Urinary Potassium and Kidney Function Decline in the Population—Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurements
2.2. Statistical Analyses
3. Results
3.1. Descriptive Statistics
3.2. Analyses by Sex- and Age- Controlled uK/Cr Quintiles
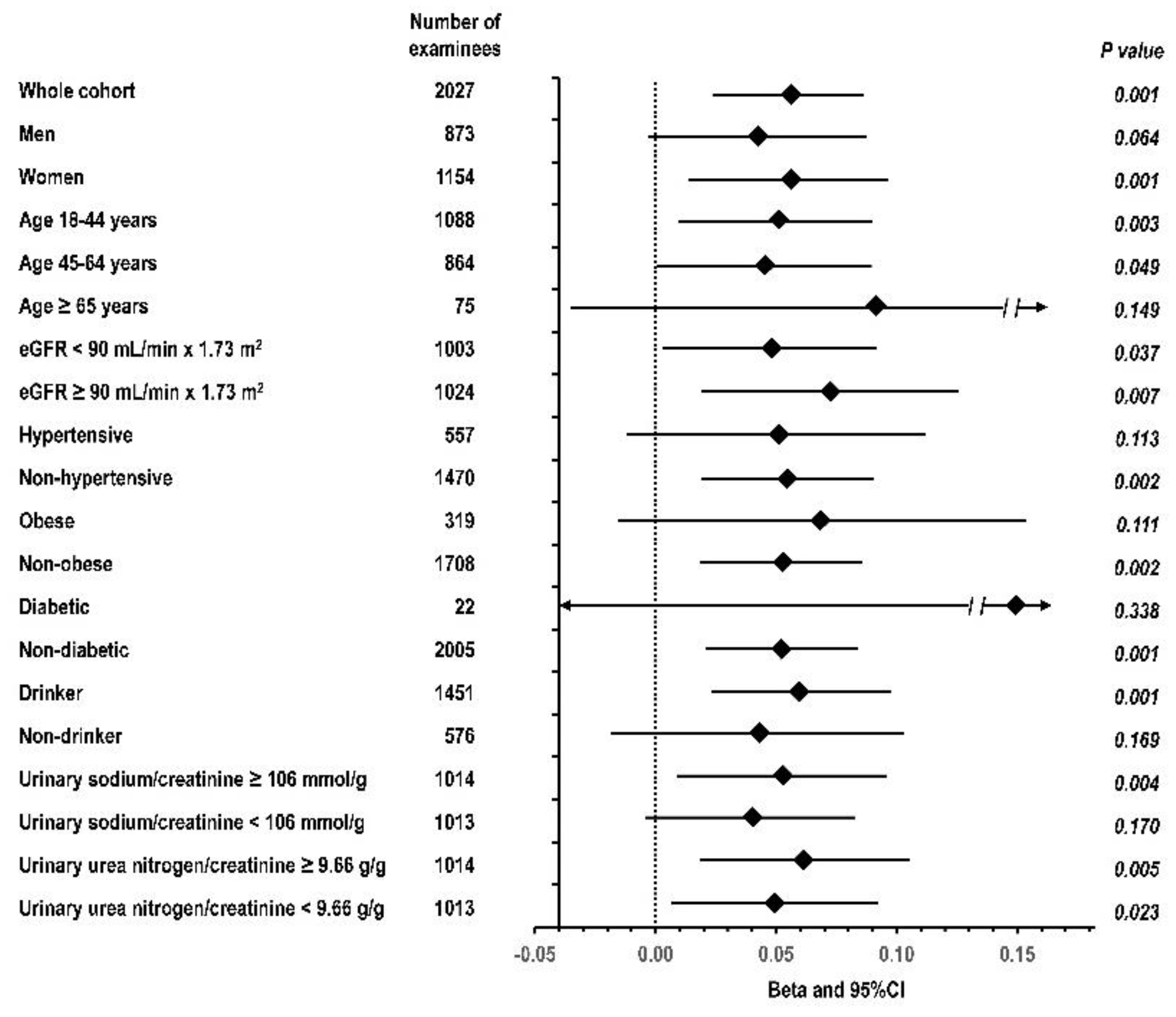
3.3. Multivariable Regression Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Pugh, D.; Gallacher, P.J.; Dhaun, N. Management of Hypertension in Chronic Kidney Disease. Drugs 2019, 79, 365–379. [Google Scholar] [CrossRef]
- Chung, S.; Kim, G.-H. Use of Anti-Diabetic Agents in Non-Diabetic Kidney Disease: From Bench to Bedside. Life 2021, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Levey, A.S. Dietary protein and renal function. J. Am. Soc. Nephrol. 1993, 3, 1723–1737. [Google Scholar] [CrossRef]
- Cheng, C.-J.; Kuo, E.; Huang, C.-L. Extracellular Potassium Homeostasis: Insights from Hypokalemic Periodic Paralysis. Semin. Nephrol. 2013, 33, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Hoorn, E.J.; Gritter, M.; Cuevas, C.A.; Fenton, R.A. Regulation of the Renal NaCl Cotransporter and Its Role in Potassium Homeostasis. Physiol. Rev. 2020, 100, 321–356. [Google Scholar] [CrossRef]
- Kieneker, L.M.; Bakker, S.J.; de Boer, R.A.; Navis, G.J.; Gansevoort, R.T.; Joosten, M.M. Low potassium excretion but not high sodium excretion is associated with increased risk of developing chronic kidney disease. Kidney Int. 2016, 90, 888–896. [Google Scholar] [CrossRef]
- Deriaz, D.; Guessous, I.; Vollenweider, P.; Devuyst, O.; Burnier, M.; Bochud, M.; Ponte, B. Estimated 24-h urinary sodium and sodium-to-potassium ratio are predictors of kidney function decline in a population-based study. J. Hypertens. 2019, 37, 1853–1860. [Google Scholar] [CrossRef]
- Tabara, Y.; Takahashi, Y.; Setoh, K.; Kawaguchi, T.; Kosugi, S.; Nakayama, T.; Matsuda, F. The Nagahama Study Group Prognostic Significance of Spot Urine Na/K for Longitudinal Changes in Blood Pressure and Renal Function: The Nagahama Study. Am. J. Hypertens. 2017, 30, 899–906. [Google Scholar] [CrossRef]
- Smyth, A.; Griffin, M.; Yusuf, S.; Mann, J.F.; Reddan, D.; Canavan, M.; Newell, J.; O’Donnell, M. Diet and Major Renal Outcomes: A Prospective Cohort Study. The NIH-AARP Diet and Health Study. J. Ren. Nutr. 2016, 26, 288–298. [Google Scholar] [CrossRef]
- Mirmiran, P.; Nazeri, P.; Bahadoran, Z.; Khalili-Moghadam, S.; Azizi, F. Dietary Sodium to Potassium Ratio and the Incidence of Chronic Kidney Disease in Adults: A Longitudinal Follow-Up Study. Prev. Nutr. Food Sci. 2018, 23, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.-Y.; Gritter, M.; Vogt, L.; De Borst, M.H.; Rotmans, J.; Hoorn, E.J. Dietary potassium and the kidney: Lifesaving physiology. Clin. Kidney J. 2020, 13, 952–968. [Google Scholar] [CrossRef] [PubMed]
- Mun, K.H.; Yu, G.I.; Choi, B.Y.; Kim, M.K.; Shin, M.-H.; Shin, D.H. Association of Dietary Potassium Intake with the Development of Chronic Kidney Disease and Renal Function in Patients with Mildly Decreased Kidney Function: The Korean Multi-Rural Communities Cohort Study. Med. Sci. Monit. 2019, 25, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.-I.; Haneda, M.; Koya, D.; Kondo, K.; Tanaka, S.; Arima, H.; Kume, S.; Nakazawa, J.; Chin-Kanasaki, M.; Ugi, S.; et al. Urinary Potassium Excretion and Renal and Cardiovascular Complications in Patients with Type 2 Diabetes and Normal Renal Function. Clin. J. Am. Soc. Nephrol. 2015, 10, 2152–2158. [Google Scholar] [CrossRef]
- Nagata, T.; Sobajima, H.; Ohashi, N.; Hirakawa, A.; Katsuno, T.; Yasuda, Y.; Matsuo, S.; Tsuboi, N.; Maruyama, S. Association between 24 h Urinary Sodium and Potassium Excretion and Estimated Glomerular Filtration Rate (eGFR) Decline or Death in Patients with Diabetes Mellitus and eGFR More than 30 mL/min/1.73 m2. PLoS ONE 2016, 11, e0152306. [Google Scholar] [CrossRef]
- Engberink, R.H.O.; van den Born, B.J.H.; Peters-Sengers, H.; Vogt, L. Long-term potassium intake and associated renal and cardiovascular outcomes in the clinical setting. Clin. Nutr. 2020, 39, 3671–3676. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Park, J.T.; Yoo, T.-H.; Lee, J.; Chung, W.; Lee, K.-B.; Chae, D.-W.; Ahn, C.; Kang, S.-W.; Choi, K.H.; et al. Urinary Potassium Excretion and Progression of CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A.; Dunkler, D.; Gao, P.; Teo, K.; Yusuf, S.; O’Donnell, M.; Mann, J.F.; Clase, C.M. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int. 2014, 86, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Leonberg-Yoo, A.K.; Tighiouart, H.; Levey, A.S.; Beck, G.J.; Sarnak, M.J. Urine potassium excretion, kidney failure, and mortality in CKD. Am. J. Kidney Dis. 2017, 69, 341–349. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Mills, K.T.; Appel, L.J.; Yang, W.; Chen, J.; Lee, B.T.; Rosas, S.E.; Porter, A.; Makos, G.; Weir, M.R.; et al. Urinary Sodium and Potassium Excretion and CKD Progression. J. Am. Soc. Nephrol. 2015, 27, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Laurenzi, M.; Cirillo, M.; Angeletti, M.; Buongiorno, A.; Morisi, G.; Panarelli, W.; Panfili, M.; Stamler, J.; Terradura, O.; Trevisan, M.; et al. Gubbio population study: Baseline findings. Nutr. Metab. Cardiovasc. Dis. 1991, 1, S1–S18. [Google Scholar]
- Cirillo, M.; Terradura-Vagnarelli, O.; Mancini, M.; Menotti, A.; Zanchetti, A.; Laurenzi, M. Cohort profile: The Gubbio Population Study. Int. J. Epidemiol. 2013, 43, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, M.; Laurenzi, M.; Panarelli, W.; Stamler, J. Urinary sodium to potassium ratio and urinary stone disease. Kidney Int. 1994, 46, 1133–1139. [Google Scholar] [CrossRef][Green Version]
- Cirillo, M.; Lombardi, C.; Laurenzi, M.; De Santo, N.G.; For The Gubbio Study Research Group. Relation of urinary urea to blood pressure: Interaction with urinary sodium. J. Hum. Hypertens. 2002, 16, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, M.; Lombardi, C.; Chiricone, D.; De Santo, N.G.; Zanchetti, A.; Bilancio, G. Protein intake and kidney function in the middle-age population: Contrast between cross-sectional and longitudinal data. Nephrol. Dial. Transplant. 2014, 29, 1733–1740. [Google Scholar] [CrossRef]
- Cirillo, M.; Zingone, F.; Lombardi, C.; Cavallo, P.; Zanchetti, A.; Bilancio, G. Population-based dose–response curve of glomerular filtration rate to dietary protein intake. Nephrol. Dial. Transplant. 2015, 30, 1156–1162. [Google Scholar] [CrossRef]
- Cirillo, M.; Cavallo, P.; Bilancio, G.; Lombardi, C.; Vagnarelli, O.T.; Laurenzi, M. Low Protein Intake in the Population: Low Risk of Kidney Function Decline but High Risk of Mortality. J. Ren. Nutr. 2018, 28, 235–244. [Google Scholar] [CrossRef]
- Cirillo, M.; Bilancio, G.; Cavallo, P.; Palladino, R.; Terradura-Vagnarelli, O.; Laurenzi, M. Sodium intake and kidney function in the general population: An observational, population-based study. Clin. Kidney J. 2020, 14, 647–655. [Google Scholar] [CrossRef]
- Cirillo, M.; Cavallo, P.; Palladino, R.; Terradura-Vagnarelli, O.; Zulli, E.; Villa, R.; Veneziano, R.; Laurenzi, M. Relationship of the intake of water and other beverages with renal endpoints: Cross-sectional and longitudinal data—Observational, population-based study. J. Ren. Nutr. 2021, in press. [Google Scholar]
- Myers, G.L.; Miller, W.G.; Coresh, J.; Fleming, J.; Greenberg, N.; Greene, T.; Hostetter, T.; Levey, A.S.; Panteghini, M.; Welch, M.; et al. Recommendations for improving serum creatinine measurement: A report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin. Chem. 2006, 52, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Cirillo, M.; Ciacci, C.; De Santo, N.G. Age, Renal Tubular Phosphate Reabsorption, and Serum Phosphate Levels in Adults. New Engl. J. Med. 2008, 359, 864–866. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, M.; Bilancio, G.; Cavallo, P.; Giordano, F.; Iesce, G.; Costanzo, S.; De Curtis, A.; Di Castelnuovo, A.; Iacoviello, L. Reduced Kidney Function and Relative Hypocalciuria—Observational, Cross-Sectional, Population-Based Data. J. Clin. Med. 2020, 9, 4133. [Google Scholar] [CrossRef]
- Mercado, C.I.; Cogswell, M.E.; Loria, C.M.; Liu, K.; Allen, N.; Gillespie, C.; Wang, C.-Y.; De Boer, I.H.; Wright, J. Validity of predictive equations for 24-h urinary potassium excretion based on timing of spot urine collection among adults: The MESA and CARDIA Urinary Sodium Study and NHANES Urinary Sodium Calibration Study. Am. J. Clin. Nutr. 2018, 108, 532–547. [Google Scholar] [CrossRef]
- Moore-Ede, M.C.; Herd, A.J.L. Renal electrolyte circadian rhythms: Independence from feeding and activity patterns. Am. J. Physiol. 1977, 232, F128–F135. [Google Scholar] [CrossRef]
- Peng, Y.-G.; Feng, J.-J.; Zhang, Y.; Li, K.; Cai, S.-Y.; Yan, R.-H.; Peng, X.-X. Cosinor-rhythmometry for 24-h urinary sodium, potassium, creatinine excretion in the Chinese adult population. Chin. Med. J. 2021, 134, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, M.; Lombardi, C.; Luciano, M.G.; Bilancio, G.; Anastasio, P.; De Santo, N.G. Estimation of GFR: A Comparison of New and Established Equations. Am. J. Kidney Dis. 2010, 56, 802–804. [Google Scholar] [CrossRef]
- Ix, J.H.; Wassel, C.L.; Stevens, L.A.; Beck, G.J.; Froissart, M.; Navis, G.; Rodby, R.; Torres, V.E.; Zhang, Y.; Greene, T.; et al. Equations to Estimate Creatinine Excretion Rate: The CKD Epidemiology Collaboration. Clin. J. Am. Soc. Nephrol. 2010, 6, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, M.; Lanti, M.P.; Menotti, A.; Laurenzi, M.; Mancini, M.; Zanchetti, A.; De Santo, N.G. Definition of Kidney Dysfunction as a Cardiovascular Risk FactorUse of Urinary Albumin Excretion and Estimated Glomerular Filtration Rate. Arch. Intern. Med. 2008, 168, 617–624. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bingham, S.A. Urine Nitrogen as a Biomarker for the Validation of Dietary Protein Intake. J. Nutr. 2003, 133, 921S–924S. [Google Scholar] [CrossRef]
- Siani, A.; Iacoviello, L.; Giorgione, N.; Iacone, R.; Strazzullo, P. Comparison of variability of urinary sodium, potassium, and calcium in free-living men. Hypertension 1989, 13, 38–42. [Google Scholar] [CrossRef]
- Elliott, P.; Stamler, J.; Nichols, R.; Dyer, A.R.; Stamler, R.; Kesteloot, H.; Marmot, M. Intersalt revisited: Further analyses of 24 h sodium excretion and blood pressure within and across populations. BMJ 1996, 312, 1249–1253. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef]
- Suga, S.-I.; Phillips, M.I.; Ray, P.E.; Raleigh, J.A.; Vio, C.; Kim, Y.-G.; Mazzali, M.; Gordon, K.L.; Hughes, J.; Johnson, R.J. Hypokalemia induces renal injury and alterations in vasoactive mediators that favor salt sensitivity. Am. J. Physiol. Physiol. 2001, 281, F620–F629. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.E.; Suga, S.-I.; Liu, X.-H.; Huang, X.; Johnson, R.J. Chronic potassium depletion induces renal injury, salt sensitivity, and hypertension in young rats. Kidney Int. 2001, 59, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Kramers, B.J.; Koorevaar, I.W.; Drenth, J.P.; De Fijter, J.W.; Neto, A.G.; Peters, D.J.; Vart, P.; Wetzels, J.F.; Zietse, R.; Gansevoort, R.T.; et al. Salt, but not protein intake, is associated with accelerated disease progression in autosomal dominant polycystic kidney disease. Kidney Int. 2020, 98, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Health Information of Potassium. Available online: https://ods.od.nih.gov/factsheets/Potassium-HealthProfessional/ (accessed on 7 August 2021).
- Kovesdy, C.P.; Matsushita, K.; Sang, Y.; Brunskill, N.J.; Carrero, J.J.; Chodick, G.; Hasegawa, T.; Heerspink, H.L.; Hirayama, A.; Landman, G.W.D.; et al. Serum potassium and adverse outcomes across the range of kidney function: A CKD Prognosis Consortium meta-analysis. Eur. Hear. J. 2018, 39, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
| Whole Cohort | Quintile * of uK/Cr at Exam-1 | p for Trend ¶ | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| N | 2027 | 402 | 439 | 377 | 406 | 403 | |
| Women, % | 56.9% | 57.2% | 52.6% | 61.5% | 56.9% | 57.1% | 0.576 |
| Age, years | 42.9 ± 13.3 | 42.6 | 42.0 | 44.4 | 42.8 | 43.0 | 0.439 |
| uK/Cr †, mmol/g | 28.6 (21.7/38.6) | 17.3 | 23.3 | 29.4 | 36.2 | 60.3 | <0.001 |
| Serum potassium, mmol/L | 4.04 ± 0.36 | 4.05 | 4.03 | 4.03 | 4.05 | 4.02 | 0.589 |
| Body mass index, kg/m3 | 26.1 ± 4.2 | 26.2 | 25.6 | 26.2 | 25.9 | 26.8 | 0.015 |
| Estimated urinary creatinine, g/d | 1.26 ± 0.31 | 1.27 | 1.27 | 1.23 | 1.26 | 1.28 | 0.993 |
| Systolic pressure, mmHg | 127 ± 18 | 127 | 127 | 128 | 126 | 127 | 0.378 |
| Diastolic pressure, mmHg | 76 ± 11 | 77 | 76 | 77 | 75 | 76 | 0.287 |
| Antihypertensive drug treatment, % | 9.0% | 7.7% | 9.8% | 9.3% | 9.1% | 8.9% | 0.710 |
| Diuretic, % | 7.7% | 6.7% | 8,7% | 8.8% | 6.9% | 7.7% | 0.981 |
| Potassium sparing diuretic, % | 2.2% | 1.7% | 1.8% | 2.4% | 2.2% | 3.0% | 0.213 |
| Inhibitor/blocker renin-angiotensin system, % | 0.4% | 0.2% | 0.7% | 0.3% | 0.7% | 0.0% | 0.639 |
| Diabetes, % | 1.1% | 1.0% | 0.2% | 1.1% | 1.0% | 2.2% | 0.044 |
| Smoking, % | 15.5% | 16.2% | 13.4% | 16.2% | 16.5% | 15.4% | 0.768 |
| Habitual alcohol intake, g/d | 11.9 (0.0/35.7) | 16.4 | 20.8 | 19.4 | 22.1 | 23.2 | 0.001 |
| Urine sodium/creatinine †, mmol/g | 106 (74/151) | 96 | 108 | 119 | 123 | 162 | <0.001 |
| Urine urea nitrogen/creatinine, mmol/g | Not measured | Not measured | --- | ||||
| Whole Cohort | Quintile * of uK/Cr at Exam-2 | p for Trend ¶ | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| N | 2027 | 398 | 399 | 401 | 401 | 428 | |
| Women, % | 56.9% | 56.5% | 56.4% | 55.6% | 56.1% | 59.8% | 0.400 |
| Age, years | 48.9 ± 13.3 | 48.4 | 48.6 | 49.2 | 49.0 | 49.0 | 0.467 |
| uK/Cr †, mmol/g | 24.3 (18.7/32.9) | 14.8 | 20.2 | 24.6 | 30.2 | 47.0 | <0.001 |
| Serum potassium, mmol/L | 4.12 ± 0.34 | 4.10 | 4.10 | 4.12 | 4.11 | 4.17 | 0.005 |
| Body mass index, kg/m3 | 26.9 ± 4.3 | 26.8 | 26.7 | 26.7 | 26.9 | 27.2 | 0.149 |
| Estimated urinary creatinine, g/d | 1.25 ± 0.31 | 1.26 | 1.25 | 1.25 | 1.26 | 1.24 | 0.652 |
| Systolic pressure, mmHg | 125 ± 18 | 123 | 125 | 126 | 126 | 126 | 0.016 |
| Diastolic pressure, mmHg | 76 ± 10 | 75 | 75 | 76 | 76 | 75 | 0.359 |
| Antihypertensive drug treatment, % | 14.9% | 12.3% | 15.0% | 13.7% | 16.7% | 16.8% | 0.056 |
| Diuretic, % | 7.5% | 4.5% | 9.8% | 6.7% | 7.0% | 9.3% | 0.093 |
| Potassium sparing diuretic, % | 3.4% | 3.3% | 2.8% | 4.0% | 4.0% | 3.0% | 0.804 |
| Inhibitor/blocker renin-angiotensin system, % | 4.2% | 2.5% | 4.0% | 4.5% | 5.7% | 4.4% | 0.083 |
| Diabetes, % | 3.3% | 2.8% | 3.5% | 4.7% | 2.5% | 3.0% | 0.858 |
| Smoking, % | 31.6% | 32.7% | 34.1% | 34.2% | 28.9% | 28.3% | 0.053 |
| Habitual alcohol intake, g/d | 11.9 (0.0/35.7) | 20.8 | 22.2 | 24.2 | 22.9 | 22.4 | 0.400 |
| Urine sodium/creatinine †, mmol/g | 103 (68/146) | 82 | 101 | 113 | 122 | 156 | <0.001 |
| Urine urea nitrogen/creatinine †, mmol/g | 9.66 (7.50/12.26) | 8.71 | 9.62 | 10.56 | 10.66 | 12.64 | <0.001 |
| Quintile * of uK/Cr at Exam-1 | p for Trend ¶ | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Number of examinees | 402 | 439 | 377 | 406 | 403 | |
| Time from exam 1 to exam 2, years | 5.96 | 5.90 | 5.97 | 5.89 | 6.03 | 0.372 |
| eGFR, mL/min × 1.73 m2 | ||||||
| at exam-1 | 90.6 | 91.8 | 87.8 | 90.4 | 91.3 | 0.990 |
| at exam-2 | 86.5 | 88.0 | 85.1 | 88.6 | 88.8 | 0.023 |
| change from exam-1 to exam-2 | −4.06 | −3.75 | −1.78 | −1.79 | −2.54 | 0.033 |
| yearly change from exam-1 to exam-2 | −0.737 | −0.671 | −0.248 | −0.314 | −0.460 | 0.026 |
| Quintile * of uK/Cr at Exam-2 | ||||||
| 1 | 2 | 3 | 4 | 5 | ||
| Number of examinees | 398 | 399 | 401 | 401 | 428 | |
| Time from exam 2 to exam 3, years | 13.5 | 13.5 | 13.3 | 13.3 | 13.0 | 0.001 |
| eGFR, mL/min × 1.73 m2 | ||||||
| at exam-2 | 86.5 | 87.6 | 88.4 | 87.6 | 88.0 | 0.171 |
| at exam-3 | 75.0 | 76.4 | 76.7 | 77.5 | 78.0 | 0.002 |
| change from exam-2 to exam-3 | −11.50 | −11.24 | −11.66 | −10.16 | −10.04 | 0.009 |
| yearly change from exam-2 to exam-3 | −0.887 | −0.870 | −0.908 | −0.788 | −0.799 | 0.032 |
| Quintile * of Mean uK/Cr at Exam−1 and Exam−2 | ||||||
| 1 | 2 | 3 | 4 | 5 | ||
| Number of examinees | 395 | 447 | 378 | 405 | 402 | |
| Time from exam-1 to exam-3, years | 19.3 | 19.1 | 19.3 | 19.3 | 19.4 | 0.241 |
| eGFR, mL/min × 1.73 m2 | ||||||
| at exam-1 | 90.2 | 89.1 | 90.8 | 91.7 | 90.3 | 0.363 |
| at exam-3 | 75.3 | 75.3 | 77.4 | 77.7 | 77.6 | 0.003 |
| change from exam-1 to exam-3 | −14.89 | −13.88 | −13.34 | −13.20 | −12.69 | 0.034 |
| yearly change from exam-1 to exam-3 | −0.788 | −0.733 | −0.701 | −0.683 | −0.666 | 0.020 |
| Type of Model | Independent Variable | Model | Dependent Variable | Beta (95%CI) |
|---|---|---|---|---|
| Cross-sectional | log transformed uK/Cr at exam-1 | 1 a | eGFR at exam-1 | −0.020 ns (−0.059/0.019) |
| log transformed uK/Cr at exam-2 | 2 b | eGFR at exam-2 | 0.024 ns (−0.013/0.056) | |
| Longitudinal | log transformed uK/Cr at exam-1 | 3 c | Yearly eGFR change from exam-1 to exam-2 | 0.051 ** (0.018/0.084) |
| log transformed uK/Cr at exam-2 | 4 d | Yearly eGFR change from exam-2 to exam-3 | 0.048 * (0.005/0.091) | |
| mean of log transformed uK/Cr at exam-1 and exam-2 | 5 e | Yearly eGFR change from exam-1 to exam-3 | 0.056 *** (0.027/0.087) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, M.; Bilancio, G.; Cavallo, P.; Palladino, R.; Zulli, E.; Villa, R.; Veneziano, R.; Laurenzi, M. Urinary Potassium and Kidney Function Decline in the Population—Observational Study. Nutrients 2021, 13, 2747. https://doi.org/10.3390/nu13082747
Cirillo M, Bilancio G, Cavallo P, Palladino R, Zulli E, Villa R, Veneziano R, Laurenzi M. Urinary Potassium and Kidney Function Decline in the Population—Observational Study. Nutrients. 2021; 13(8):2747. https://doi.org/10.3390/nu13082747
Chicago/Turabian StyleCirillo, Massimo, Giancarlo Bilancio, Pierpaolo Cavallo, Raffaele Palladino, Enrico Zulli, Rachele Villa, Rosangela Veneziano, and Martino Laurenzi. 2021. "Urinary Potassium and Kidney Function Decline in the Population—Observational Study" Nutrients 13, no. 8: 2747. https://doi.org/10.3390/nu13082747
APA StyleCirillo, M., Bilancio, G., Cavallo, P., Palladino, R., Zulli, E., Villa, R., Veneziano, R., & Laurenzi, M. (2021). Urinary Potassium and Kidney Function Decline in the Population—Observational Study. Nutrients, 13(8), 2747. https://doi.org/10.3390/nu13082747







