Healthy Eating Is Associated with Sarcopenia Risk in Physically Active Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Assessment of Dietary Intake
2.3. Assessment of Anthropometry and Components of Sarcopenia Risk
2.4. Assessment of Adherence to PA Guidelines
2.5. Assessment of High-Sensitivity C-Reactive Protein (hs-CRP)
2.6. Statistical Analysis
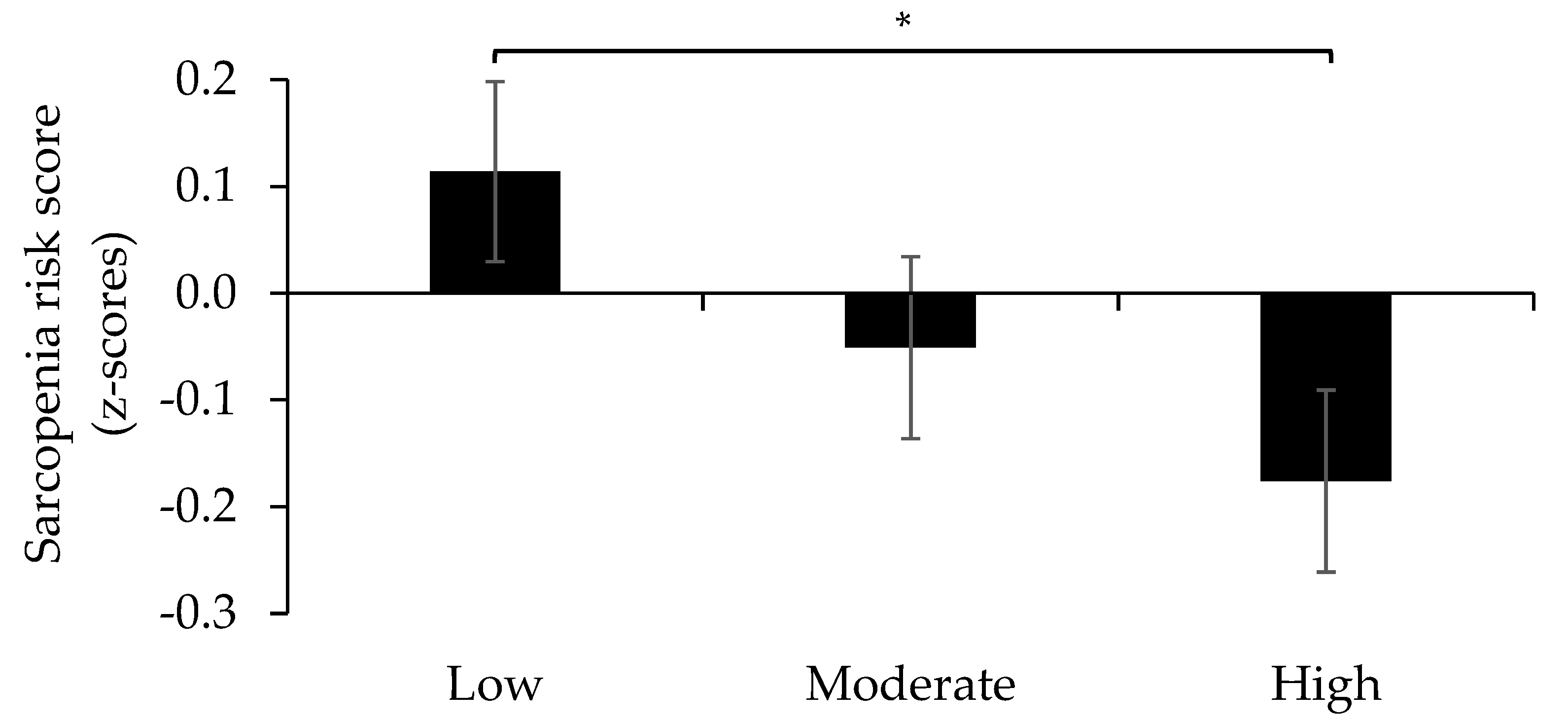
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Bahat, G.; Tufan, A.; Tufan, F.; Kilic, C.; Akpinar, T.S.; Kose, M.; Erten, N.; Karan, M.A.; Cruz-Jentoft, A.J. Cut-off points to identify Sarcopenia Accord. to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clin. Nutr. 2016, 35, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Makanae, Y.; Fujita, S. Role of exercise and nutrition in the prevention of sarcopenia. J. Nutr. Sci. Vitaminol. 2015, 61, S125–S127. [Google Scholar] [CrossRef] [Green Version]
- Anton, S.; Hida, A.; Mankowski, R.; Layne, A.; Solberg, L.; Mainous, A.; Buford, T. Nutrition and Exercise in Sarcopenia. Curr. Protein Pept. Sci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Pardo, P.J.; González-Gálvez, N.; López-Vivancos, A.; Espeso-García, A.; Martínez-Aranda, L.M.; Gea-García, G.M.; Orquín-Castrillón, F.J.; Carbonell-Baeza, A.; Jiménez-García, J.D.; Velázquez-Díaz, D.; et al. Sarcopenia, diet, physical activity and obesity in european middle-aged and older adults: The lifeage study. Nutrients 2021, 13, 8. [Google Scholar] [CrossRef]
- Kiesswetter, E.; Sieber, C.C.; Volkert, D. Protein intake in older people: Why, how much and how? Zeitschrift Gerontologie Geriatrie 2020, 53, 285–289. [Google Scholar] [CrossRef]
- Beasley, J.M.; Deierlein, A.L.; Morland, K.B.; Granieri, E.C.; Spark, A. Is meeting the recommended dietary allowance (RDA) for protein related to body composition among older adults?: Results from the Cardiovascular Health of Seniors and Built Environment Study. J. Nutr. Health Aging 2016, 20, 790–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baum, J.; Wolfe, R. The Link between Dietary Protein Intake, Skeletal Muscle Function and Health in Older Adults. Healthcare 2015, 3, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, A.; Rojas, D.M.; Kadi, F. Impact of meeting different guidelines for protein intake on muscle mass and physical function in physically active older women. Nutrients 2018, 10, 1156. [Google Scholar] [CrossRef] [Green Version]
- Robinson, S.M.; Reginster, J.Y.; Rizzoli, R.; Shaw, S.C.; Kanis, J.A.; Bautmans, I.; Bischoff-Ferrari, H.; Bruyère, O.; Cesari, M.; Dawson-Hughes, B.; et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin. Nutr. 2018, 37, 1121–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, I.; Shand, C.; Cooper, C.; Robinson, S.; Baird, J. Diet quality and sarcopenia in older adults: A systematic review. Nutrients 2018, 10, 308. [Google Scholar] [CrossRef] [Green Version]
- Schoenfeld, B.J.; Ogborn, D.; Krieger, J.W. Effects of Resistance Training Frequency on Measures of Muscle Hypertrophy: A Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Veen, J.; Montiel-Rojas, D.; Nilsson, A.; Kadi, F. Engagement in muscle-strengthening activities lowers sarcopenia risk in older adults already adhering to the aerobic physical activity guidelines. Int. J. Environ. Res. Public Health 2021, 18, 989. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Wåhlin-Larsson, B.; Wilkinson, D.J.; Strandberg, E.; Hosford-Donovan, A.; Atherton, P.J.; Kadi, F. Mechanistic Links Underlying the Impact of C-Reactive Protein on Muscle Mass in Elderly. Cell. Physiol. Biochem. 2017, 44, 267–278. [Google Scholar] [CrossRef]
- Bergens, O.; Nilsson, A.; Kadi, F. Cardiorespiratory Fitness Does Not Offset Adiposity-Related Systemic Inflammation in Physically Active Older Women. J. Clin. Endocrinol. Metab. 2019, 104, 4119–4126. [Google Scholar] [CrossRef]
- Johansson, I.; Hallmans, G.; Wikman, Å.; Biessy, C.; Riboli, E.; Kaaks, R. Validation and calibration of food-frequency questionnaire measurements in the Northern Sweden Health and Disease cohort. Public Health Nutr. 2002, 5, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Van Guelpen, B.; Hultdin, J.; Johansson, M.; Hallmans, G.; Stattin, P. Validity of food frequency questionnaire estimated intakes of folate and other B vitamins in a region without folic acid fortification. Eur. J. Clin. Nutr. 2010, 64, 905–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkvist, A.; Klingberg, S.; Nilsson, L.M.; Wennberg, M.; Renström, F.; Hallmans, G.; Boman, K.; Johansson, I. Longitudinal 10-year changes in dietary intake and associations with cardio-metabolic risk factors in the Northern Sweden Health and Disease Study. Nutr. J. 2017, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the prot-age study group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Montiel-Rojas, D.; Nilsson, A.; Santoro, A.; Bazzocchi, A.; de Groot, L.C.P.G.M.; Feskens, E.J.M.; Berendsen, A.A.M.; Madej, D.; Kaluza, J.; Pietruszka, B.; et al. Fighting sarcopenia in ageing european adults: The importance of the amount and source of dietary proteins. Nutrients 2020, 12, 3601. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.; Wåhlin-Larsson, B.; Kadi, F. Physical activity and not sedentary time per se influences on clustered metabolic risk in elderly community-dwelling women. PLoS ONE 2017, 12, e0175496. [Google Scholar] [CrossRef] [PubMed]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; Mcdowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Wareham, N.J.; Jakes, R.W.; Rennie, K.L.; Mitchell, J.; Hennings, S.; Day, N.E. Validity and repeatability of the EPIC-Norfolk physical activity questionnaire. Int. J. Epidemiol. 2002, 31, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Na, W.; Kim, J.; Chung, B.H.; Jang, D.J.; Sohn, C. Relationship between diet quality and sarcopenia in elderly Koreans: 2008–2011 Korea national health and nutrition examination survey. Nutr. Res. Pract. 2020, 14, 352–364. [Google Scholar] [CrossRef]
- Perälä, M.M.; Von Bonsdorff, M.B.; Männistö, S.; Salonen, M.K.; Simonen, M.; Kanerva, N.; Rantanen, T.; Pohjolainen, P.; Eriksson, J.G. The healthy Nordic diet predicts muscle strength 10 years later in old women, but not old men. Age Ageing 2017, 46, 588–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, R.; Motlagh, A.D.; Heshmat, R.; Esmaillzadeh, A.; Payab, M.; Yousefinia, M.; Siassi, F.; Pasalar, P.; Baygi, F. Diet and its relationship to sarcopenia in community dwelling iranian elderly: A cross sectional study. Nutrition 2015, 31, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Leung, J.; Woo, J. A Prospective Cohort Study to Examine the Association Between Dietary Patterns and Sarcopenia in Chinese Community-Dwelling Older People in Hong Kong. J. Am. Med. Dir. Assoc. 2016, 17, 336–342. [Google Scholar] [CrossRef]
- Granic, A.; Mendonça, N.; Sayer, A.A.; Hill, T.R.; Davies, K.; Siervo, M.; Mathers, J.C.; Jagger, C. Effects of dietary patterns and low protein intake on sarcopenia risk in the very old: The Newcastle 85+ study. Clin. Nutr. 2020, 39, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Breen, L.; Stokes, K.A.; Churchward-Venne, T.A.; Moore, D.R.; Baker, S.K.; Smith, K.; Atherton, P.J.; Phillips, S.M. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J. Clin. Endocrinol. Metab. 2013, 98, 2604–2612. [Google Scholar] [CrossRef] [Green Version]
- Coelho-Júnior, H.J.; Milano-Teixeira, L.; Rodrigues, B.; Bacurau, R.; Marzetti, E.; Uchida, M. Relative protein intake and physical function in older adults: A systematic review and meta-analysis of observational studies. Nutrients 2018, 10, 1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Nilsson, A.; Bergens, O.; Kadi, F. Physical Activity Alters Inflammation in Older Adults by Different Intensity Levels. Med. Sci. Sports Exerc. 2018, 50, 1502–1507. [Google Scholar] [CrossRef]
- Welch, A.A. Nutritional influences on age-related skeletal muscle loss. Proc. Nutr. Soc. 2014, 73, 16–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.S.; Kim, K.M. Association of vegetables and fruits consumption with sarcopenia in older adults: The fourth Korea national health and nutrition examination survey. Age Ageing 2015, 44, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Montiel-Rojas, D.; Santoro, A.; Nilsson, A.; Franceschi, C.; Capri, M.; Bazzocchi, A.; Battista, G.; de Groot, L.C.P.G.M.; Feskens, E.J.M.; Berendsen, A.A.M.; et al. Beneficial role of replacing dietary saturated fatty acids with polyunsaturated fatty acids in the prevention of sarcopenia: Findings from the nu- age cohort. Nutrients 2020, 12, 3079. [Google Scholar] [CrossRef]
- Montiel-Rojas, D.; Nilsson, A.; Santoro, A.; Franceschi, C.; Bazzocchi, A.; Battista, G.; de Groot, L.C.P.G.M.; Feskens, E.J.M.; Berendsen, A.; Pietruszka, B.; et al. Dietary fibre may mitigate sarcopenia risk: Findings from the NU-AGE cohort of older european adults. Nutrients 2020, 12, 1075. [Google Scholar] [CrossRef]
- Molag, M.L.; De Vries, J.H.M.; Ocké, M.C.; Dagnelie, P.C.; Van Den Brandt, P.A.; Jansen, M.C.J.F.; Van Staveren, W.A.; Van’t Veer, P. Design characteristics of food frequency questionnaires in relation to their validity. Am. J. Epidemiol. 2007, 166, 1468–1478. [Google Scholar] [CrossRef] [Green Version]



| Men | Women | |
|---|---|---|
| Anthropometrics | ||
| Height (cm) | 178.2 ± 6 | 164.6 ± 5.8 * |
| Weight (kg) | 79.9 ± 11.1 | 63.4 ± 9.0 * |
| BMI (kg/m2) | 25.1 ± 3.0 | 23.4 ± 3.2 * |
| Waist circumference (cm) | 93.4 ± 9.9 | 78.9 ± 8.5 * |
| Sarcopenia Risk | ||
| Skeletal Mass Index (%) | 34.6± 3.1 | 26.9 ± 3.4 * |
| Hand grip per body weight (g·kg−1 bodyweight) | 0.56 ± 0.10 | 0.45 ± 0.08 * |
| 5 Sit-to-Stand test (s) | 10.1 ± 1.9 | 10.2 ± 2.3 |
| Low HDS (n = 69) | Moderate HDS (n = 61) | High HDS (n = 61) | |
| Favorable food groups | |||
| Vegetables | 81.5 (36.7–148.5) | 125.2 (75.8–200.6) | 176.6 (113.4–247) |
| Fruit | 110.6 (70.6–196.4) | 165.4 (90.3–239.7) | 236.8 (161.9–317.2) |
| Fish | 24.8 (20.3–32.7) | 29.5 (18.1–45.0) | 35.9 (24–46.3) |
| Whole grain | 55.9 (35.1–68.0) | 57.8 (38.4–78.4) | 67.4 (50–91.4) |
| Unfavorable food groups | |||
| Sugar sweetened beverages | 34.5 (12.7–107.1) | 19.1 (1.3–89.7) | 12.6 (1–54.3) |
| Red/processed meat | 55.1 (41.8–74.7) | 48.5 (29.7–62.9) | 36.1 (13.4–54.8) |
| Desserts and sweets | 14.4 (9.6–28.7) | 11.1 (7.4–23.0) | 9.7 (5.0 –13.1) |
| Fried potatoes | 14.0 (8.0 –21.4) | 8.2 (0.5–17.1) | 8.2 (0.53–13.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaioannou, K.-G.; Nilsson, A.; Nilsson, L.M.; Kadi, F. Healthy Eating Is Associated with Sarcopenia Risk in Physically Active Older Adults. Nutrients 2021, 13, 2813. https://doi.org/10.3390/nu13082813
Papaioannou K-G, Nilsson A, Nilsson LM, Kadi F. Healthy Eating Is Associated with Sarcopenia Risk in Physically Active Older Adults. Nutrients. 2021; 13(8):2813. https://doi.org/10.3390/nu13082813
Chicago/Turabian StylePapaioannou, Konstantinos-Georgios, Andreas Nilsson, Lena Maria Nilsson, and Fawzi Kadi. 2021. "Healthy Eating Is Associated with Sarcopenia Risk in Physically Active Older Adults" Nutrients 13, no. 8: 2813. https://doi.org/10.3390/nu13082813
APA StylePapaioannou, K.-G., Nilsson, A., Nilsson, L. M., & Kadi, F. (2021). Healthy Eating Is Associated with Sarcopenia Risk in Physically Active Older Adults. Nutrients, 13(8), 2813. https://doi.org/10.3390/nu13082813









