Sodium Decanoate Improves Intestinal Epithelial Barrier and Antioxidation via Activating G Protein-Coupled Receptor-43
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Samples Collection
2.3. Chemical and Reagents
2.4. Laboratory Analysis
2.4.1. Cell Viability
2.4.2. Intestinal Morphology
2.4.3. Expression of Tight Junction Proteins and GPR-43
2.4.4. Antioxidant Indexes and Cytokines in Serum
2.4.5. Volatile Fatty Acids Concentration
2.4.6. Microbial Community
2.5. Statistical Analysis
3. Results
3.1. Optimal Treatment Concentrations of Sodium Decanoate and Its Effects on Tight Junction Proteins of IPEC-J2
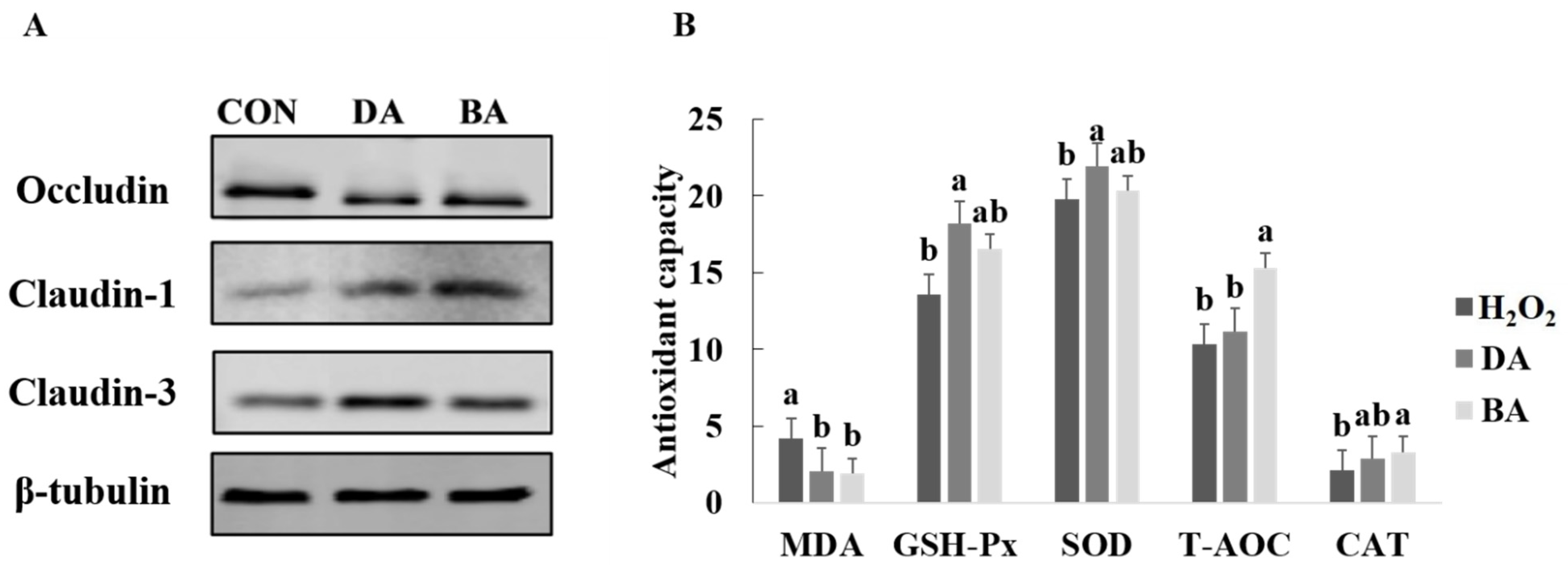
3.2. Effects of Sodium Decanoate on Antioxidant Capacity and Tight Junction Protein Expression in an H2O2 Oxidant Damage Model of IPEC-J2
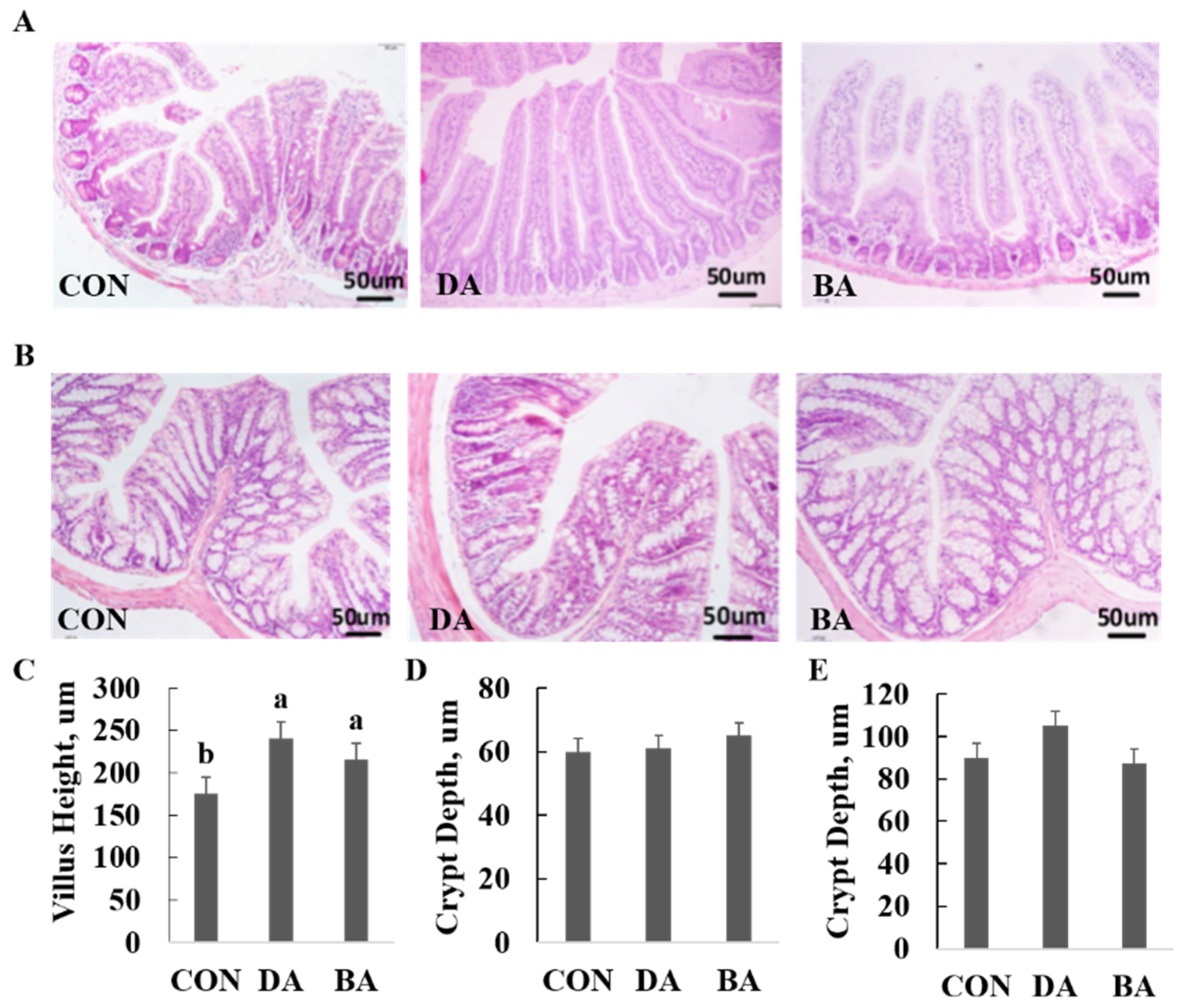
3.3. Effects of Sodium Decanoate on Growth Performance, Intestinal Morphology, and Intestinal Barrier Functions in a Mice Model
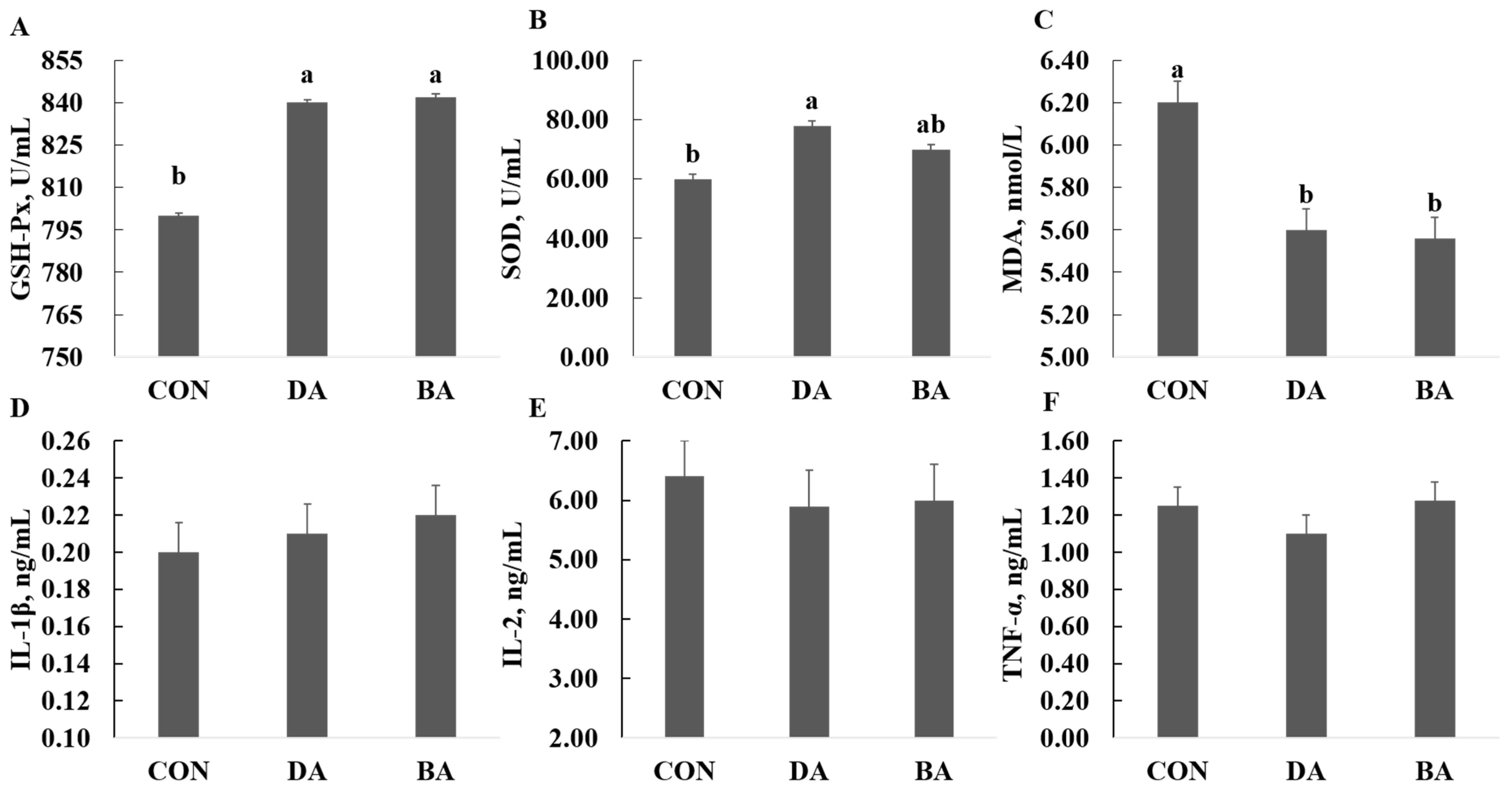
3.4. Effects of Sodium Decanoate on Serum Antioxidant and Immunological Indexes in a Mice Model
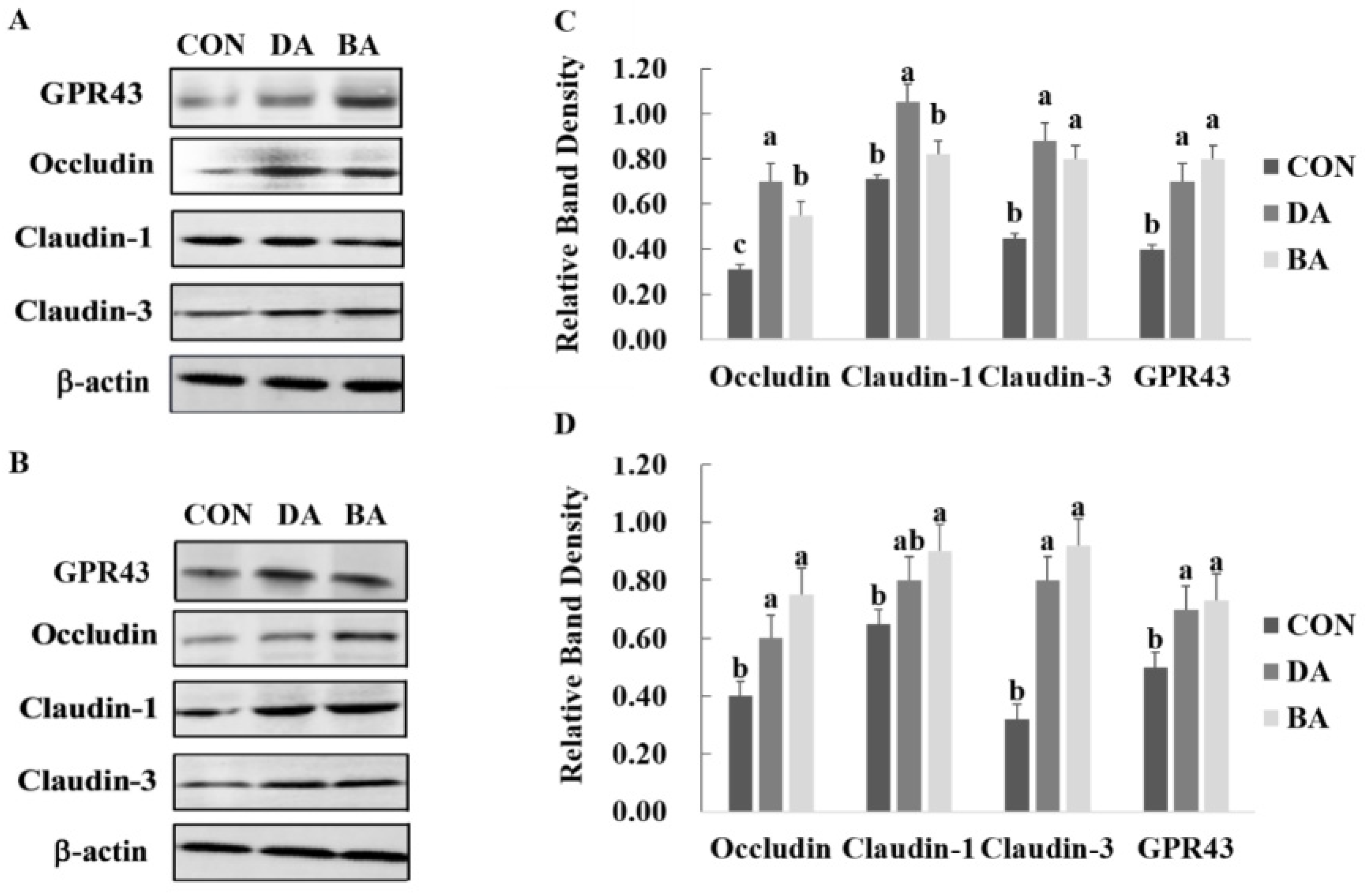
3.5. Effects of Sodium Decanoate on Expression of Tight Junction Proteins and GPR-43 in the Intestine of Mice
3.6. Effects of Sodium Decanoate on Gut Microbiota and Their Metabolites in a Mice Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meale, S.J.; Chaves, A.V.; He, M.L.; Guan, L.L.; McAllister, T.A. Effects of various dietary lipid additives on lamb performance, carcass characteristics, adipose tissue fatty acid composition, and wool characteristics. J. Anim. Sci. 2015, 93, 3110–3120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprong, R.C.; Hulstein, M.F.; van der Meer, R. Bactericidal activities of milk lipids. Antimicrob. Agents Chemother. 2001, 45, 1298–1301. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Huang, N.; Xiong, J.; Wei, H.; Jiang, S.; Peng, J. Caprylic acid and nonanoic acid upregulate endogenous host defense peptides to enhance intestinal epithelial immunological barrier function via histone deacetylase inhibition. Int. Immunopharmacol. 2018, 65, 303–311. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanczakowska, E. The use of medium-chain fatty acids in piglet feeding—A review. Ann. Anim. Sci. 2017, 17, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Zentek, J.; Ferrara, F.; Pieper, R.; Tedin, L.; Meyer, W.; Vahjen, W. Effects of dietary combinations of organic acids and medium chain fatty acids on the gastrointestinal microbial ecology and bacterial metabolites in the digestive tract of weaning piglets. J. Anim. Sci. 2013, 91, 3200–3210. [Google Scholar] [CrossRef]
- Tanaka, S.; Saitoh, O.; Tabata, K.; Matsuse, R.; Kojima, K.; Sugi, K.; Nakagawa, M.; Teranishi, T.; Uchida, K.; Hirata, I.; et al. Medium-chain fatty acids stimulate interleukin-8 production in Caco-2 cells with different mechanisms from long-chain fatty acids. J. Gastroenterol. Hepatol. 2001, 16, 748–754. [Google Scholar] [CrossRef]
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.W.; Harper, D. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Smith, F.; Clark, J.E.; Overman, B.L.; Tozel, C.C.; Huang, J.H.; Rivier, J.E.F.; Blisklager, A.T.; Moeser, A.J. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 298, G352–G363. [Google Scholar] [CrossRef] [Green Version]
- Moeser, A.J.; Pohl, C.S.; Rajput, M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr. 2017, 3, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Nohr, M.K.; Egerod, K.L.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Moller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H. Activation of GPR109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Kuang, Y.; Wang, Y.; Zhang, Y.; Song, Y.; Zhang, X.; Lin, Y.; Che, L.; Xu, S.; Wu, D.; Xue, B.; et al. Effects of dietary combinations of organic acids and medium chain fatty acids as a replacement of zinc oxide on growth, digestibility and immunity of weaned pigs. Anim. Feed Sci. Technol. 2015, 208, 145–157. [Google Scholar] [CrossRef]
- Tao, S.; Duanmu, Y.; Dong, H.; Tian, J.; Ni, Y.; Zhao, R. A high-concentrate diet induced colonic epithelial barrier disruption is associated with the activating of cell apoptosis in lactating goats. BMC Vet. Res. 2014, 10, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Zhao, J.B.; Wang, C.L.; Guo, P.T.; Lu, W.; Wang, C.; Liu, L.; Johnston, L.J.; Zhao, Y.; Wu, X.; et al. Dietary corn bran altered the diversity of microbial communities and cytokine production in weaned pigs. Front. Microbiol. 2018, 9, 2090. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Yang, L.; Zhang, L.; Wang, T. Effect of medium-chain triglycerides on growth performance, nutrient digestibility, plasma metabolites and antioxidant capacity in weanling pigs. Anim. Nutr. 2015, 1, 12–18. [Google Scholar] [CrossRef]
- Devi, S.M.; Kim, I.H. Effect of medium chain fatty acids (MCFA) and probiotic (Enterococcus faecium) supplementation on the growth performance, digestibility and blood profiles in weanling pigs. Vet. Med. 2014, 59, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Van der Hoeven-Hangoor, E.; van der Vossen, J.M.; Schuren, F.H.J.; Verstegen, M.W.A.; de Oliveira, J.E.; Montijn, R.C.; Hendriks, W.H. Ileal microbiota composition of broilers fed various commercial diet compositions. Poult. Sci. 2013, 92, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.W.; Prosser, Z.; Chee, E.Y.W.; Hansen, C.F.; Dunshea, F.R.; Mullan, B.P.; Pluske, J.R. Dietary stimulation of the endogenous somatotropic axis in weaner and grower-finisher pigs using medium chain triglycerides and cysteamine hydrochloride. J. Anim. Sci. Biotechnol. 2017, 7, 405–416. [Google Scholar] [CrossRef] [Green Version]
- Zicker, M.C.; Silveira, A.L.M.; Lacerda, D.R.; Rodrigues, D.F.; Oliveira, C.T.; Cordeiro, L.M.S.; Lima, L.C.F.; Santos, S.H.S.; Teixeira, M.M.; Ferreira, A.V.M. Virgin coconut oil is effective to treat metabolic and inflammatory dysfunction induced by high refined carbohydrate-containing-diet in mice. J. Nutr. Biochem. 2018, 63, 117–128. [Google Scholar] [CrossRef]
- Decuypere, J.A.; Dierick, N.A. The combined use of triacylglycerols containing medium-chain fatty acids and exogenous lipolytic enzymes as an alternative to in-feed antibiotics in piglets: Concept, possibilities and limitations. an overview. Nutr. Res. Rev. 2003, 16, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Corpeleijn, W.E.; Riedijk, M.A.; Zhou, Y.; Schierbeek, H.; Huang, Y.; Chen, C.; van Goudoever, J.B. Almost all enteral aspartate is taken up in first-pass metabolism in enterally fed preterm infants. Clin. Nutr. 2010, 29, 341–346. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Xie, F.; He, H.; Johnston, L.J.; Dai, X.; Wu, C.; Ma, X. Dietary fiber-derived short-chain fatty acids: A potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes. Rev. 2021. [Google Scholar] [CrossRef]
- De Keyser, K.; Dierick, N.; Kanto, U.; Hongsapak, T.; Buyens, G.; Kuterna, L.; Vanderbeke, E. Medium-chain glycerides affect gut morphology, immune-and goblet cells in post-weaning piglets: In vitro fatty acid screening with Escherichia coli and in vivo consolidation with LPS challenge. J. Anim. Physiol. Anim. Nutr. 2019, 103, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Zhan, T.; Zhao, Q.; Tang, C.; Zhang, K.; Han, Y.; Zhang, J. Effects of mixed organic acids and medium chain fatty acids as antibiotic alternatives on the performance, serum immunity, and intestinal health of weaned piglets orally challenged with Escherichia coli K88. Anim. Feed Sci. Technol. 2020, 269, 114617. [Google Scholar] [CrossRef]
- Xu, X.; Chen, S.; Wang, H.; Tu, Z.; Wang, S.; Wang, X.; Zhu, H.; Wang, C.; Zhu, J.; Liu, Y. Medium-chain TAG improve intestinal integrity by suppressing toll-like receptor 4, nucleotide binding oligomerisation domain proteins and necroptosis signalling in weanling piglets challenged with lipopolysaccharide. Br. J. Nutr. 2018, 119, 1019–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Ma, N.; Song, P.; He, T.; Levesque, C.; Bai, Y.; Zhang, A.; Ma, X. Grape Seed Proanthocyanidin Affects Lipid Metabolism via Changing Gut Microflora and Enhancing Propionate Production in Weaned Pigs. J. Nutr. 2019, 149, 1523–1532. [Google Scholar] [CrossRef]
- Malapaka, R.R.; Khoo, S.; Zhang, J.; Choi, J.H.; Zhou, X.E.; Xu, Y.; Gong, Y.; Li, J.; Yong, E.; Chalmers, M.J.; et al. Identification and mechanism of 10-carbon fatty acid as modulating ligand of peroxisome proliferator-activated receptors. J. Biol. Chem. 2012, 287, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Liberato, M.V.; Nascimento, A.S.; Ayers, S.D.; Lin, J.Z.; Cvore, A.; Silveira, R.L.; Martinez, L.; Souza, P.C.T.; Saidemberg, D.; Deng, T.; et al. Medium chain fatty acids are selective peroxisome proliferator activated receptor (PPAR) γ activators and pan-PPAR partial agonists. PLoS ONE 2012, 7, e36297. [Google Scholar] [CrossRef] [Green Version]
- Kono, H.; Fujii, H.; Asakawa, M.; Maki, A.; Amemiya, H.; Hirai, Y.; Matsuda, M.; Yamamoto, M. Medium-chain triglycerides enhance secretory IgA expression in rat intestine after administration of endotoxin. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G1081–G1089. [Google Scholar] [CrossRef] [Green Version]
- Tan, P.; Fu, H.Y.; Ma, X. Design, optimization, and nanotechnology of antimicrobial peptides: From exploration to applications. Nano Today 2021, 39. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhao, Y.T.; Zhao, T.C. Histone deacetylases and mechanisms of regulation of gene expression (histone deacetylases in cancer). Crit. Rev. Oncog. 2015, 20, 35. [Google Scholar] [CrossRef]
- Chang, P.; Zuckermann, A.M.; Williams, S.; Close, A.J.; Cano-Jaimez, M.; McEvoy, J.P.; Spencer, J.; Walker, M.C.; Williams, R.S.B. Seizure control by derivatives of medium chain fatty acids associated with the ketogenic diet show novel branching-point structure for enhanced potency. J. Pharmacol. Exp. Ther. 2015, 352, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghayour-Mobarhan, M.; Saber, H.; Ferns, G.A.A. The potential role of heat shock protein 27 in caediovascular disease. Clin. Chim. Acta 2012, 312, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Valle-González, E.R.; Jackman, J.A.; Yoon, B.K. Characterizing how acidic pH conditions affect the membrane-disruptive activities of lauric acid and glycerol monolaurate. Langmuir 2018, 34, 13745–13753. [Google Scholar] [CrossRef]
- Thormar, H.; Hilmarsson, H.; Bergsson, G. Stable concentrated emulsions of the 1-monoglyceride of capric acid (monocaprin) with microbicidal activities against the food-borne bacteria Campylobacter jejuni, Salmonella spp. and Escherichia coli. Appl. Environ. Microb. 2006, 72, 522–526. [Google Scholar] [CrossRef] [Green Version]
- Schlievert, P.M.; Peterson, M.L.; Kaufmann, G.F. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS ONE 2012, 7, e40350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Vallespín, B.; Vahjen, W.; Zentek, J. Effects of medium chain fatty acids on the structure and immune response of IPEC-J2 cells. Cytotechnology 2016, 68, 1925–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zentek, J.; Buchheit-Renko, S.; Ferrara, F.; Vahjen, W.; van Kessel, A.G.; Pieper, R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim. Health Res. Rev. 2011, 12, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.P.; Collins, D.A.; Pierson, F.W.; Mahsoub, H.M.; Sriranganathan, N.; Persia, M.E.; Karnezos, T.P.; Sims, M.D.; Dalloul, R.A. Investigation of medium chain fatty Acid feed supplementation for reducing Salmonella typhimurium colonization in turkey poults. Foodborne Pathog. Dis. 2017, 14, 531–536. [Google Scholar] [CrossRef]
- Guyard-Nicodeme, M.; Keita, A.; Quesne, S.; Amelot, M.; Poezevara, T.; Le Berre, B.; Sanchez, J.; Vesseur, P.; Martin, A.; Medel, P.; et al. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period. Poult. Sci. 2016, 95, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.T.; Chen, J.W.; Rathod, J.; Jiang, Y.; Tsai, P.; Hung, Y.; Ko, W.C.; Sabia, D.; Huang, I.H. Lauric acid is an inhibitor of Clostridium difficile growth in vitro and reduces inflammation in a mouse infection model. Front. Microbiol. 2017, 8, 2635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagato, E.; Pozzi, C.; Bertocchi, A.; Schioppa, T.; Saccheri, F.; Guglietta, S.; Fosso, B.; Melocchi, L.; Nizzoli, G.; Troisi, J.; et al. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat. Microbiol. 2020, 5, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Usta-Gorgun, B.; Yilmaz-Ersan, L. Short-chain fatty acids production by Bifidobacterium species in the presence of salep. Electron. J. Biotechnol. 2020, 47, 29–35. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.S.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Ma, N.; Ma, X. Dietary amino acids and the gut-microbiome-immune axis: Physiological metabolism and therapeutic prospects. Compr. Rev. Food Sci. Food Saf. 2019, 18, 221–242. [Google Scholar]
- Miletta, M.C.; Petkovic, V.; Eble, A.; Ammann, R.A.; Fluck, C.E.; Mullis, P.E. Butyrate increases intracellular calcium levels and enhances growth hormone release from rat anterior pituitary cells via the G-protein coupled receptors GPR 41 and 43. PLoS ONE 2014, 9, e107388. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
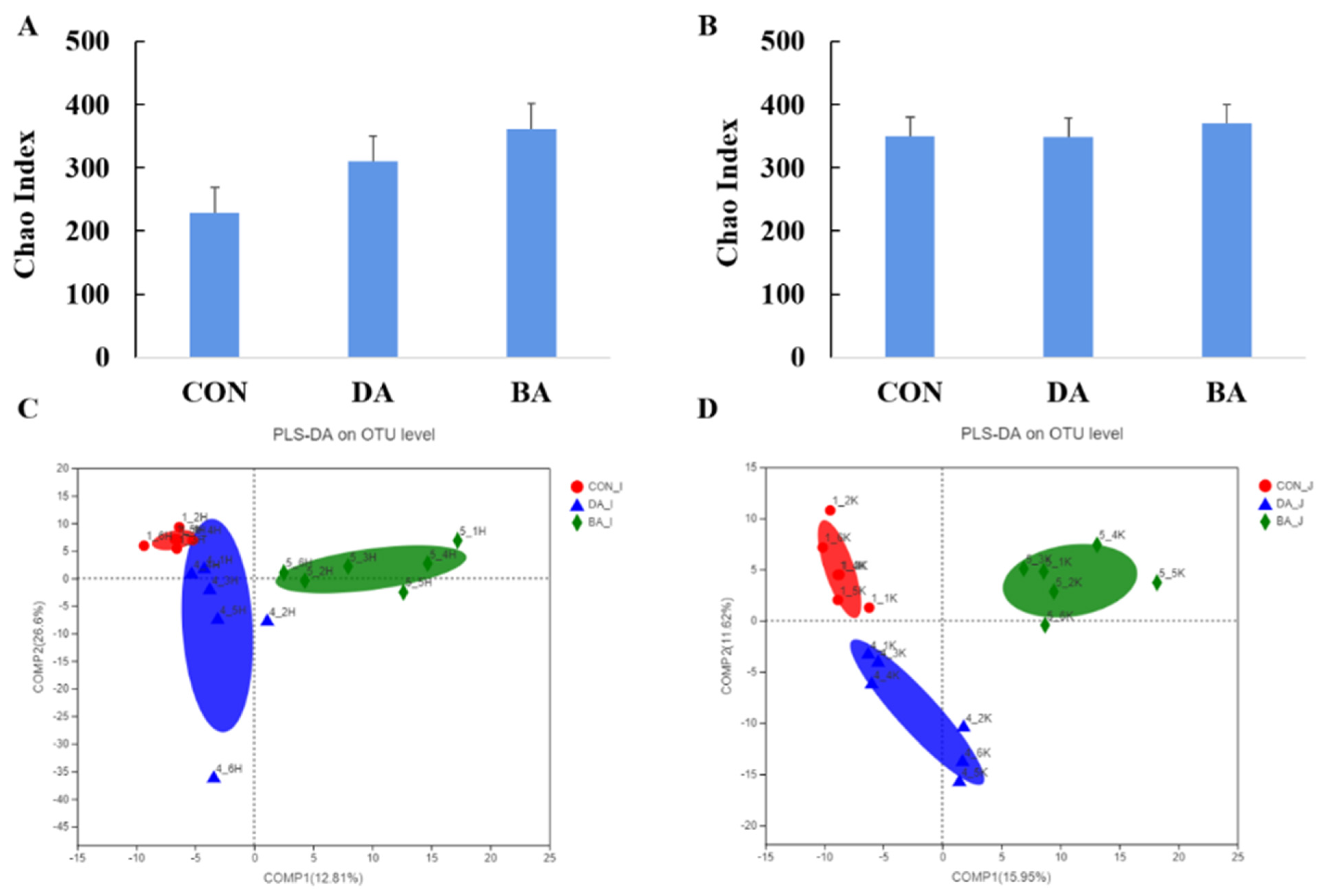
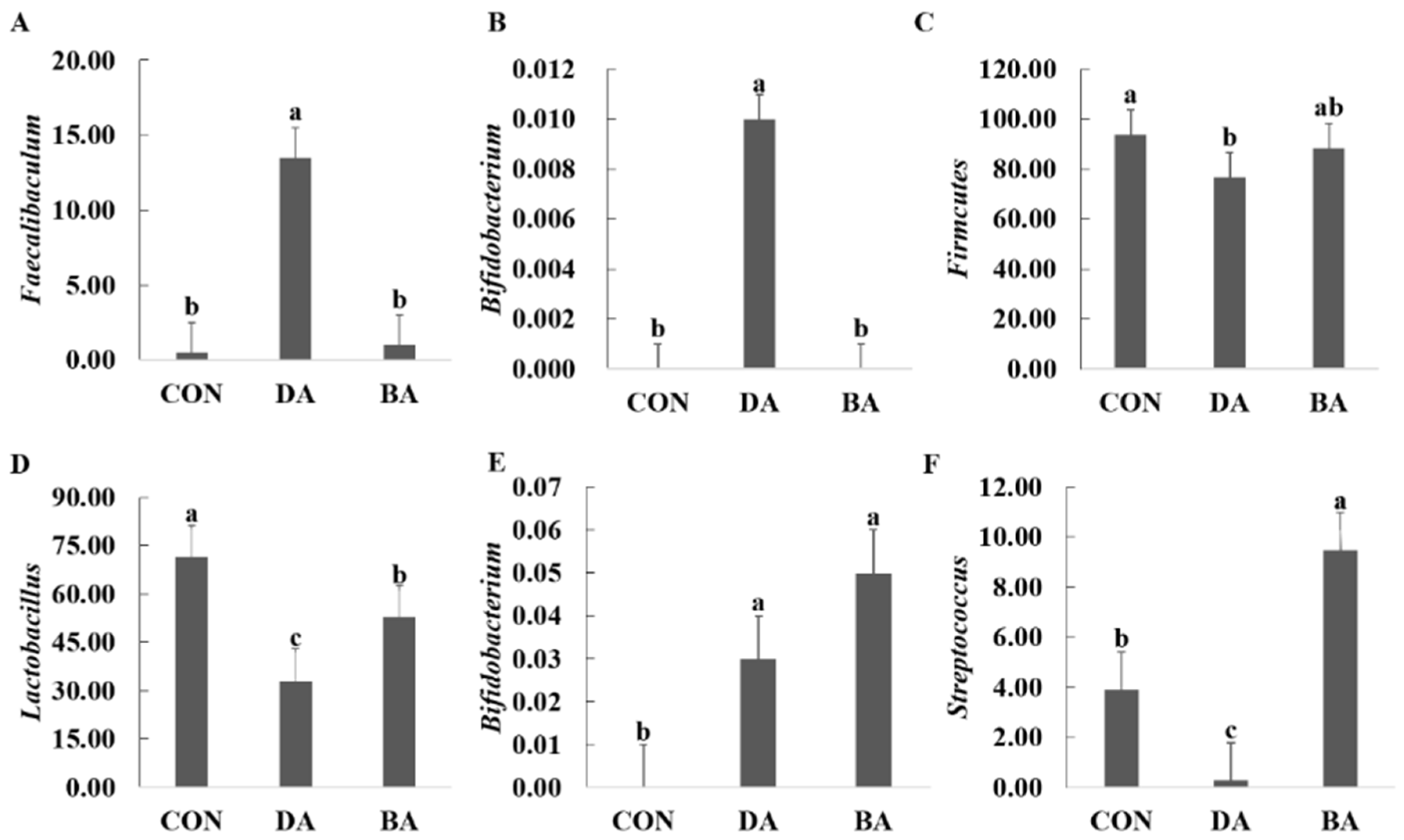
| Items | CON | DA | BA | p Value |
|---|---|---|---|---|
| Initial weight, (g) | 13.52 ± 0.51 | 13.68 ± 0.60 | 13.67 ± 0.55 | >0.05 |
| Final weight, (g) | 27.54 ± 0.65 a | 28.83 ± 0.59 b | 28.45 ± 0.56 b | <0.05 |
| ADG, (g/day) | 0.50 ± 0.01 a | 0.54 ± 0.01 b | 0.53 ± 0.01 b | <0.05 |
| ADFI, (g/day) | 2.65 ± 0.15 | 2.62 ± 0.08 | 2.58 ± 0.10 | >0.05 |
| F/G | 5.30 ± 0.09 a | 4.85 ± 0.12 b | 4.87 ± 0.08 b | <0.05 |
| Variable | CON | DA | BA | p Value |
|---|---|---|---|---|
| Ileal digesta, mg/g | ||||
| Acetic acid | 1.12 ± 0.03 a | 1.40 ± 0.02 b | 1.62 ± 0.03 c | <0.05 |
| Propionic acid | 0.50 ± 0.01 a | 0.58 ± 0.01 b | 0.62 ± 0.01 b | <0.05 |
| Butyric acid | 0.26 ± 0.02 a | 0.36 ± 0.01 b | 0.38 ± 0.01 b | <0.05 |
| Total VFA | 1.88 ± 0.06 a | 2.34 ± 0.07 b | 2.62±0.08 b | <0.05 |
| Colonic digesta, mg/g | ||||
| Acetic acid | 1.90 ± 0.02 a | 2.25 ± 0.03 b | 2.36 ± 0.03 b | <0.05 |
| Propionic acid | 1.15 ± 0.03 a | 1.53 ± 0.02 b | 1.65 ± 0.03 b | <0.05 |
| Butyric acid | 0.64 ± 0.02 a | 0.82 ± 0.02 b | 0.80 ± 0.02 a,b | <0.05 |
| Total VFA | 3.09 ± 0.06 a | 4.60 ± 0.08 b | 4.81 ± 0.09 b | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Hu, J.; Ma, X. Sodium Decanoate Improves Intestinal Epithelial Barrier and Antioxidation via Activating G Protein-Coupled Receptor-43. Nutrients 2021, 13, 2756. https://doi.org/10.3390/nu13082756
Zhao J, Hu J, Ma X. Sodium Decanoate Improves Intestinal Epithelial Barrier and Antioxidation via Activating G Protein-Coupled Receptor-43. Nutrients. 2021; 13(8):2756. https://doi.org/10.3390/nu13082756
Chicago/Turabian StyleZhao, Jinbiao, Jinhua Hu, and Xi Ma. 2021. "Sodium Decanoate Improves Intestinal Epithelial Barrier and Antioxidation via Activating G Protein-Coupled Receptor-43" Nutrients 13, no. 8: 2756. https://doi.org/10.3390/nu13082756
APA StyleZhao, J., Hu, J., & Ma, X. (2021). Sodium Decanoate Improves Intestinal Epithelial Barrier and Antioxidation via Activating G Protein-Coupled Receptor-43. Nutrients, 13(8), 2756. https://doi.org/10.3390/nu13082756








