Effect of Protein-Rich Breakfast on Subsequent Energy Intake and Subjective Appetite in Children and Adolescents: Systematic Review and Meta–Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Selection Criteria
2.3. Data Extraction
2.4. Appraisal of the Quality of Studies
2.5. Data Synthesis
3. Results
3.1. Literature Search and Screening
3.2. Study Design Characteristics
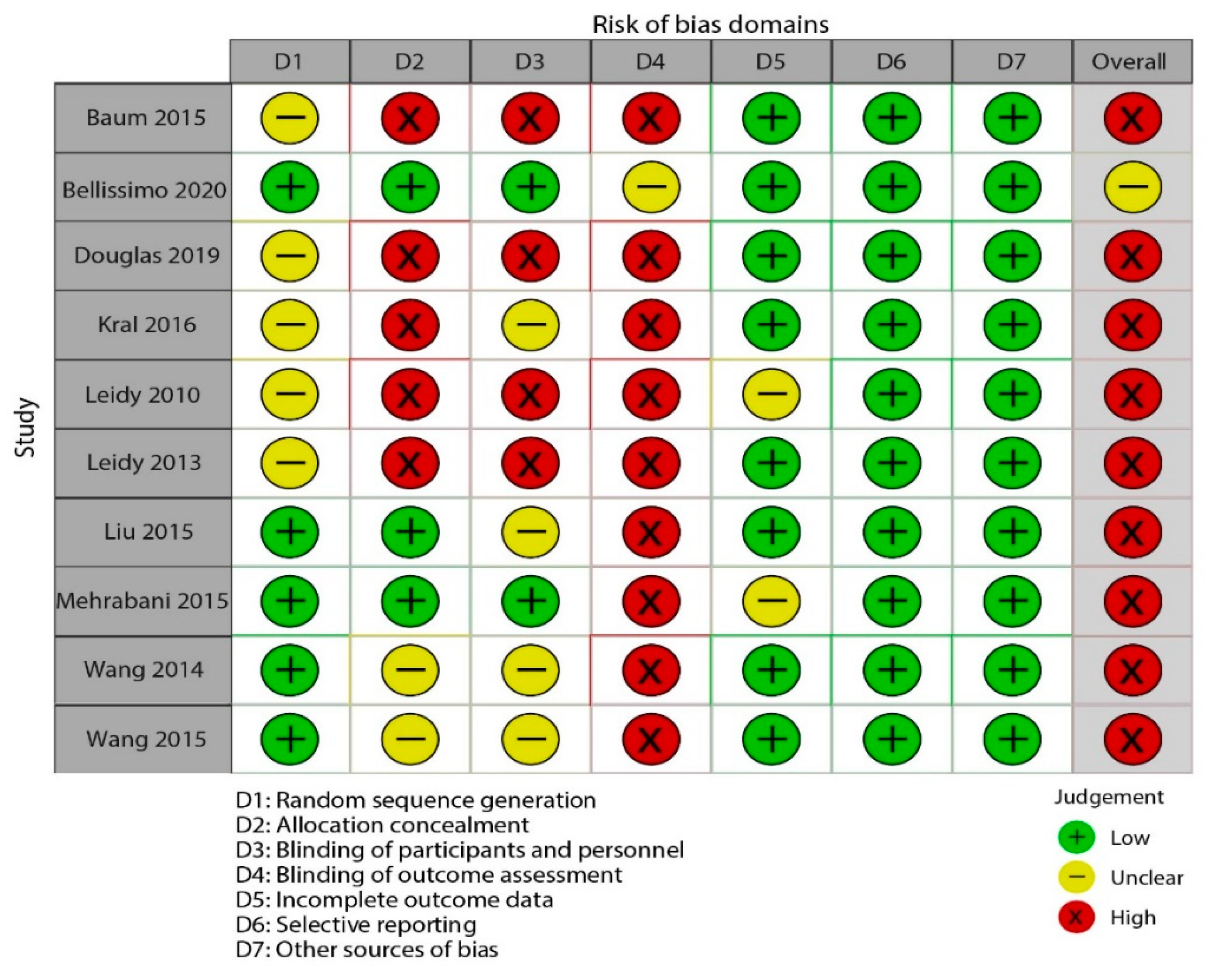
3.3. Risk of Bias across Studies
3.4. Findings from Meta–Analysis
3.4.1. Protein–Rich Breakfast and Subsequent Energy Intake
3.4.2. Breakfast and Subjective Appetite
Protein–Rich Breakfast and Fullness
Protein–Rich Breakfast and Hunger
4. Discussion
4.1. Principal Findings
4.2. Quality of Evidence
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- LBD Double Burden of Malnutrition Collaborators. Mapping local patterns of childhood overweight and wasting in low- and middle-income countries between 2000 and 2017. Nat. Med. 2020, 26, 750–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Strategy on Diet, Physical Activity and Health; WHO Technical Report; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Cavalcanti-De-Albuquerque, J.P.; Bober, J.; Zimmer, M.R.; Dietrich, M. Regulation of substrate utilization and adiposity by Agrp neurons. Nat. Commun. 2019, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Spiegelman, B.M. Towards a molecular understanding of adaptive thermogenesis. Nature 2000, 404, 652–660. [Google Scholar] [CrossRef]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef] [Green Version]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef] [Green Version]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Zolotarjova, J.; Velde, G.T.; Vreugdenhil, A.C.E. Effects of multidisciplinary interventions on weight loss and health outcomes in children and adolescents with morbid obesity. Obes. Rev. 2018, 19, 931–946. [Google Scholar] [CrossRef] [Green Version]
- Coles, N.; Birken, C.; Hamilton, J. Emerging treatments for severe obesity in children and adolescents. BMJ 2016, 354, i4116. [Google Scholar] [CrossRef]
- O’Neil, C.E.; Byrd-Bredbenner, C.; Hayes, D.; Jana, L.; Klinger, S.E.; Stephenson-Martin, S. The role of breakfast in health: Definition and criteria for a quality breakfast. J. Acad. Nutr. Diet. 2014, 114, S8–S26. [Google Scholar] [CrossRef]
- Larsen, T.M.; Dalskov, S.-M.; van Baak, M.; Jebb, S.A.; Papadaki, A.; Pfeiffer, A.F.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunešová, M.; Pihlsgård, M.; et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N. Engl. J. Med. 2010, 363, 2102–2113. [Google Scholar] [CrossRef] [Green Version]
- Baum, J.I.; Gray, M.; Binns, A. Breakfasts higher in protein increase postprandial energy expenditure, increase fat oxidation, and reduce hunger in overweight children from 8 to 12 years of age. J. Nutr. 2015, 145, 2229–2235. [Google Scholar] [CrossRef]
- Liu, A.G.; Puyau, R.S.; Han, H.; Johnson, W.D.; Greenway, F.L.; Dhurandhar, N.V. The effect of an egg breakfast on satiety in children and adolescents: A randomized crossover trial. J. Am. Coll. Nutr. 2015, 34, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Kral, T.V.; Bannon, A.L.; Chittams, J.L.; Moore, R.H. Comparison of the satiating properties of egg- versus cereal grain-based breakfasts for appetite and energy intake control in children. Eat. Behav. 2016, 20, 14–20. [Google Scholar] [CrossRef]
- Leidy, H.J.; Racki, E.M. The addition of a protein-rich breakfast and its effects on acute appetite control and food intake in ‘breakfast-skipping’ adolescents. Int. J. Obes. 2010, 34, 1125–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S. The Effects of High-Protein Breakfast on Food Intake, Appetite and Body Weight. Ph.D. Thesis, Chinese PLA Medical College, Beijing, China, 2014. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kant, A.K.; Graubard, B.I. 40-year trends in meal and snack eating behaviors of american adults. J. Acad. Nutr. Diet. 2015, 115, 50–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellissimo, N.; Fansabedian, T.; Wong, V.; de Zepetnek, J.T.; Brett, N.; Schwartz, A.; Cassin, S.; Suitor, K.; Rousseau, D. Effect of increasing the dietary protein content of breakfast on subjective appetite, short-term food intake and diet-induced thermogenesis in children. Nutrients 2020, 12, 3025. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.M.; Byers, A.W.; Leidy, H.J. Habitual breakfast patterns do not influence appetite and satiety responses in normal vs. high-protein breakfasts in overweight adolescent girls. Nutrients 2019, 11, 1223. [Google Scholar] [CrossRef] [Green Version]
- Leidy, H.J.; Ortinau, L.C.; Douglas, S.M.; Hoertel, H.A. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, “breakfast-skipping,” late-adolescent girls. Am. J. Clin. Nutr. 2013, 97, 677–688. [Google Scholar] [CrossRef]
- Mehrabani, S.; Safavi, S.M.; Mehrabani, S.; Asemi, M.; Feizi, A.; Bellissimo, N.; Salehi-Abargouei, A. Effects of low-fat milk consumption at breakfast on satiety and short-term energy intake in 10- to 12-year-old obese boys. Eur. J. Nutr. 2015, 55, 1389–1396. [Google Scholar] [CrossRef]
- Wang, S.; Yang, L.; Lu, J.; Mu, Y. High-protein breakfast promotes weight loss by suppressing subsequent food intake and regulating appetite hormones in obese chinese adolescents. Horm. Res. Paediatr. 2014, 83, 19–25. [Google Scholar] [CrossRef]
- Rosato, V.; Edefonti, V.C.; Parpinel, M.; Milani, G.P.; Mazzocchi, A.; DeCarli, A.; Agostoni, C.; Ferraroni, M. Energy contribution and nutrient composition of breakfast and their relations to overweight in free-living individuals: A systematic review. Adv. Nutr. 2016, 7, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Gosby, A.K.; Conigrave, A.D.; Raubenheimer, D.; Simpson, S.J. Protein leverage and energy intake. Obes. Rev. 2014, 15, 183–191. [Google Scholar] [CrossRef]
- De Carvalho, K.M.B.; Pizato, N.; Botelho, P.B.; Dutra, E.S.; Gonçalves, V.S.S. Dietary protein and appetite sensations in individuals with overweight and obesity: A systematic review. Eur. J. Nutr. 2020, 59, 2317–2332. [Google Scholar] [CrossRef] [PubMed]
- Nepocatych, S.; Melson, C.E.; Madzima, T.A.; Balilionis, G. Comparison of the effects of a liquid breakfast meal with varying doses of plant-based soy protein on appetite profile, energy metabolism and intake. Appetite 2019, 141, 104322. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Kassis, A.; Godin, J.-P.; Moille, S.E.; Nielsen-Moennoz, C.; Groulx, K.; Oguey-Araymon, S.; Praplan, F.; Beaumont, M.; Sauser, J.; Monnard, I.; et al. Effects of protein quantity and type on diet induced thermogenesis in overweight adults: A randomized controlled trial. Clin. Nutr. 2019, 38, 1570–1580. [Google Scholar] [CrossRef]
- Leidy, H.J.; Gwin, J.A.; Roenfeldt, C.A.; Zino, A.Z.; Shafer, R.S. Evaluating the intervention-based evidence surrounding the causal role of breakfast on markers of weight management, with specific focus on breakfast composition and size. Adv. Nutr. 2016, 7, 563S–575S. [Google Scholar] [CrossRef] [Green Version]
- Gwin, J.A.; Leidy, H.J. A review of the evidence surrounding the effects of breakfast consumption on mechanisms of weight management. Adv. Nutr. 2018, 9, 717–725. [Google Scholar] [CrossRef]
- Hill, C.M.; Morrison, C.D. The protein leverage hypothesis: A 2019 update for obesity. Obesity 2019, 27, 1221. [Google Scholar] [CrossRef] [PubMed]
- Tentolouris, N.; Pavlatos, S.; Kokkinos, A.; Perrea, D.; Pagoni, S.; Katsilambros, N. Diet-induced thermogenesis and substrate oxidation are not different between lean and obese women after two different isocaloric meals, one rich in protein and one rich in fat. Metabolism 2008, 57, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, J.; Frederiksen, R.; Hoppe, C.; Hvid, R.; Astrup, A. The effect of milk proteins on appetite regulation and diet-induced thermogenesis. Eur. J. Clin. Nutr. 2012, 66, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Moosavian, S.P.; Haghighatdoost, F. Dietary energy density and appetite: A systematic review and meta-analysis of clinical trials. Nutrients 2020, 69, 110551. [Google Scholar] [CrossRef] [PubMed]
- Dougkas, A.; Östman, E. Protein-enriched liquid preloads varying in macronutrient content modulate appetite and appetite-regulating hormones in healthy adults. J. Nutr. 2016, 146, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Stubbs, R.J.; Hughes, D.A.; Johnstone, A.; Rowley, E.; Reid, C.; Elia, M.; Stratton, R.; Delargy, H.; King, N.; Blundell, J.E. The use of visual analogue scales to assess motivation to eat in human subjects: A review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br. J. Nutr. 2000, 84, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Crone, E.A.; Dahl, R.E. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012, 13, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Veldhorst, M.A.; Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; van Vught, A.J.; Westerterp, K.R.; Engelen, M.P.; Brummer, R.-J.M.; Deutz, N.; Westerterp-Plantenga, M.S. Dose-dependent satiating effect of whey relative to casein or soy. Physiol. Behav. 2009, 96, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Sievert, K.; Hussain, S.M.; Page, M.; Wang, Y.; Hughes, H.J.; Malek, M.; Cicuttini, F.M. Effect of breakfast on weight and energy intake: Systematic review and meta-analysis of randomised controlled trials. BMJ 2019, 364, l42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohanmoo, A.; Faghih, S.; Akhlaghi, M. Effect of short- and long-term protein consumption on appetite and appetite-regulating gastrointestinal hormones, a systematic review and meta-analysis of randomized controlled trials. Physiol. Behav. 2020, 226, 113123. [Google Scholar] [CrossRef]





| PICOS | Descriptions |
|---|---|
| Participants | Children and adolescents older than 7 and younger than 19 years; Both sexes; All nationalities |
| Interventions | The intervention group consumed a protein-rich breakfast; No restrictions regarding the dose or intervention duration were applied. |
| Control/ Comparator group | The control group consumed a normal protein or traditional breakfast; |
| Outcomes | Subsequent energy intake or subjective appetite components (fullness and hunger) |
| Setting | Randomized controlled or crossover trials |
| 1 | ((((“Breakfast”[Mesh]) OR (((((((((((((Breakfasts[Title/Abstract]) OR (Breakfast Time[Title/Abstract])) OR (Breakfast Times[Title/Abstract])) OR (Time, Breakfast[Title/Abstract])) OR (Times, Breakfast[Title/Abstract])) OR (Morning Meal[Title/Abstract])) OR (Meals, Morning[Title/Abstract])) OR (Morning Meals[Title/Abstract])) OR (meal timing[Title/Abstract])) OR (Cereal[Title/Abstract])) OR (RTEC[Title/Abstract])) OR (Ready To Eat Cereals[Title/Abstract])) OR (breakfast cereal[Title/Abstract]))) AND ((“Breakfast”[Mesh]) OR (((((((((((((Breakfasts[Title/Abstract]) OR (Breakfast Time[Title/Abstract])) OR (Breakfast Times[Title/Abstract])) OR (Time, Breakfast[Title/Abstract])) OR (Times, Breakfast[Title/Abstract])) OR (Morning Meal[Title/Abstract])) OR (Meals, Morning[Title/Abstract])) OR (Morning Meals[Title/Abstract])) OR (meal timing[Title/Abstract])) OR (Cereal[Title/Abstract])) OR (RTEC[Title/Abstract])) OR (Ready To Eat Cereals[Title/Abstract])) OR (breakfast cereal[Title/Abstract])))) |
| 2 | (((((“Child, Preschool”[Mesh]) OR (“Adolescent”[Mesh])) OR (“Minors”[Mesh])) OR (“Students”[Mesh])) OR (((((((((((((((((((((((((((Preschool Child[Title/Abstract]) OR (Children, Preschool[Title/Abstract])) OR (Preschool Children[Title/Abstract])) OR (Children[Title/Abstract])) OR (Adolescence[Title/Abstract])) OR (Teens[Title/Abstract])) OR (Teens[Title/Abstract])) OR (Teenagers[Title/Abstract])) OR (Teenager[Title/Abstract])) OR (Youth[Title/Abstract])) OR (Youths[Title/Abstract])) OR (Adolescents, Female[Title/Abstract])) OR (Adolescent, Female[Title/Abstract])) OR (Female Adolescent[Title/Abstract])) OR (Adolescents, Male[Title/Abstract])) OR (Female Adolescents[Title/Abstract])) OR (Adolescent, Male[Title/Abstract])) OR (Male Adolescent[Title/Abstract])) OR (Male Adolescents[Title/Abstract])) OR (juvenile adult[Title/Abstract])) OR (Minor[Title/Abstract])) OR (Minors[Title/Abstract])) OR (Student[Title/Abstract])) OR (School Enrollment[Title/Abstract])) OR (Enrollment, School[Title/Abstract])) OR (Enrollments, School[Title/Abstract])) OR (School Enrollments[Title/Abstract])))) |
| 3 | ((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti])) |
| 4 | 1 AND 2 AND 3 |
| 5 | Filters: from 1 January 1990–1 January 2021 |
| Author (Country, Year) | Study Design (Duration) | Participants | Intervention Group (Composition of the Whole Breakfast) | Control Group (Composition of the Whole Breakfast) | Composition of the Intervention Breakfast | Subsequent Lunch Intake | Appetite (Mean ± SD/SE *) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Population 1 | Age (Mean ± SD) | BMI (Mean ± SD) | Intervention (mm) | Control (mm) | ||||||
| Baum (US, 2015) [13] | Crossover (9 days) | [n = 16; 44%], Nonoverweight | 9.9 ± 1.2 | 16.7 ± 1.6 | PRO (344 kcal, 18 g protein, 45 g CHO, 16 g sugars, 1 g fiber, 10.5 g fat) | CHO 2 (327 kcal, 3 g protein, 55 g CHO, 39 g sugars, 0.5 g fiber, 11 g fat) | Egg whites, butter, orange juice, white bread | Buffet-style meal served at 240 min | Hunger Post–breakfast: 32.8 ± 8.2 * Pre–lunch: 77.6 ± 3.5 * Fullness Post–breakfast: 68.9 ± 7.5 * Pre–lunch: 23.3 ± 4.2 * | Hunger Post–breakfast: 50.8 ± 6.1 * Pre–lunch: 82.6 ± 4.3 * Fullness Post–breakfast: 42.7 ± 6.1 * Pre–lunch: 21.1 ± 5.3 * |
| [n = 13; 46%], Overweight/obese | 9.5 ± 1.4 | 22.7 ± 4.0 | PRO (344 kcal, 18 g protein, 45 g CHO, 16 g sugars, 1 g fiber, 10.5 g fat) | CHO * (327 kcal, 3 g protein, 55 g CHO, 39 g sugars, 0.5 g fiber, 11 g fat) | Egg whites, butter, orange juice, white bread | Buffet-style meal served at 240 min | Hunger Post–breakfast: 37.0 ± 9.4 * Pre–lunch: 85.2 ± 5.0 * Fullness Post–breakfast: 70.0 ± 9.0 * Pre–lunch: 13.0 ± 4.7 * | Hunger Post–breakfast: 41.5 ± 10.1 * Pre–lunch: 81.6 ± 7.0 * Fullness Post–breakfast: 38.2 ± 9.2 * Pre–lunch: 15.4 ± 6.3 * | ||
| Bellissimo (Canada, 2020) [20] | Crossover (25 days) | [n = 17; 47%], Nonoverweight | 12.0 ± 1.65 | 20.8 ± 3.7 | HP (450 kcal, 45 g protein, 30 g CHO, 2 g fiber, 17 g fat)MP (450 kcal, 30 g protein, 45 g CHO, 3 g fiber, 17 g fat)LP (450 kcal, 15 g protein, 61 g CHO, 5 g fiber, 17 g fat) | C (450 kcal, 7 g protein, 69 g CHO, 3 g fiber, 17 g fat) | Egg yolk, egg whites, butter, cheese, home fries, ketchup | Pizza lunch according to one’s preference served at 210 min | NA | NA |
| Douglas (US, 2019) [21] | Crossover (15 days) | [n = 19, 100%], Overweight | 19 ± 1 | 29.0 ± 3.8 | SKIP-HP (350 kcal, 35 g protein) | SKIP-NP (350 kcal, 13 g protein) | Yogurt parfaits, bagels, breakfast burritos, cereals, etc. | NA | Hunger Post–breakfast: 9.9 ± 9.7 Pre–lunch: 50.8 ± 19.7 Fullness Post–breakfast: 72.0 ± 22.2 Pre–lunch: 28.3 ± 16.8 | Hunger Post–breakfast: 12.7 ± 15.9 Pre–lunch: 61.1 ± 20.5 Fullness Post–breakfast: 83. 5 ± 11.3 Pre–lunch: 30.2 ± 22.5 |
| [n = 18, 100%], Overweight | 19 ± 1 | 28.9 ± 2.9 | CONSUME-HP (350 kcal, 35 g protein) | CONSUME-NP (350 kcal, 13 g protein) | Yogurt parfaits, bagels, breakfast burritos, cereals, etc. | NA | Hunger Post–breakfast: 10.3 ± 17.9 Pre–lunch: 70.8 ± 14.6 Fullness Post–breakfast: 80.9 ± 14.9 Pre-lunch: 21.0 ± 14.5 | Hunger Post–breakfast: 7.3 ± 9.2 Pre–lunch: 46.4 ± 22.5 Fullness Post–breakfast: 75.8 ± 19.3 Pre–lunch: 34.8 ± 17.8 | ||
| Kral (US, 2016) [15] | Crossover (3 weeks) | [n = 40, 47.5%], Overweight/Nonoverweight | 9.4 ± 0.8 | NA Overweight or obese (45%) | Egg (350 kcal, protein % energy: 21) | Oatmeal (350 kcal, protein % energy: 14%)Cereal (350 kcal, protein % energy: 8%) | Scrambled eggs (prepared with 1/8 tsp. table salt), toasted whole wheat bread, diced peaches, and milk (1% fat) | Lunch (chicken nuggets, macaroni and cheese, green beans (prepared with 3 g of salted butter), ketchup, applesauce, chocolate chip cookies, and milk) served at 180 min | Hunger Post–breakfast: 19.0 ± 4.4 * Pre–lunch: 83.1 ± 4.4 * Fullness Post–breakfast: 60.4 ± 4.9 * Pre–lunch: 8.4 ± 4.9 * | Hunger (Oarmal) Post–breakfast: 14.1 ± 3.5 * Pre–lunch: 69.4 ± 4.9 * Fullness (Oarmal) Post–breakfast: 59.0 ± 5.5 * Pre–lunch: 16.0 ± 3.8 * Hunger (Cereal) Post–breakfast: 22.5 ± 5.5 * Pre–lunch: 77.0 ± 4.6 * Fullness (Cereal) Post–breakfast: 57. 7 ± 6.0 * Pre–lunch: 14.4 ± 7.1 * |
| Leidy (UK, 2010) [16] | Crossover (17 days) | [n = 13, 46%], Overweight/Nonoverweight | 14.3 ± 1.1 | 23.5 ± 3.6 | PR * (512 ± 26 kcal, 49.1 ± 2.5 g protein, 62.8 ± 3.2 g CHO, 30.7 ± 1.6 g sugar, 2.1 ± 0.1 g fiber, 7.5 ± 0.4 g fat) | PN * (513 ± 26 kcal, 18.1 ± 0.9 g protein, 95.3 ± 4.9 g CHO, 31.1 ± 1.6 g sugar, 2.0 ± 0.1 g fiber, 7.5 ± 0.4 g fat) | Whey Pancakes (whey protein powder, skim milk, margarine, egg–whites, butter, etc.) | Buffet lunch served at 240 min | Fullness Post–breakfast: 54.7 ± 8.1 * Pre–lunch: 32.1 ± 5.8 * | Fullness Post–breakfast: 48.7 ± 6.0 * Pre–lunch: 18.5 ± 4.0 * |
| Leidy (US, 2013) [22] | Crossover (5 weeks) | [n = 20, 100%], Overweight | 19 ± 4.5 | 28.6 ± 3.1 | HP (350 kcal, 35.1 g protein, 35.1 g CHO, 18 g sugar, 6.1 g fiber, 7.8 g fat) | NP (350 kcal, 13 g protein, 57 g CHO, 18 g sugar, 6.1 g fiber, 7.8 g fat) | Egg, Beef, Dairy, Plant–based, etc. | NA | Hunger Post–breakfast: 7.1 ± 5. 7 * Pre–lunch: 45.3 ± 3.6 * Fullness Post–breakfast: 76.3 ± 2.2 * Pre–lunch: 35.0 ± 2.9 * | Hunger Post–breakfast: 10.5 ± 2.3 * Pre–lunch: 49.7 ± 5.3 * Fullness Post–breakfast: 71.0 ± 5.1 * Pre–lunch: 28.0 ± 3.6 * |
| Liu (US, 2015) [14] | Parallel (9 days) | [n = 15, 60%], Overweight/Nonoverweight | 15.6 ± 4.26 | NA Overweight or obese (40%) | Egg (342 kcal, 16.8 g protein, 32.2 g CHO, 16.6 g fat) | Bagel (336 kcal, 11 g protein, 48.6 g CHO, 10 g fat) | Scrambled, toast, jelly | Lunch (baked chicken, macaroni and cheese, green beans, mandarin oranges, rolls, and milk) served at 180 min | Hunger Post–breakfast: 23.0 ± 6.0 * Pre–lunch: 42.2 ± 6.2 * Fullness Post–breakfast: 66.4 ± 6. 9 * Pre–lunch: 49.6 ± 7.3 * | Hunger Post–breakfast: 25.2 ± 6.2 * Pre–lunch: 49.0 ± 6.0 * Fullness Post–breakfast: 68.44 ± 6. 7 * Pre–lunch: 49.55 ± 4. 9 * |
| Mehrabani (Iran, 2015) [23] | Crossover (16 days) | [n = 34, 0%], Overweight | 11.14 ± 0.8 | 27.62 ± 2.7 | LFM (401.24 kcal, 19.08 g protein, 49.055 g CHO, 0.458 g fiber, 15.407 g fat) | W (297.74 kcal, 10.931 g protein, 37.185 g CHO, 0.458 g fiber, 12.779 g fat) AJ (411.44 kcal, 11.276 g protein, 65.195 g CHO, 1.016 g fiber, 13.022 g fat) | Low–fat milk, Iranian whole wheat bread, Walnut, Low–fat cheese | Buffet-style meal served at 300 min | Hunger Post–breakfast: 12.6 ± 1.0 * Pre–lunch: 72.6 ± 1.0 * Fullness Post–breakfast: 86.4 ± 0.9 * Pre–lunch: 21.1 ± 1.4 * | Hunger (W) Post–breakfast: 22.8 ± 1.6 * Pre–lunch: 79.4 ± 1.0 * Fullness (W) Post–breakfast: 74.0 ± 1.5 * Pre–lunch: 16.4 ± 0.9 * Hunger (AJ) Post–breakfast: 14.7 ± 1.2 * Pre–lunch: 75.9 ± 0.8 * Fullness (AJ) Post–breakfast: 83.5 ± 1.0 * Pre–lunch: 19.5 ± 1.3 * |
| Wang (China, 2014) [17] | Parallel (9 days) | [n = 56, 46%], Overweight | 14.1 ± 2.1 | 32.2 ± 1.7 | Egg (386 kcal, 12.2 g protein, 29.3 g CHO, 15.9 g fat) | Steamed bread (386 kcal, 8.2 g protein, 44.7 g CHO, 11.5 g fat) | Boiled eggs, White rice, Milk | Lunch (pork with Chinese cabbage, apple, and rice, etc.) served at 240 min | Hunger Post–breakfast: 23.2 ± 0.2 Pre–lunch: 41.1 ± 0.4 Fullness Post–breakfast: 64, 9 ± 0.7 Pre–lunch: 45.2 ± 0.6 | Hunger Post–breakfast: 23.1 ± 0.2 Pre–lunch: 52.3 ± 0.5 Fullness Post–breakfast: 65.0 ± 0.8 Pre–lunch: 35.1 ± 0.8 |
| Wang (China, 2015) [24] | Parallel (3 months) | [n = 156, 49%], Overweight | 14.3 ± 2.2 | 32.0 ± 1.7 | Egg (386 kcal, 12.2 g protein, 29.3 g CHO, 15.9 g fat) | Steamed bread (386 kcal, 8.2 g protein, 44.7 g CHO, 11.5 g fat) | Boiled eggs, White rice, Milk | Lunch (pork with Chinese cabbage, apple, and rice, etc.) served at 240 min | Hunger Post–breakfast: 22.1 ± 0.1 Pre–lunch: 40.6 ± 0.6 Fullness: Post–breakfast: 65.1 ± 0.8 Pre–lunch: 45.0 ± 0.6 | Hunger Post–breakfast: 22.30 ± 0.3 Pre–lunch: 51.20 ± 0.3 Fullness: Post–breakfast: 64.9 ± 0.9 Pre–lunch: 34.9 ± 0.9 |
| Study ID | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessors | Incomplete Outcome Data | Selective Reporting | Other Bias | Overall Quality |
|---|---|---|---|---|---|---|---|---|
| Baum 2015 | Unclear risk | High risk | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Bellissimo2020 | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | Unclear risk |
| Douglas2019 | Unclear risk | High risk | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Kral2016 | Unclear risk | High risk | Unclear risk | High risk | Low risk | Low risk | Low risk | High risk |
| Leidy2010 | Unclear risk | High risk | High risk | High risk | Unclear risk | Low risk | Low risk | High risk |
| Leidy2013 | Unclear risk | High risk | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Liu2015 | Low risk | Low risk | Unclear risk | High risk | Low risk | Low risk | Low risk | High risk |
| Mehrabani2015 | Low risk | Low risk | Low risk | High risk | Unclear risk | Low risk | Low risk | High risk |
| Wang2014 | Low risk | Unclear risk | Unclear risk | High risk | Low risk | Low risk | Low risk | High risk |
| Wang2015 | Low risk | Unclear risk | Unclear risk | High risk | Low risk | Low risk | Low risk | High risk |
| Number of Comparisons | WMD (95% CI) | Heterogeneity I2 (%) | p between | ||
|---|---|---|---|---|---|
| Study–design | |||||
| Crossover | 10 | −116.9 (−145.6, −88.3) | 75% | p < 0.0001 | |
| Parallel | 3 | −114.1 (−124.9, −103.4) | 0% | 0.66 | |
| Sex | |||||
| Girl | 0 | ||||
| Boy | 2 | −125.3 (−156.8, −93.9) | 97% | p < 0.0001 | |
| Both | 11 | −113.2 (−123.9, −102.6) | 0% | 0.82 | |
| Economic status of country | |||||
| High–income country | 9 | −70.09 (−137.8, −2.4) | 0% | 0.83 | |
| Medium–and–low–income country | 4 | −115.49 (−125.7, −105.3) | 90% | p < 0.0001 | |
| Baseline body mass index (BMI) | |||||
| Non–overweight/Overweight | 8 | −76.70 (−145.5, −8.0) | 0% | 0.87 | |
| Overweight/Obese | 5 | −115.31 (−125.5, −105.1) | 88% | p < 0.0001 | |
| Number of Comparisons | WMD (95% CI) | Heterogeneity I2 (%) | p between | ||
|---|---|---|---|---|---|
| Subgroup analyses for fullness and protein–rich breakfast (post–breakfast) | |||||
| Study–design | |||||
| Crossover | 10 | 6.0 (4.1, 7.9) | 76% | p < 0.0001 | |
| Parallel | 3 | 0.1 (−0.1, 0.3) | 0% | 0.46 | |
| Sex | |||||
| Girl | 3 | −0.3 (−6.7, 6.2) | 65% | 0.06 | |
| Boy | 2 | 6.4 (4.3, 8.5) | 95% | p < 0.0001 | |
| Both | 7 | −0.1 (−0.5, 0.3) | 58% | 0.03 | |
| Economic status of country | |||||
| High–income country | 9 | 2.5 (−2.2, 7.1) | 57% | 0.02 | |
| Medium–and low– income country | 4 | 0.2 (−0.0, 0.4) | 95% | p < 0.0001 | |
| Baseline body mass index (BMI) | |||||
| Non–overweight/Overweight | 5 | 5.9 (−1.8, 13.5) | 29% | 0.23 | |
| Overweight/Obese | 8 | 0.2 (−0.0, 0.4) | 90% | p < 0.0001 | |
| Subgroup analyses for fullness and protein–rich breakfast (pre–lunch) | |||||
| Study–design | |||||
| Crossover | 10 | 2.4 (0.3, 4.5) | 53% | 0.02 | |
| Parallel | 3 | 10.1 (9. 9, 10.3) | 0% | 0.52 | |
| Sex | |||||
| Girl | 3 | −1.8 (−7.9, 4.2) | 76% | 0.01 | |
| Boy | 2 | 3.4 (1.0, 5.8) | 37% | 0.21 | |
| Both | 8 | 10.1 (9.9, 10.3) | 59% | 0.02 | |
| Economic status of country | |||||
| High–income country | 9 | −0.8 (−5.1, 3.4) | 46% | 0.06 | |
| Medium–and low– income country | 4 | 10.1 (9.9, 10.3) | 90% | P < 0.0001 | |
| Baseline body mass index (BMI) | |||||
| Non–overweight/Overweight | 5 | 0.6 (−5.9, 6.9) | 33% | 0.2 | |
| Overweight/Obese | 8 | 10.0 (9.8, 10.2) | 88% | P < 0.0001 | |
| Number of Comparisons | WMD (95% CI) | Heterogeneity I2 (%) | p between | ||
|---|---|---|---|---|---|
| Study–design | |||||
| Crossover | 9 | −3.8 (−5.5, −2.0) | 78% | p < 0.0001 | |
| Parallel | 3 | −10.8 (−10.9, −10.6) | 88% | 0.0002 | |
| Sex | |||||
| Girl | 3 | 3.5 (−3.7, 10.8) | 88% | 0.0002 | |
| Boy | 2 | −4.9 (−6.8, −3.0) | 70% | 0.07 | |
| Both | 7 | −10.8 (−10.9, −10.6) | 86% | p < 0.0001 | |
| Economic status of country | |||||
| High–income country | 8 | 2.9 (−1.7, 7.5) | 70% | 0.0002 | |
| Medium–and low– income country | 4 | −10.7 (−10.9, −10.6) | 95% | p < 0.0001 | |
| Baseline body mass index (BMI) | |||||
| Non–overweight/Overweight | 4 | 2.3 (−4.0, 8.7) | 52% | 0.1 | |
| Overweight/Obese | 8 | −10.7 (−10.9, −10.6) | 92% | p < 0.0001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, M.; Zhang, Y.; Long, Z.; He, Y. Effect of Protein-Rich Breakfast on Subsequent Energy Intake and Subjective Appetite in Children and Adolescents: Systematic Review and Meta–Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 2840. https://doi.org/10.3390/nu13082840
Qiu M, Zhang Y, Long Z, He Y. Effect of Protein-Rich Breakfast on Subsequent Energy Intake and Subjective Appetite in Children and Adolescents: Systematic Review and Meta–Analysis of Randomized Controlled Trials. Nutrients. 2021; 13(8):2840. https://doi.org/10.3390/nu13082840
Chicago/Turabian StyleQiu, Meijuan, Yu Zhang, Zheng Long, and Yuna He. 2021. "Effect of Protein-Rich Breakfast on Subsequent Energy Intake and Subjective Appetite in Children and Adolescents: Systematic Review and Meta–Analysis of Randomized Controlled Trials" Nutrients 13, no. 8: 2840. https://doi.org/10.3390/nu13082840
APA StyleQiu, M., Zhang, Y., Long, Z., & He, Y. (2021). Effect of Protein-Rich Breakfast on Subsequent Energy Intake and Subjective Appetite in Children and Adolescents: Systematic Review and Meta–Analysis of Randomized Controlled Trials. Nutrients, 13(8), 2840. https://doi.org/10.3390/nu13082840






