Decreased Fatty Acid Transporter FABP1 and Increased Isoprostanes and Neuroprostanes in the Human Term Placenta: Implications for Inflammation and Birth Weight in Maternal Pre-Gestational Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ethics
2.3. Biological Samples
2.4. Chemical and Reagents
2.5. Fatty Acid Transporter Proteins in Placental Tissue
2.5.1. Quantitative Real-Time PCR (qPCR)
2.5.2. Western Blotting
2.6. Cytokines in Plasma and Placental Tissue
2.7. Maternal Lipoprotein Profile
2.8. Ion Mobility QTOF LC/MS Lipid Profile Analysis of Placenta Samples
Chromatogram Pre-Processing and Lipid Annotation
2.9. Isoprostanoids in Placental Tissue
2.10. Statistical Analyses
3. Results
3.1. General Characteristics of the Mothers and Newborns
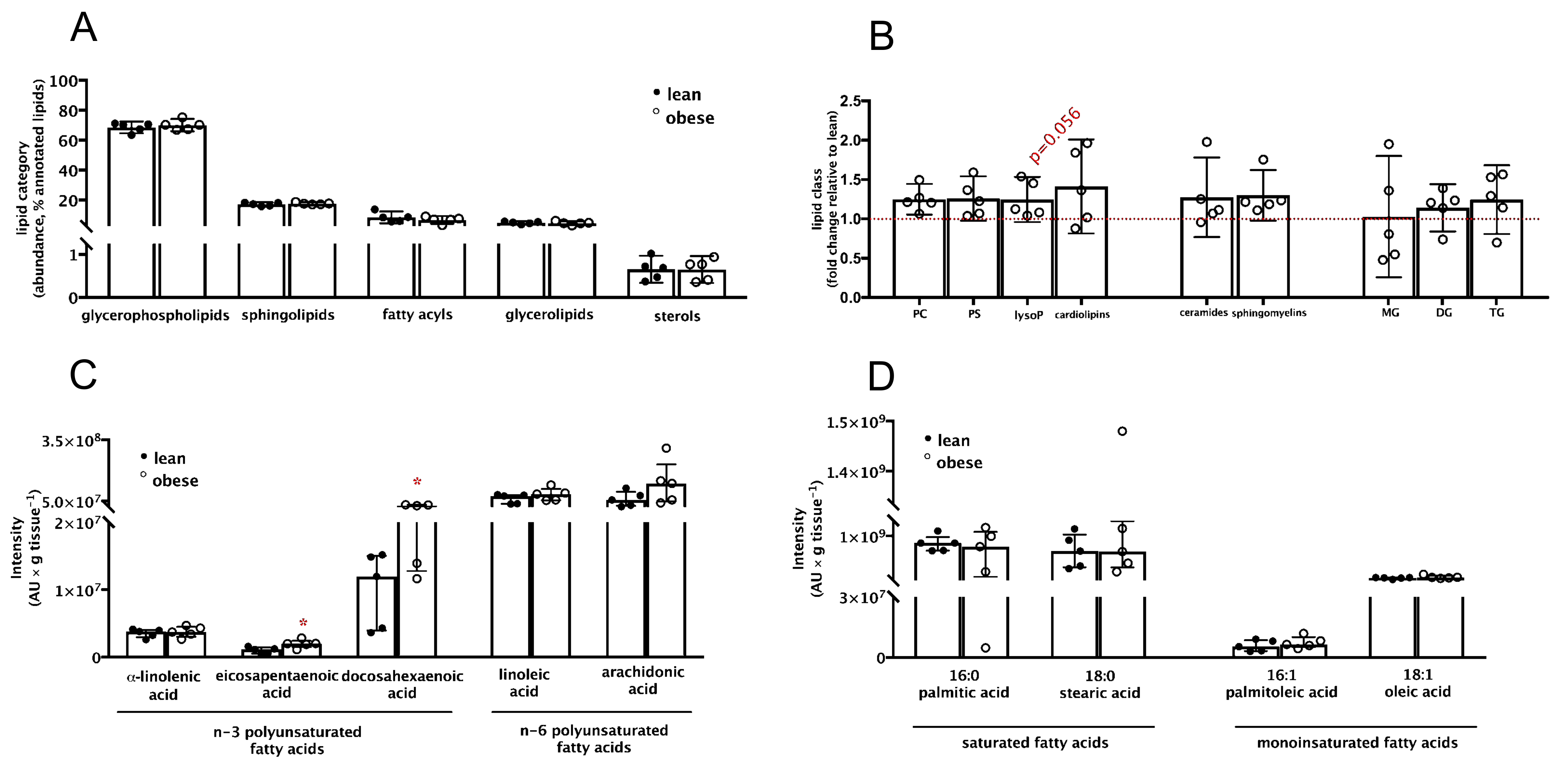
3.2. Placental Lipid Profile Suggests Alterations in Long-Chain Polyunsaturated Fatty Acids Abundance despite No Apparent Signs of Inflammation and Dyslipidemia in Maternal Pre-Gestational Obesity
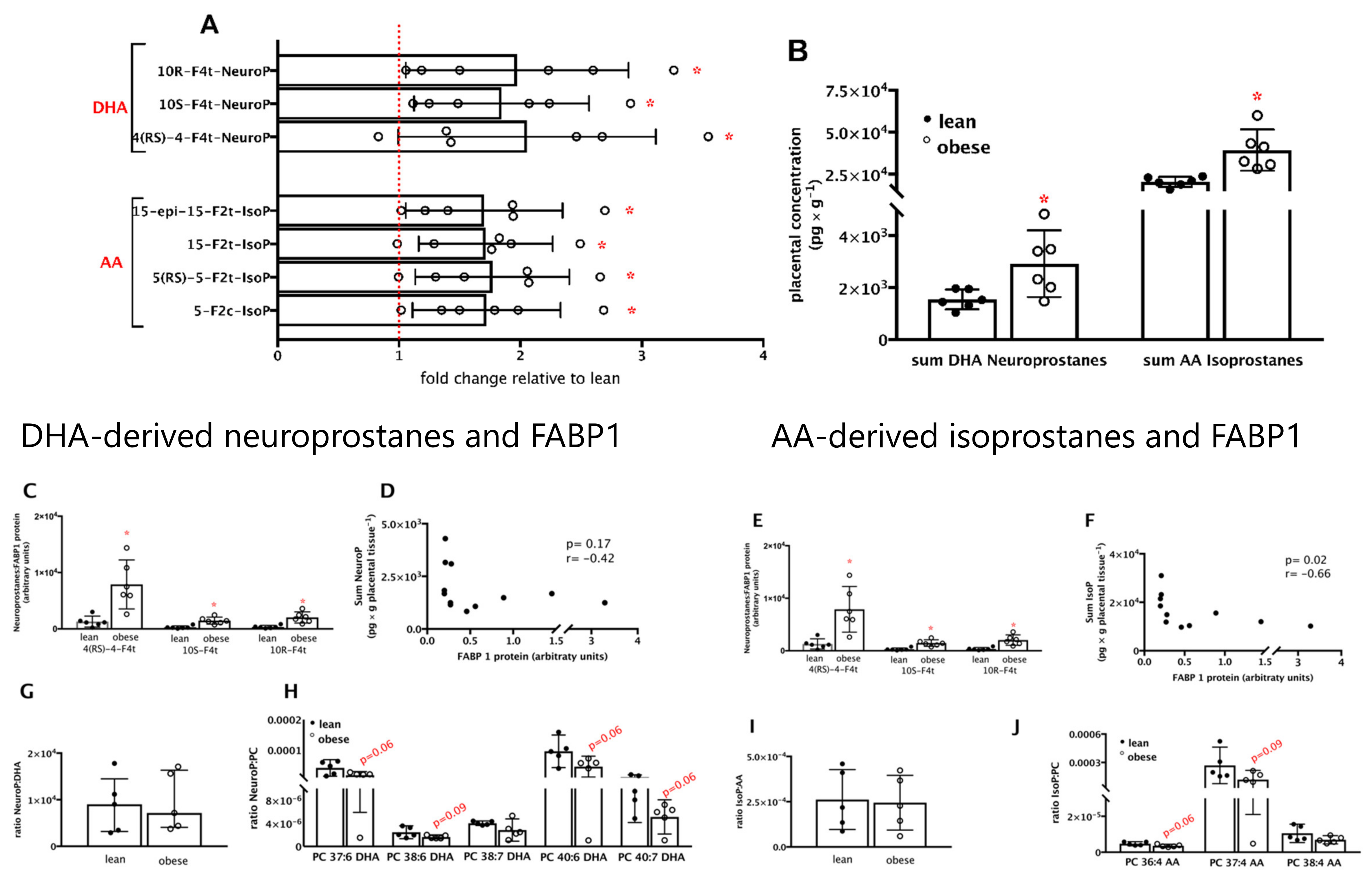
3.3. Fatty Acid Transporter Protein FABP1 Is Decreased and Negatively Associated with Polyunsaturated Fatty Acids in Placentas from Women with Pre-Gestational Obesity
3.4. Neuroprostanes and Isoprostanes Are Increased and Negatively Associated with FABP1 Protein in Placentas from Women with Pre-Gestational Obesity
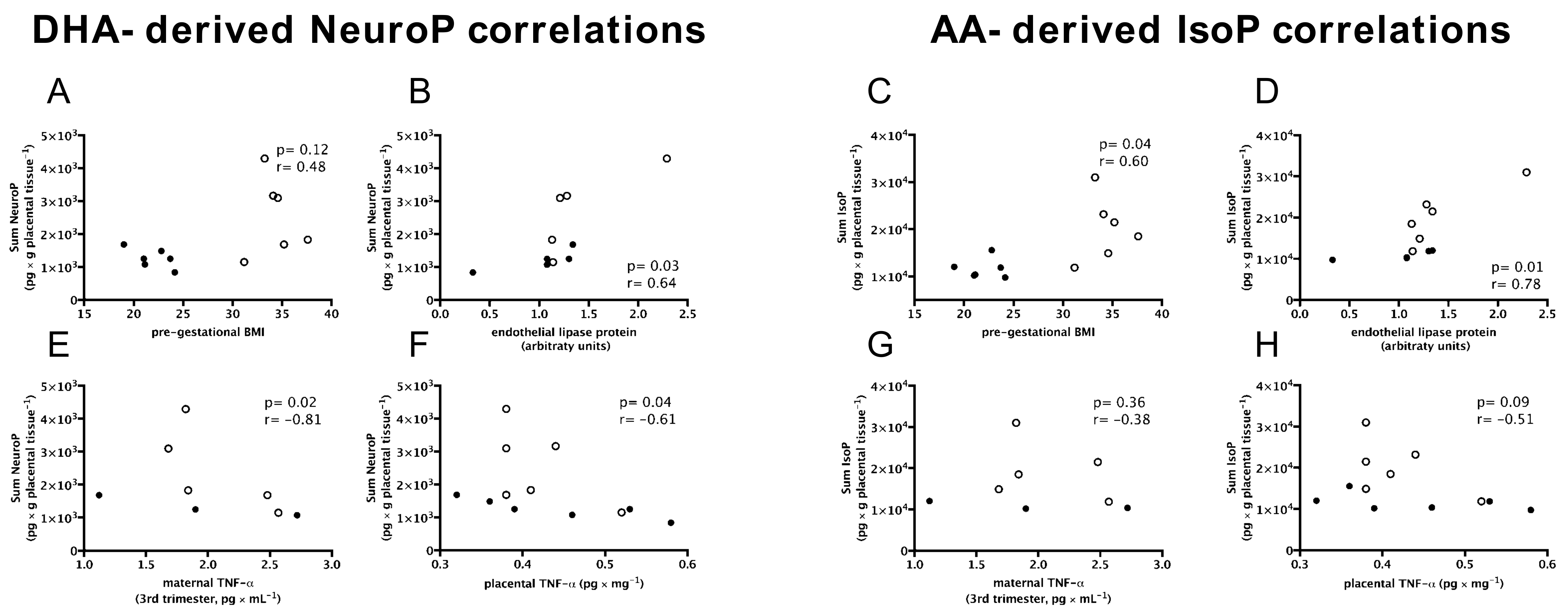
3.5. DHA-Derived Neuroprostanes and AA-Derived Isoprostanes Are Positively Associated with Maternal Pre-Gestational BMI and Endothelial Lipase Protein; and DHA-Derived Neuroprostanes Only Are Negatively Associated with Inflammation and Birth Weight
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef]
- Brazil. Vigilancia de Fatores de Risco e Proteção Para Doenças Crônicas por Inquérito Telefônico. In Brasilia; 2018. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2018_vigilancia_fatores_risco.pdf (accessed on 4 April 2021).
- Dai, H.; Alsalhe, T.A.; Chalghaf, N.; Riccò, M.; Bragazzi, N.L.; Wu, J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: An analysis of the Global Burden of Disease Study. PLoS Med. 2020, 17, e1003198. [Google Scholar] [CrossRef]
- Schneider, B.C.; Menezes, A.M.B.; Wehrmeister, F.C.; Gonçalves, H. Gestational weight gain and childhood body mass index across three generations: Results from the 1993 Pelotas (Brazil) Birth Cohort. Pediatr. Obes. 2021, 16, e12760. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, H.; Jiang, Y.; Mzayek, F.; Arshad, H.; Karmaus, W. Maternal Birth Weight and BMI Mediate the Transgenerational Effect of Grandmaternal BMI on Grandchild’s Birth Weight. Obesity 2020, 28, 647–654. [Google Scholar] [CrossRef]
- Lahti-Pulkkinen, M.; Bhattacharya, S.; Räikkönen, K.; Osmond, C.; Norman, J.; Reynolds, R.M. Intergenerational Transmission of Birth Weight Across 3 Generations. Am. J. Epidemiol. 2018, 187, 1165–1173. [Google Scholar] [CrossRef]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalan, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, R.S.; Luxwolda, M.F.; Offringa, P.J.; Boersma, E.R.; Dijck-Brouwer, D.J.; Muskiet, F.A. Fetal intrauterine whole body linoleic, arachidonic and docosahexaenoic acid contents and accretion rates. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 13–20. [Google Scholar] [CrossRef]
- Uauy, R.; Hoffman, D.R.; Peirano, P.; Birch, D.; Birch, E. Essential fatty acids in visual and brain development. Lipids 2001, 36, 885–895. [Google Scholar] [CrossRef]
- Innis, S.M. Fatty acids and early human development. Early Hum. Dev. 2007, 83, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Perazzolo, S.; Hirschmugl, B.; Wadsack, C.; Desoye, G.; Lewis, R.; Sengers, B.G. The influence of placental metabolism on fatty acid transfer to the fetus. J. Lipid Res. 2017, 58, 443–454. [Google Scholar] [CrossRef]
- Gázquez, A.; Prieto-Sánchez, M.T.; Blanco-Carnero, J.E.; van Harskamp, D.; Perazzolo, S.; Oosterink, J.E.; Demmelmair, H.; Schierbeek, H.; Sengers, B.G.; Lewis, R.; et al. In vivokinetic study of materno-fetal fatty acid transfer in obese and normal weight pregnant women. J. Physiol. 2019, 597, 4959–4973. [Google Scholar] [CrossRef]
- Lewis, R.M.; Childs, C.E.; Calder, P.C. New perspectives on placental fatty acid transfer. Prostaglandins Leukot. Essent. Fat. Acids 2018, 138, 24–29. [Google Scholar] [CrossRef]
- Thornburg, K.L.; Kolahi, K.S.; Valent, A.M. What’s so special about lipid transport in the human placenta? J. Physiol. 2019, 597, 4863–4864. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental function in maternal obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef]
- Gázquez, A.; Prieto-Sánchez, M.T.; Blanco-Carnero, J.E.; Ruíz-Palacios, M.; Nieto, A.; van Harskamp, D.; Oosterink, J.E.; Schierbeek, H.; van Goudoever, J.B.; Demmelmair, H.; et al. Altered materno-fetal transfer of 13C-polyunsaturated fatty acids in obese pregnant women. Clin. Nutr. 2020, 39, 1101–1107. [Google Scholar] [CrossRef]
- Hirschmugl, B.; Perazzolo, S.; Sengers, B.G.; Lewis, R.M.; Gruber, M.; Desoye, G.; Wadsack, C. Placental mobilization of free fatty acids contributes to altered materno-fetal transfer in obesity. Int. J. Obes. 2021, 45, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Hirschmugl, B.; Desoye, G.; Catalano, P.; Klymiuk, I.; Scharnagl, H.; Payr, S.; Kitzinger, E.; Schliefsteiner, C.; Lang, U.; Wadsack, C.; et al. Maternal obesity modulates intracellular lipid turnover in the human term placenta. Int. J. Obes. 2017, 41, 317–323. [Google Scholar] [CrossRef]
- Calabuig-Navarro, V.; Haghiac, M.; Minium, J.; Glazebrook, P.; Ranasinghe, G.C.; Hoppel, C.; De-Mouzon, S.H.; Catalano, P.; O’Tierney-Ginn, P. Effect of Maternal Obesity on Placental Lipid Metabolism. Endocrinology 2017, 158, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.T.; Demmelmair, H.; Krauss-Etschmann, S.; Nathan, P.; Dehmel, S.; Padilla, M.C.; Rueda, R.; Koletzko, B.; Campoy, C. Maternal BMI and gestational diabetes alter placental lipid transporters and fatty acid composition. Placenta 2017, 57, 144–151. [Google Scholar] [CrossRef]
- Duttaroy, A.K.; Basak, S. Maternal dietary fatty acids and their roles in human placental development. Prostaglandins Leukot. Essent. Fat. Acids 2020, 155, 102080. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Bernlohr, D.A. Metabolic functions of FABPs—Mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 2015, 11, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Lager, S.; Ramirez, V.I.; Gaccioli, F.; Jang, B.; Jansson, T.; Powell, T.L. Protein expression of fatty acid transporter 2 is polarized to the trophoblast basal plasma membrane and increased in placentas from overweight/obese women. Placenta 2016, 40, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Dubé, E.; Gravel, A.; Martin, C.; Desparois, G.; Moussa, I.; Ethier-Chiasson, M.; Forest, J.-C.; Giguère, Y.; Masse, A.; Lafond, J. Modulation of Fatty Acid Transport and Metabolism by Maternal Obesity in the Human Full-Term Placenta1. Biol. Reprod. 2012, 87, 14. [Google Scholar] [CrossRef]
- Scifres, C.M.; Chen, B.; Nelson, D.M.; Sadovsky, Y. Fatty Acid Binding Protein 4 Regulates Intracellular Lipid Accumulation in Human Trophoblasts. J. Clin. Endocrinol. Metab. 2011, 96, E1083–E1091. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.D.; Polakoski, K.L.; Huang, X.; Sadovsky, Y.; Nelson, D. The release of 15-hydroxyeicosatetraenoic acid by human placental trophoblast is increased in preeclampsia. Am. J. Obstet. Gynecol. 1998, 178, 54–58. [Google Scholar] [CrossRef]
- Bilodeau, J.-F. Review: Maternal and placental antioxidant response to preeclampsia–Impact on vasoactive eicosanoids. Placenta 2014, 35, S32–S38. [Google Scholar] [CrossRef]
- WHO. Consultation on Obesity (1999: Geneva, Switzerland) & World Health Organization. (2000). Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation, World Health Organization. Available online: https://apps.who.int/iris/handle/10665/42330 (accessed on 1 July 2021).
- Institute of Medicine and National Research Council. Weight Gain during Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press: Washington, DC, USA, 2009; pp. 73–83. ISBN 9780309149150. [Google Scholar]
- Villar, J.; Ismail, L.C.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross–Sectional Study of the INTERGROWTH–21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Burton, G.; Sebire, N.; Myatt, L.; Tannetta, D.; Wang, Y.-L.; Sadovsky, Y.; Staff, A.; Redman, C. Optimising sample collection for placental research. Placenta 2014, 35, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Durand, T.; Cracowski, J.-L.; Guy, A.; Rossi, J.-C. Syntheses and preliminary pharmacological evaluation of the two epimers of the 5-F2t-isoprostane. Bioorg. Med. Chem. Lett. 2001, 11, 2495–2498. [Google Scholar] [CrossRef]
- Durand, T.; Guy, A.; Vidal, J.-P.; Rossi, J.-C. Total Synthesis of (15R)- and (15S)-F2t-Isoprostanes by a Biomimetic Process Using the Cyclization of Acyclic Dihydroxylated Octa-5,7-dienyl Radicals. J. Org. Chem. 2002, 67, 3615–3624. [Google Scholar] [CrossRef] [PubMed]
- Oger, C.; Brinkmann, Y.; Bouazzaoui, S.; Durand, T.; Galano, J.-M. Stereocontrolled Access to Isoprostanes via a Bicyclo[3.3.0]octene Framework. Org. Lett. 2008, 10, 5087–5090. [Google Scholar] [CrossRef] [PubMed]
- Oger, C.; Bultel-Poncé, V.; Guy, A.; Balas, L.; Durand, T.; Rossi, J.-C.; Galano, J.-M. The Handy Use of Brown’s P2-Ni Catalyst for a Skipped Diyne Deuteration: Application to the Synthesis of a [D4]-Labeled F4t-Neuroprostane. Chem. A. Eur. J. 2010, 16, 13976–13980. [Google Scholar] [CrossRef]
- Guy, A.; Oger, C.; Heppekausen, J.; Signorini, C.; De Felice, C.; Fürstner, A.; Durand, T.; Galano, J.-M. Oxygenated Metabolites ofn-3 Polyunsaturated Fatty Acids as Potential Oxidative Stress Biomarkers: Total Synthesis of 8-F3t-IsoP, 10-F4t-NeuroP and [D4]-10-F4t-NeuroP. Chem. A Eur. J. 2014, 20, 6374–6380. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cook, D.B.; McLucas, B.C.; Montoya, L.A.; Brotski, C.M.; Das, S.; Miholits, M.; Sebata, T.H. Multiplexing protein and gene level measurements on a single Luminex platform. Methods 2019, 158, 27–32. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Liggi, S.; Hinz, C.; Hall, Z.; Santoru, M.L.; Poddighe, S.; Fjeldsted, J.; Atzori, L.; Griffin, J.L. KniMet: A pipeline for the processing of chromatography–mass spectrometry metabolomics data. Metabolomics 2018, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.; Le Faouder, P.; Vigor, C.; Oger, C.; Galano, J.-M.; Dray, C.; Lee, J.C.-Y.; Valet, P.; Gladine, C.; Durand, T.; et al. Simultaneous quantitative profiling of 20 isoprostanoids from omega-3 and omega-6 polyunsaturated fatty acids by LC–MS/MS in various biological samples. Anal. Chim. Acta 2016, 921, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef]
- Ahmed, O.S.; Galano, J.-M.; Pavlickova, T.; Revol-Cavalier, J.; Vigor, C.; Lee, J.C.-Y.; Oger, C.; Durand, T. Moving forward with isoprostanes, neuroprostanes and phytoprostanes: Where are we now? Essays Biochem. 2020, 64, 463–484. [Google Scholar] [CrossRef]
- Wallace, M.K.; Shivappa, N.; Wirth, M.D.; Hébert, J.R.; Huston-Gordesky, L.; Alvarado, F.; Mouzon, S.H.-D.; Catalano, P.M. Longitudinal Assessment of Relationships Between Health Behaviors and IL-6 in Overweight and Obese Pregnancy. Biol. Res. Nurs. 2021, 23, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.; Lager, S.; Ramirez, V.I.; Gaccioli, F.; Dudley, D.J.; Jansson, T.; Powell, T. Increasing Maternal Body Mass Index Is Associated with Systemic Inflammation in the Mother and the Activation of Distinct Placental Inflammatory Pathways1. Biol. Reprod. 2014, 90, 129. [Google Scholar] [CrossRef] [PubMed]
- Martino, J.; Sebert, S.; Segura, M.T.; García-Valdés, L.; Florido, J.; Padilla, M.C.; Marcos, A.; Rueda, R.; McArdle, H.J.; Budge, H.; et al. Maternal Body Weight and Gestational Diabetes Differentially Influence Placental and Pregnancy Outcomes. J. Clin. Endocrinol. Metab. 2016, 101, 59–68. [Google Scholar] [CrossRef]
- Nogues, P.; Dos Santos, E.; Couturier-Tarrade, A.; Berveiller, P.; Arnould, L.; Lamy, E.; Grassin-Delyle, S.; Vialard, F.; Dieudonne, M.-N. Maternal Obesity Influences Placental Nutrient Transport, Inflammatory Status, and Morphology in Human Term Placenta. J. Clin. Endocrinol. Metab. 2021, 106, e1880–e1896. [Google Scholar] [CrossRef]
- Fowden, A.L.; Camm, E.; Sferruzzi-Perri, A. Effects of Maternal Obesity On Placental Phenotype. Curr. Vasc. Pharmacol. 2020, 19, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef]
- Lewis, R.; Desoye, G. Placental Lipid and Fatty Acid Transfer in Maternal Overnutrition. Ann. Nutr. Metab. 2017, 70, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Easton, Z.J.W.; Regnault, T.R.H. The Impact of Maternal Body Composition and Dietary Fat Consumption upon Placental Lipid Processing and Offspring Metabolic Health. Nutrients 2020, 12, 3031. [Google Scholar] [CrossRef]
- Wang, G.; Bonkovsky, H.L.; de Lemos, A.; Burczynski, F.J. Recent insights into the biological functions of liver fatty acid binding protein 1. J. Lipid Res. 2015, 56, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Schachtrup, C.; Emmler, T.; Bleck, B.; Sandqvist, A.; Spener, F. Functional analysis of peroxisome-proliferator-responsive element motifs in genes of fatty acid-binding proteins. Biochem. J. 2004, 382, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, C.; Borrmann, C.M.; Börchers, T.; Spener, F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors-and-mediated gene expression via liver fatty acid binding protein: A signaling path to the nucleus. Proc. Natl. Acad. Sci. USA 2001, 98, 2323–2328. [Google Scholar] [CrossRef]
- Peng, L.; Yang, H.; Ye, Y.; Ma, Z.; Kuhn, C.; Rahmeh, M.; Mahner, S.; Makrigiannakis, A.; Jeschke, U.; Von Schönfeldt, V. Role of Peroxisome Proliferator-Activated Receptors (PPARs) in Trophoblast Functions. Int. J. Mol. Sci. 2021, 22, 433. [Google Scholar] [CrossRef]
- Schaiff, W.T.; Carlson, M.G.; Smith, S.D.; Levy, R.; Nelson, D.M.; Sadovsky, Y. Peroxisome Proliferator-Activated Receptor? Modulates Differentiation of Human Trophoblast in a Ligand-Specific Manner 1. J. Clin. Endocrinol. Metab. 2000, 85, 3874–3881. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; Glazebrook, P.; Ranasinghe, G.C.; Haghiac, M.; Calabuig-Navarro, V.; Minium, J.; O’Tierney-Ginn, P. Fatty acid transporter expression and regulation is impaired in placental macrovascular endothelial cells in obese women. J. Matern. Neonatal Med. 2017, 32, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.; Lindsey, F.; Zhong, Y.; Thakali, K.; Badger, T.; Andres, A.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity is associated with a lipotoxic placental environment. Placenta 2014, 35, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.J.; Premji, M.; Netherton, S.; Coruzzi, J.; Lu-Chao, H.; Cox, P.G. Vasoconstrictor actions of isoprostanes via tyrosine kinase and Rho kinase in human and canine pulmonary vascular smooth muscles. Br. J. Pharmacol. 2001, 132, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M.; Makita, N.; Roberts, L.J.; Morrow, J.D.; Takahashi, K.; Badr, K.F. Evidence for the existence of F2-isoprostane receptors on rat vascular smooth muscle cells. Am. J. Physiol. Physiol. 1993, 264, C1619–C1624. [Google Scholar] [CrossRef]
- Musiek, E.; Brooks, J.D.; Joo, M.; Brunoldi, E.; Porta, A.; Zanoni, G.; Vidari, G.; Blackwell, T.S.; Montine, T.J.; Milne, G.; et al. Electrophilic Cyclopentenone Neuroprostanes Are Anti-inflammatory Mediators Formed from the Peroxidation of the ω-3 Polyunsaturated Fatty Acid Docosahexaenoic Acid. J. Biol. Chem. 2008, 283, 19927–19935. [Google Scholar] [CrossRef]
- Bosviel, R.; Joumard-Cubizolles, L.; Chinetti-Gbaguidi, G.; Bayle, D.; Copin, C.; Hennuyer, N.; Duplan, I.; Staels, B.; Zanoni, G.; Porta, A.; et al. DHA-derived oxylipins, neuroprostanes and protectins, differentially and dose-dependently modulate the inflammatory response in human macrophages: Putative mechanisms through PPAR activation. Free. Radic. Biol. Med. 2017, 103, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Galano, J.; Leung, H.H.; Balas, L.; Oger, C.; Durand, T.; Lee, J.C. Nonenzymatic oxygenated metabolite of docosahexaenoic acid, 4(RS)-4-F4t-neuroprostane, acts as a bioactive lipid molecule in neuronal cells. FEBS Lett. 2020, 594, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Schild, R.L.; Schaiff, W.T.; Carlson, M.G.; Cronbach, E.J.; Nelson, D.M.; Sadovsky, Y. The Activity of PPARγ in Primary Human Trophoblasts Is Enhanced by Oxidized Lipids. J. Clin. Endocrinol. Metab. 2002, 87, 1105–1110. [Google Scholar] [CrossRef][Green Version]
- Brien, M.; LaRose, J.; Greffard, K.; Julien, P.; Bilodeau, J. Increased placental phospholipase A 2 gene expression and free F 2-isoprostane levels in response to oxidative stress in preeclampsia. Placenta 2017, 55, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Varastehpour, A.; Radaelli, T.; Minium, J.; Ortega-Senovilla, H.; Herrera, E.; Catalano, P.; Mouzon, S.H.-D. Activation of Phospholipase A2 Is Associated with Generation of Placental Lipid Signals and Fetal Obesity. J. Clin. Endocrinol. Metab. 2006, 91, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Gauster, M.; Hiden, U.; Van Poppel, M.; Frank, S.; Wadsack, C.; Mouzon, S.H.-D.; Desoye, G. Dysregulation of Placental Endothelial Lipase in Obese Women With Gestational Diabetes Mellitus. Diabetes 2011, 60, 2457–2464. [Google Scholar] [CrossRef]
- Voerman, E.; Santos, S.; Golab, B.P.; Amiano, P.; Ballester, F.; Barros, H.; Bergström, A.; Charles, M.-A.; Chatzi, L.; Chevrier, C.; et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef] [PubMed]
- Josefson, J.L.; Scholtens, D.M.; Kuang, A.; Catalano, P.M.; Lowe, L.P.; Dyer, A.R.; Petito, L.C.; Lowe, W.L.; Metzger, B.E. Newborn Adiposity and Cord Blood C-Peptide as Mediators of the Maternal Metabolic Environment and Childhood Adiposity. Diabetes Care 2021, 44, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.L.; Barner, K.; Madi, L.; Armstrong, M.; Manke, J.; Uhlson, C.; Jansson, T.; Ferchaud-Roucher, V. Sex-specific responses in placental fatty acid oxidation, esterification and transfer capacity to maternal obesity. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2021, 1866, 158861. [Google Scholar] [CrossRef]
- Alvarado, F.L.; Calabuig-Navarro, V.; Haghiac, M.; Puchowicz, M.; Tsai, P.-J.S.; O’Tierney-Ginn, P. Maternal obesity is not associated with placental lipid accumulation in women with high omega-3 fatty acid levels. Placenta 2018, 69, 96–101. [Google Scholar] [CrossRef]
- Monthé-Drèze, C.; Sen, S.; Mouzon, S.H.-D.; Catalano, P.M. Effect of Omega-3 Supplementation in Pregnant Women with Obesity on Newborn Body Composition, Growth and Length of Gestation: A Randomized Controlled Pilot Study. Nutrients 2021, 13, 578. [Google Scholar] [CrossRef] [PubMed]

| Mothers | ||
|---|---|---|
| Lean (n = 6) | Obese (n = 6) | |
| Maternal age (years) a | 26.0 (19.0–32.0) | 26.5 (20.0–31.0) |
| Maternal pre-gestational BMI (kg/m2) a | 22.0 (19.0–24.2) | 34.3 (31.2–37.6) * |
| Gestational weight gain (kg) a | 11.6 (10.1–21.3) | 6.6 (1.5–19.0) |
| Gestational age (weeks) | 40.5 (38.0–41.0) | 39.0 (38.0–41.0) |
| Complications of pregnancy (other than pre-gestational obesity) | none | none |
| Delivery mode (n; %) | vaginal (2; 33); c/s B (4; 66) | vaginal (1; 17); c/s B (5; 83) |
| Newborn | ||
| Placental weight (g) a | 485.0 (400.0–555.0) | 480.0 (480.0–575.0) |
| Placental efficiency (birth weight:placental weight ratio) | 6.9 (6.1–10.0) | 6.9 (6.4–7.4) |
| Birth weight (kg) a | 3.3 (3.1–4.0) | 3.4 (3.0–4.0) |
| Birth length (cm) a | 49.5 (46.0–53.5) | 48.5 (45.0–52.0) |
| Dependent Variables | Independent Variables | β Coefficients | Adj. R2 | Estimated Error (%) 1 | p2 | ||
|---|---|---|---|---|---|---|---|
| Value | SE | p-Value | |||||
| Σ Neuroprostanes in placenta Model 1 | Pre-gestational BMI | 1.64 × 102 | 2.61 × 101 | 0.0004 | 93.88 | 20.1 | 0.0000 |
| TNF-α, placenta | −5.56 × 103 | 1.59 × 103 | 0.0101 | ||||
| Gestational weight gain | ̶ | ̶ | ns | ̶ | ̶ | ns | |
| IL-6, placenta | ̶ | ̶ | ns | ̶ | ̶ | ns | |
| Endothelial lipase, protein | ̶ | ̶ | ns | ̶ | ̶ | ns | |
| FABP-1, protein | ̶ | ̶ | ns | ̶ | ̶ | ns | |
| Σ Isoprostanes in placenta Model 2 | Endothelial lipase, protein | 1.21 × 104 | 1.12 × 103 | 0.0000 | 93.65 | 22.9 | 0.0000 |
| Pre-gestational BMI | ̶ | ̶ | ns | ̶ | ̶ | ns | |
| Gestational weight gain | ̶ | ̶ | ns | ̶ | ̶ | ns | |
| TNF-α, placenta | ̶ | ̶ | ns | ̶ | ̶ | ns | |
| IL-6, placenta | ̶ | ̶ | ns | ̶ | ̶ | ns | |
| Birth weight Model 3 | Pre-gestational BMI | 1.26 × 102 | 1.38 × 101 | 0.0001 | 99.07 | 6.83 | 0.0000 |
| Gestational weight gain | 7.72 × 101 | 1.71 × 101 | 0.0040 | ||||
| Σ Neuroprostanes, placenta | −4.06 × 10−1 | 1.37 × 10−1 | 0.0253 | ||||
| Σ Isoprostanes, placenta | ̶ | ̶ | ns | ̶ | ̶ | ns | |
| IL-6, placenta | ̶ | ̶ | ns | ̶ | ̶ | ns | |
| TNF-α, placenta | ̶ | ̶ | ns | ̶ | ̶ | ns | |
| Endothelial lipase, protein | ̶ | ̶ | ns | ̶ | ̶ | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belcastro, L.; Ferreira, C.S.; Saraiva, M.A.; Mucci, D.B.; Murgia, A.; Lai, C.; Vigor, C.; Oger, C.; Galano, J.-M.; Pinto, G.D.A.; et al. Decreased Fatty Acid Transporter FABP1 and Increased Isoprostanes and Neuroprostanes in the Human Term Placenta: Implications for Inflammation and Birth Weight in Maternal Pre-Gestational Obesity. Nutrients 2021, 13, 2768. https://doi.org/10.3390/nu13082768
Belcastro L, Ferreira CS, Saraiva MA, Mucci DB, Murgia A, Lai C, Vigor C, Oger C, Galano J-M, Pinto GDA, et al. Decreased Fatty Acid Transporter FABP1 and Increased Isoprostanes and Neuroprostanes in the Human Term Placenta: Implications for Inflammation and Birth Weight in Maternal Pre-Gestational Obesity. Nutrients. 2021; 13(8):2768. https://doi.org/10.3390/nu13082768
Chicago/Turabian StyleBelcastro, Livia, Carolina S. Ferreira, Marcelle A. Saraiva, Daniela B. Mucci, Antonio Murgia, Carla Lai, Claire Vigor, Camille Oger, Jean-Marie Galano, Gabriela D. A. Pinto, and et al. 2021. "Decreased Fatty Acid Transporter FABP1 and Increased Isoprostanes and Neuroprostanes in the Human Term Placenta: Implications for Inflammation and Birth Weight in Maternal Pre-Gestational Obesity" Nutrients 13, no. 8: 2768. https://doi.org/10.3390/nu13082768
APA StyleBelcastro, L., Ferreira, C. S., Saraiva, M. A., Mucci, D. B., Murgia, A., Lai, C., Vigor, C., Oger, C., Galano, J.-M., Pinto, G. D. A., Griffin, J. L., Torres, A. G., Durand, T., Burton, G. J., Sardinha, F. L. C., & El-Bacha, T. (2021). Decreased Fatty Acid Transporter FABP1 and Increased Isoprostanes and Neuroprostanes in the Human Term Placenta: Implications for Inflammation and Birth Weight in Maternal Pre-Gestational Obesity. Nutrients, 13(8), 2768. https://doi.org/10.3390/nu13082768







