41) | |||
| No | 211 (78.73) | 254 (88.19) | 130 (75.58) | 156 (86.19) | 81 (84.38) | 98 (91.59) | |||
| Missing | 1 (0.37) | 0 (0.0) | |||||||
| Regular exercise [n (%)] | <0.001 | 0.079 | <0.001 | ||||||
| Yes | 95 (35.45) | 150 (52.08) | 69 (40.12) | 89 (49.17) | 26 (27.08) | 61 (57.01) | |||
| No | 173 (64.55) | 137 (47.57) | 103 (59.88) | 91 (50.28) | 70 (72.92) | 46 (42.99) | |||
| Missing | 0 (0.00) | 1 (0.35) | 0 (0.00) | 1 (0.55) | |||||
| Educational level [n (%)] | <0.001 | <0.001 | 0.001 | ||||||
| Middle school | 92 (34.33) | 42 (14.58) | 58 (33.72) | 25 (13.81) | 34 (35.42) | 17 (15.89) | |||
| High school | 116 (43.28) | 86 (29.86) | 77 (44.77) | 43 (23.76) | 39 (40.63) | 43 (40.19) | |||
| College or more | 58 (21.64) | 148 (51.39) | 36 (20.93) | 103 (56.91) | 22 (22.92) | 45 (42.06) | |||
| Missing | 2 (0.75) | 12 (4.17) | 1 (0.58) | 10 (5.52) | 1 (1.04) | 2 (1.87) | |||
| Occupation [n (%)] | 0.037 | 0.004 | 0.017 | ||||||
| Group 1: Professionals, administrative management | 44 (16.42) | 60 (20.83) | 37 (21.51) | 45 (24.86) | 7 (7.29) | 15 (14.02) | |||
| Group 2: Office, sales and service positions | 72 (26.87) | 98 (34.03) | 46 (26.74) | 74 (40.88) | 26 (27.08) | 24 (22.43) | |||
| Group 3: Agriculture, laborer | 65 (24.25) | 47 (16.32) | 51 (29.65) | 43 (23.76) | 14(14.58) | 4 (3.74) | |||
| Group 4: Unemployed and others | 85 (31.72) | 83 (28.82) | 37 (21.51) | 19 (10.50) | 48 (50.00) | 64 (59.81) | |||
| Missing | 2 (0.75) | 0 (0.00) | 1 (0.58) | 0 (0.00) | 1 (1.04) | 0 (0.00) | |||
| Marital status [n (%)] | 0.319 | 0.249 | 0.864 | ||||||
| Married | 234 (87.31) | 245 (85.07) | 155 (90.12) | 157 (86.74) | 79 (82.29) | 88 (82.24) | |||
| Others (single, divorced, separated, widowed, cohabitating) | 32 (11.94) | 43 (14.93) | 16 (9.30) | 24 (13.26) | 16 (16.67) | 19 (17.76) | |||
| Missing | 2 (0.75) | 0 (0.00) | 1 (0.58) | 0 (0.00) | 1 (1.04) | 0 (0.00) | |||
| Monthly income [n (%)] * | <0.001 | <0.001 | 0.084 | ||||||
| <200 | 79 (29.48) | 46 (15.97) | 49 (28.49) | 21 (11.60) | 30 (31.25) | 25 (23.36) | |||
| 200–400 | 101 (37.69) | 114 (39.58) | 70 (40.70) | 80 (44.20) | 31 (32.29) | 34 (31.78) | |||
| ≥400 | 59 (22.01) | 110 (38.19) | 34 (19.77) | 64 (35.36) | 25 (26.04) | 46 (42.99) | |||
| Missing | 29 (10.82) | 18 (6.25) | 19 (11.05) | 16 (8.84) | 10 (10.42) | 2 (1.87) | |||
| H. pylori infection | <0.001 | 0.008 | 0.004 | ||||||
| Positive | 267 (99.63) | 269 (93.40) | 171 (99.42) | 171 (94.48) | 96 (100.00) | 98 (91.59) | |||
| Negative | 1 (0.37) | 19 (6.60) | 1 (0.58) | 10 (5.52) | 0 (0.00) | 9(8.41) | |||
| Missing | |||||||||
| Lauren’s classification *** | NA | NA | NA | ||||||
| Intestinal | 105 (39.18) | NA | 89 (51.74) | NA | 16 (16.67) | NA | |||
| Diffuse | 109 (40.67) | NA | 51 (29.65) | NA | 58 (60.42) | NA | |||
| Mixed | 36 (13.43) | NA | 21 (12.21) | NA | 15 (15.63) | NA | |||
| Missing | 18 (6.72) | NA | 11 (6.40) | NA | 7 (7.29) | NA | |||
| Total energy intake (kcal/day) | 1934.24 ± 624.91 | 1766.35 ± 554.67 | <0.001 | 2057.70 ± 643.65 | 1839.30 ± 542.53 | 0.001 | 1713.03 ± 524.18 | 1642.95 ± 555.62 | 0.358 |
| Food groups intakes of networks (g/day) | |||||||||
| Vegetables and seafood | 326.50 ± 168.60 | 358.50 ± 213.70 | 0.049 | 937.30 ± 182.10 | 942.40 ± 192.90 | 0.800 | 374.50 ± 191.50 | 385.70 ± 215.10 | 0.698 |
| Meat and beverages | 424.00 ± 4835.70 | 467.60 ± 4652.60 | 0.914 | NA | NA | NA | NA | ||
| Meat and snacks | NA | NA | 663.50 ± 6069.00 | 340.60 ± 3077.20 | 0.526 | NA | NA | ||
| Meats | NA | NA | NA | NA | 80.70 ± 119.90 | 94.67 ± 147.60 | 0.458 | ||
| Snacks | 61.45 ± 113.60 | 77.73 ± 178.30 | 0.197 | NA | NA | 77.98 ± 144.90 | 124.50 ± 265.00 | 0.117 | |
| Dairy | 120.80 ± 269.60 | 183.20 ± 377.60 | 0.025 | NA | NA | 190.40 ± 384.80 | 264.10 ± 422.10 | 0.197 | |
| Fruits | 135.00 ± 159.90 | 192.60 ± 207.80 | <0.001 | 116.00 ± 148.40 | 154.60 ± 176.40 | 0.027 | 169.00 ± 174.50 | 256.70 ± 239.80 | 0.003 |
| Dietary Patterns | No. of Controls | No. of Patients | Model I OR (95% CI) | Model II OR (95% CI) | Model III OR (95% CI) |
|---|---|---|---|---|---|
| Vegetables and seafood | |||||
| T1 (Low) | 95 (33.0) | 97 (36.2) | 1.00 | 1.00 | 1.00 |
| T2 (Medium) | 96 (33.3) | 97 (36.2) | 0.99 (0.66–1.48) | 1.05 (0.66–1.66) | 1.05 (0.66–1.67) |
| T3 (High) | 97 (33.7) | 74 (27.6) | 0.75 (0.50–1.13) | 0.74 (0.46–1.20) | 0.74 (0.45–1.20) |
| p for trend | 0.142 | 0.180 | 0.186 | ||
| Meat and beverages | |||||
| T1 (Low) | 96 (33.3) | 109 (40.7) | 1.00 | 1.00 | 1.00 |
| T2 (Medium) | 95 (33.0) | 87 (32.5) | 0.81 (0.54–1.20) | 1.02 (0.63–1.66) | 1.06 (0.65–1.72) |
| T3 (High) | 97 (33.7) | 72 (26.9) | 0.65 (0.43–0.98) | 1.17 (0.63–2.18) | 1.17 (0.63–2.19) |
| p for trend | 0.056 | 0.579 | 0.628 | ||
| Snacks | |||||
| T1 (Low) | 95 (33.0) | 92 (34.3) | 1.00 | 1.00 | 1.00 |
| T2 (Medium) | 96 (33.3) | 106 (40.0) | 1.14 (0.77–1.70) | 1.26 (0.80–1.98) | 1.34 (0.85–2.13) |
| T3 (High) | 97 (33.7) | 70 (26.1) | 0.75 (0.50–1.14) | 1.28 (0.75–2.16) | 1.30 (0.77–2.22) |
| p for trend | 0.087 | 0.455 | 0.441 | ||
| Dairy | |||||
| T1 (Low) | 96 (33.3) | 133 (50.0) | 1.00 | 1.00 | 1.00 |
| T2 (Medium) | 95 (33.0) | 67 (25.0) | 0.51 (0.34–0.77) | 0.58 (0.36–0.92) | 0.57 (0.36–0.91) |
| T3 (High) | 97 (33.7) | 68 (25.4) | 0.51 (0.34–0.76) | 0.71 (0.43–1.17) | 0.70 (0.43–1.17) |
| p for trend | 0.007 | 0.380 | 0.378 | ||
| Fruits | |||||
| T1 (Low) | 95 (33.0) | 129 (48.1) | 1.00 | 1.00 | 1.00 |
| T2 (Medium) | 96 (33.3) | 87 (32.5) | 0.67 (0.45–0.98) | 0.85 (0.54–1.33) | 0.87 (0.55–1.37) |
| T3 (High) | 97 (33.7) | 52 (19.4) | 0.40 (0.26–0.61) | 0.45 (0.27–0.74) | 0.47 (0.28–0.77) |
| p for trend | <0.001 | 0.001 | 0.003 |
| Dietary Patterns | No. of Controls | No. of Patients | Model I OR (95% CI) | Model II OR (95% CI) | Model III OR (95% CI) |
|---|---|---|---|---|---|
| Vegetables and seafood | |||||
| T1 (Low) | 35 (32.7) | 34 (35.4) | 1.00 | 1.00 | 1.00 |
| T2 (Medium) | 37 (34.6) | 30 (31.3) | 0.84 (0.43–1.64) | 0.87 (0.40–1.87) | 0.84 (0.38–1.86) |
| T3 (High) | 35 (32.7) | 32 (33.3) | 0.94 (0.48–1.84) | 0.95 (0.44–2.05) | 0.91 (0.41–2.00) |
| p for trend | 0.911 | 0.925 | 0.844 | ||
| Meats | |||||
| T1 (Low) | 36 (33.6) | 37 (38.5) | 1.00 | 1.00 | 1.00 |
| T2 (Medium) | 36 (33.6) | 31 (32.3) | 0.84 (0.43–1.63) | 0.84 (0.38–1.86) | 1.00 (0.44–2.26) |
| T3 (High) | 35 (32.7) | 28 (29.2) | 0.78 (0.40–1.53) | 0.75 (0.29–1.98) | 0.78 (0.29–2.06) |
| p for trend | 0.515 | 0.603 | 0.568 | ||
| Snacks | |||||
| T1 (Low) | 36 (33.6) | 34 (35.4) | 1.00 | 1.00 | 1.00 |
| T2 (Medium) | 35 (32.7) | 45 (46.9) | 1.36 (0.72–2.60) | 1.27 (0.60–2.71) | 1.51 (0.70–3.28) |
| T3 (High) | 36 (33.6) | 17 (17.7) | 0.50 (0.24–1.05) | 0.42 (0.16–1.14) | 0.45 (0.16–1.23) |
| p for trend | 0.025 | 0.051 | 0.065 | ||
| Dairy | |||||
| T1 (Low) | 36 (33.6) | 47 (49.0) | 1.00 | 1.00 | 1.00 |
| T2 (Medium) | 36 (33.6) | 28 (29.2) | 0.60 (0.31–1.15) | 0.82 (0.38–1.74) | 0.73 (0.34–1.58) |
| T3 (High) | 35 (32.7) | 21 (21.8) | 0.46 (0.23–0.92) | 0.68 (0.28–1.63) | 0.67 (0.28–1.63) |
| p for trend | 0.041 | 0.411 | 0.421 | ||
| Fruits | |||||
| T1 (Low) | 36 (33.6) | 60 (62.5) | 1.00 | 1.00 | 1.00 |
| T2 (Medium) | 35 (32.7) | 15 (15.6) | 0.26 (0.12–0.54) | 0.24 (0.10–0.54) | 0.24 (0.10–0.56) |
| T3 (High) | 36 (33.6) | 21 (21.9) | 0.35 (0.18–0.69) | 0.40 (0.18–0.86) | 0.38 (0.17–0.83) |
| p for trend | 0.003 | 0.023 | 0.021 |
| MDI | No. of Controls (%) | No. of Patients (%) | Model I OR (95% CI) | Model II OR (95% CI) |
|---|---|---|---|---|
| Total | ||||
| T1(<3.18) | 96 (33.3) | 91 (33.9) | 1.00 | 1.00 |
| T2(3.18–4.52) | 97 (33.7) | 75 (27.9) | 0.82 (0.54–1.24) | 0.97 (0.60–1.57) |
| T3(≥4.52) | 95 (33.0) | 102 (38.1) | 1.13 (0.76–1.69) | 1.37 (0.86–2.17) |
| p for trend | 0.561 | 0.179 | ||
| Male | ||||
| T1(<3.25) | 60 (33.2) | 74 (43.0) | 1.00 | 1.00 |
| T2(3.25–4.48) | 60 (33.2) | 42 (24.4) | 0.57 (0.34–0.96) | 0.80 (0.43–1.52) |
| T3(≥4.48) | 61 (33.7) | 56 (32.6) | 0.74 (0.45–1.22) | 1.15 (0.63–2.11) |
| p for trend | 0.225 | 0.657 | ||
| Female | ||||
| T1(<3.04) | 36 (33.6) | 18 (18.8) | 1.00 | 1.00 |
| T2(3.04–4.52) | 36 (33.6) | 31 (32.3) | 1.72 (0.82–3.62) | 1.69 (0.71–4.02) |
| T3(≥4.52) | 35 (32.7) | 47 (48.9) | 2.69 (1.31–5.49) | 2.66 (1.19–5.99) |
| p for trend | 0.006 | 0.017 | ||
| Intestinal | ||||
| T1(<3.19) | 96 (33.3) | 37 (35.2) | 1.00 | 1.00 |
| T2(3.19–4.52) | 97 (33.7) | 31 (29.5) | 0.83 (0.48–1.44) | 1.17 (0.57–2.37) |
| T3(≥4.52) | 96 (33.3) | 37 (35.2) | 1.01 (0.59–1.73) | 1.15 (0.58–2.27) |
| p for trend | 0.992 | 0.694 | ||
| Diffuse | ||||
| T1(<3.19) | 96 (33.3) | 35 (32.1) | 1.00 | 1.00 |
| T2(3.19–4.52) | 97 (33.7) | 30 (27.5) | 0.85 (0.48–1.49) | 0.87 (0.46–1.63) |
| T3(≥4.52) | 95 (33.0) | 44 (40.4) | 1.27 (0.75–2.15) | 1.31 (0.73–2.36) |
| p for trend | 0.376 | 0.356 | ||
| Mixed | ||||
| T1(<3.15) | 96 (33.3) | 15 (41.7) | 1.00 | 1.00 |
| T2(3.15–4.50) | 97 (33.7) | 09 (25.0) | 0.59 (0.25–1.42) | 0.66 (0.24–1.81) |
| T3(≥4.50) | 95 (33.0) | 12 (33.3) | 0.81 (0.36–1.82) | 0.92 (0.37–2.31) |
| p for trend | 0.558 | 0.838 |
| Dietary Patterns | MDI [Low: <3.88] | MDI [High: ≥3.88] | |||
|---|---|---|---|---|---|
| Low | High | Low | High | p-Interaction | |
| Vegetables and seafood | |||||
| No. Controls/Patients | 44/54 | 47/40 | 46/39 | 44/39 | |
| Crude OR (95% CI) | 1.00 (ref) | 0.69 (0.39–1.24) | 0.69 (0.38–1.24) | 0.72 (0.40–1.30) | 0.337 |
| Model I OR (95% CI) | 1.00 (ref) | 0.44 (0.22–0.89) | 0.70 (0.34–1.42) | 1.02 (0.50–2.10) | 0.021 |
| Model II OR (95% CI) | 1.00 (ref) | 0.44 (0.22–0.91) | 0.63 (0.31–1.30) | 0.94 (0.45–1.95) | 0.021 |
| Meats and snacks | |||||
| No. Controls/Patients | 40/58 | 51/36 | 51/45 | 39/33 | |
| Crude OR (95% CI) | 1.00 (ref) | 0.49 (0.27–0.88) | 0.61 (0.35–1.07) | 0.58 (0.32–1.08) | 0.117 |
| Model I OR (95% CI) | 1.00 (ref) | 0.68 (0.31–1.50) | 0.82 (0.41–1.64) | 1.37 (0.59–3.13) | 0.090 |
| Model II OR (95% CI) | 1.00 (ref) | 0.66 (0.30–1.46) | 0.74 (0.37–1.50) | 1.20 (0.52–2.77) | 0.089 |
| Fruit | |||||
| No. Controls/Patients | 46/60 | 45/34 | 45/42 | 45/36 | |
| Crude OR (95% CI) | 1.00 (ref) | 0.58 (0.32–1.04) | 0.72 (0.41–1.27) | 0.61 (0.34–1.09) | 0.363 |
| Model I OR (95% CI) | 1.00 (ref) | 0.57 (0.28–1.18) | 0.92 (0.46–1.85) | 0.98 (0.48–2.03) | 0.228 |
| Model II OR (95% CI) | 1.00 (ref) | 0.63 (0.31–1.32) | 0.87 (0.43–1.77) | 0.95 (0.46–1.97) | 0.305 |
| Dietary Pattern | MDI [Low: <3.88] | MDI [High: ≥3.88] | |||
|---|---|---|---|---|---|
| Low | High | Low | High | p–Interaction | |
| Vegetables and seafood | |||||
| No. Controls/Patients | 29/18 | 25/15 | 24/27 | 29/36 | |
| Crude OR (95% CI) | 1.00 (ref) | 0.97 (0.41–2.31) | 1.81 (0.81–4.05) | 2.00 (0.93–4.30) | 0.820 |
| Model I OR (95% CI) | 1.00 (ref) | 0.92 (0.34–2.47) | 2.24 (0.89–5.65) | 1.65 (0.67–4.07) | 0.733 |
| Model II OR (95% CI) | 1.00 (ref) | 1.02 (0.36–2.86) | 1.93 (0.75–4.97) | 1.40 (0.56–3.53) | 0.620 |
| Meats | |||||
| No. Controls/Patients | 27/20 | 27/13 | 26/36 | 27/27 | |
| Crude OR (95% CI) | 1.00 (ref) | 0.65 (0.27–1.57) | 1.87 (0.87–4.03) | 1.35 (0.62–2.97) | 0.856 |
| Model I OR (95% CI) | 1.00 (ref) | 0.56 (0.18–1.66) | 2.06 (0.85–5.00) | 1.05 (0.39–2.82) | 0.896 |
| Model II OR (95% CI) | 1.00 (ref) | 0.41 (0.13–1.27) | 1.42 (0.56–3.62) | 0.70 (0.25–1.98) | 0.776 |
| Snacks | |||||
| No. Controls/Patients | 29/27 | 25/6 | 25/37 | 28/26 | |
| Crude OR (95% CI) | 1.00 (ref) | 0.26 (0.10–0.73) | 1.59 (0.77–3.30) | 0.99 (0.47–2.11) | 0.162 |
| Model I OR (95% CI) | 1.00 (ref) | 0.23 (0.07–0.74) | 1.43 (0.62–3.30) | 1.00 (0.41–2.45) | 0.120 |
| Model II OR (95% CI) | 1.00 (ref) | 0.25 (0.08–0.86) | 1.22 (0.52–2.86) | 0.86 (0.35–2.15) | 0.162 |
| Dairy | |||||
| No. Controls/Patients | 23/25 | 31/8 | 31/37 | 22/26 | |
| Crude OR (95% CI) | 1.00 (ref) | 0.24 (0.10–0.62) | 1.09 (0.52–2.30) | 1.09 (0.49–2.42) | 0.018 |
| Model I OR (95% CI) | 1.00 (ref) | 0.27 (0.08–0.82) | 1.01 (0.43–2.41) | 1.29 (0.50–3.33) | 0.025 |
| Model II OR (95% CI) | 1.00 (ref) | 0.23 (0.07–0.76) | 0.77 (0.31–1.89) | 0.98 (0.37–2.63) | 0.018 |
| Fruits | |||||
| No. Controls/Cases | 28/23 | 26/10 | 26/45 | 27/18 | |
| Crude OR (95% CI) | 1.00 (ref) | 0.47 (0.18–1.17) | 2.11 (1.01–4.39) | 0.81 (0.36–1.83) | 0.749 |
| Model I OR (95% CI) | 1.00 (ref) | 0.82 (0.28–2.34) | 3.12 (1.31–7.45) | 0.85 (0.33–2.17) | 0.119 |
| Model II OR (95% CI) | 1.00 (ref) | 0.71 (0.24–2.11) | 2.39 (0.97–5.84) | 0.65 (0.25–1.71) | 0.182 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunathilake, M.; Lee, J.; Choi, I.J.; Kim, Y.-I.; Kim, J. Effect of the Interaction between Dietary Patterns and the Gastric Microbiome on the Risk of Gastric Cancer. Nutrients 2021, 13, 2692. https://doi.org/10.3390/nu13082692
Gunathilake M, Lee J, Choi IJ, Kim Y-I, Kim J. Effect of the Interaction between Dietary Patterns and the Gastric Microbiome on the Risk of Gastric Cancer. Nutrients. 2021; 13(8):2692. https://doi.org/10.3390/nu13082692
Chicago/Turabian StyleGunathilake, Madhawa, Jeonghee Lee, Il Ju Choi, Young-Il Kim, and Jeongseon Kim. 2021. "Effect of the Interaction between Dietary Patterns and the Gastric Microbiome on the Risk of Gastric Cancer" Nutrients 13, no. 8: 2692. https://doi.org/10.3390/nu13082692
APA StyleGunathilake, M., Lee, J., Choi, I. J., Kim, Y.-I., & Kim, J. (2021). Effect of the Interaction between Dietary Patterns and the Gastric Microbiome on the Risk of Gastric Cancer. Nutrients, 13(8), 2692. https://doi.org/10.3390/nu13082692






