Dietary Protein Intake and Transition between Frailty States in Octogenarians Living in New Zealand
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Life and Living to Advanced Age Cohort Study in New Zealand (LiLACS NZ)
2.2. Dietary Protein Intake
2.3. Anthropometry Functional and Health Measures
2.4. Frailty States
Management of Missing Frailty Items
2.5. Statistical Analysis
3. Results
3.1. Number of Transitions and Baseline Characteristics by Frailty State and to Death
3.2. Protein Intake and Transitions between Frailty States and to Death
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clegg, A.; Young, J. The Frailty Syndrome. Clin. Med. 2011, 11, 72–75. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A Call to Action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Faller, J.W.; Pereira, D.d.N.; de Souza, S.; Nampo, F.K.; Orlandi, F.d.S.; Matumoto, S. Instruments for the detection of frailty syndrome in older adults: A systematic review. PLoS ONE 2019, 14, e0216166. [Google Scholar] [CrossRef] [Green Version]
- Michel, J.-P.; Cruz-Jentoft, A.J.; Cederholm, T. Frailty, Exercise and Nutrition. Clin. Geriatr. Med. 2015, 31, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.W.; Auyeung, T.-W.; Leung, J.; Kwok, T.; Woo, J. Transitions in Frailty States Among Community-Living Older Adults and Their Associated Factors. J. Am. Med. Dir. Assoc. 2014, 15, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, S.; Ligthart-Melis, G.C.; Wijers, S.L.J.; Cederholm, T.; Maier, A.B.; de van der Schueren, M.A.E. High Prevalence of Physical Frailty Among Community-Dwelling Malnourished Older Adults–A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2017, 18, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Koopman, R.; Walrand, S.; Beelen, M.; Gijsen, A.P.; Kies, A.K.; Boirie, Y.; Saris, W.H.M.; van Loon, L.J.C. Dietary Protein Digestion and Absorption Rates and the Subsequent Postprandial Muscle Protein Synthetic Response Do Not Differ between Young and Elderly Men. J. Nutr. 2009, 139, 1707–1713. [Google Scholar] [CrossRef] [Green Version]
- Coelho-Junior, H.J.; Marzetti, E.; Picca, A.; Cesari, M.; Uchida, M.C.; Calvani, R. Protein Intake and Frailty: A Matter of Quantity, Quality, and Timing. Nutrients 2020, 12, 2915. [Google Scholar] [CrossRef]
- Tieland, M.; den Berg, K.B.-V.; van Loon, L.; de Groot, L. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: Scope for improvement. Eur. J. Nutr. 2012, 51, 173–179. [Google Scholar] [CrossRef]
- Coelho-Júnior, J.H.; Rodrigues, B.; Uchida, M.; Marzetti, E. Low Protein Intake Is Associated with Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 1334. [Google Scholar] [CrossRef] [Green Version]
- Bartali, B.; Frongillo, E.A.; Bandinelli, S.; Lauretani, F.; Semba, R.D.; Fried, L.P.; Ferrucci, L. Low Nutrient Intake Is an Essential Component of Frailty in Older Persons. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2006, 61, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Junior, H.J.; Milano-Teixeira, L.; Rodrigues, B.; Bacurau, R.; Marzetti, E.; Uchida, M. Relative protein intake and physical function in older adults: A systematic review and meta-analysis of observational studies. Nutrients 2018, 10, 1330. [Google Scholar] [CrossRef] [Green Version]
- Rahi, B.; Colombet, Z.; Gonzalez-Colaço Harmand, M.; Dartigues, J.-F.; Boirie, Y.; Letenneur, L.; Feart, C. Higher Protein but Not Energy Intake Is Associated With a Lower Prevalence of Frailty Among Community-Dwelling Older Adults in the French Three-City Cohort. J. Am. Med. Dir. Assoc. 2016, 17, 672.e7–672.e11. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Ley, S.J.; Carmichael, H.E.; Plank, L.D. Body size and composition in Polynesians. Int. J. Obes. 1999, 23, 1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyall, L.; Kepa, M.; Hayman, K.; Teh, R.; Moyes, S.; Broad, J.B.; Kerse, N. Engagement and recruitment of Māori and non-Māori people of advanced age to LiLACS NZ. Aust. N. Z. J. Public Health 2013, 37, 124–131. [Google Scholar] [CrossRef] [PubMed]
- The New Zealand Institute for Plant & Food Research Limited and the Ministry of Health. New Zealand FOODfiles; 2010; Available online: https://www.foodcomposition.co.nz/foodfiles/ (accessed on 22 March 2020).
- Hirani, V.; Mindell, J. A comparison of measured height and demi-span equivalent height in the assessment of body mass index among people aged 65 years and over in England. Age Ageing 2008, 37, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Studenski, S.; Perera, S.; Wallace, D.; Chandler, J.M.; Duncan, P.W.; Rooney, E.; Fox, M.; Guralnik, J.M. Physical performance measures in the clinical setting. J. Am. Geriatr. Soc. 2003, 51, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Washburn, R.A.; McAuley, E.; Katula, J.; Mihalko, S.L.; Boileau, R.A. The Physical Activity Scale for the Elderly (PASE): Evidence for Validity. J. Clin. Epidemiol. 1999, 52, 643–651. [Google Scholar] [CrossRef]
- Teh, R.; Doughty, R.; Connolly, M.; Broad, J.; Pillai, A.; Wilkinson, T.; Edlin, R.; Jatrana, S.; Dyall, L.; Kerse, N. Agreement between self-reports and medical records of cardiovascular disease in octogenarians. J. Clin. Epidemiol. 2013, 66, 1135–1143. [Google Scholar] [CrossRef]
- Alley, D.E.; Shardell, M.D.; Peters, K.W.; McLean, R.R.; Dam, T.-T.L.; Kenny, A.M.; Fragala, M.S.; Harris, T.B.; Kiel, D.P.; Guralnik, J.M.; et al. Grip Strength Cutpoints for the Identification of Clinically Relevant Weakness. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Van Kan, G.A.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette-Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand: Including Recommended Dietary Intakes. 2006. Available online: https://www.nhmrc.gov.au/about-us/publications/nutrient-reference-values-australia-and-new-zealand-including-recommended-dietary-intakes#block-views-block-file-attachments-content-block-1 (accessed on 30 July 2021).
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. Relat. Metab. Disord 2000, 24, 1119–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meira-Machado, L.; de Uña-Alvarez, J.; Cadarso-Suárez, C.; Andersen, P.K. Multi-state models for the analysis of time-to-event data. Stat. Methods Med. Res. 2009, 18, 195–222. [Google Scholar] [CrossRef] [Green Version]
- Cox, D.R.; Miller, H.D. The Theory of Stochastic Processes; Chapman and Hall: New York, NY, USA, 1965. [Google Scholar]
- Jackson, C.H. Multi-state models for panel data: The msm package for R. J. Stat. Softw. 2011, 38, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Mendonça, N.; Kingston, A.; Granic, A.; Jagger, C. Protein intake and transitions between frailty states and to death in very old adults: The Newcastle 85+ study. Age Ageing 2019, 49, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, J.M.; Cohen, A.; Ein-Mor, E.; Maaravi, Y.; Stessman, J. Frailty, cognitive impairment and mortality among the oldest old. J. Nutr. Health Aging 2011, 15, 678–682. [Google Scholar] [CrossRef]
- Pivetta, N.R.S.; Marincolo, J.C.S.; Neri, A.L.; Aprahamian, I.; Yassuda, M.S.; Borim, F.S.A. Multimorbidity, frailty and functional disability in octogenarians: A structural equation analysis of relationship. Arch. Gerontol. Geriatr. 2020, 86, 103931. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.Q.; Theou, O.; Karnon, J.; Adams, R.J.; Visvanathan, R. Frailty prevalence in Australia: Findings from four pooled Australian cohort studies. Australas. J. Ageing 2018, 37, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Teh, R.; Kerse, N.; Kepa, M.; Doughty, R.N.; Moyes, S.; Wiles, J.; Wham, C.; Hayman, K.; Wilkinson, T.; Connolly, M.; et al. Self-rated health, health related behaviours and medical conditions of Māori and non-Māori in advanced age: LiLACS NZ. N. Z. Med. J. 2014, 127, 13–29. [Google Scholar] [PubMed]
- Lord, C.; Chaput, J.P.; Aubertin-Leheudre, M.; Labonté, M.; Dionne, I.J. Dietary animal protein intake: Association with muscle mass index in older women. J. Nutr. Health Aging 2007, 11, 383–387. [Google Scholar] [PubMed]
- Schoufour, J.D.; Franco, O.H.; Jong, J.C.K.-D.; Trajanoska, K.; Stricker, B.; Brusselle, G.; Rivadeneira, F.; Lahousse, L.; Voortman, T. The association between dietary protein intake, energy intake and physical frailty: Results from the Rotterdam Study. Br. J. Nutr. 2019, 121, 393–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wham, C.; Teh, R.; Moyes, S.A.; Rolleston, A.; Muru-Lanning, M.; Hayman, K.; Adamson, A.; Kerse, N. Macronutrient intake in advanced age: Te Puawaitanga o Nga Tapuwae Kia ora Tonu, Life and Living in Advanced Age: A Cohort Study in New Zealand (LiLACS NZ). Br. J. Nutr. 2016, 116, 1103–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margulis, B.; Tsimokha, A.; Zubova, S.; Guzhova, I. Molecular Chaperones and Proteolytic Machineries Regulate Protein Homeostasis in Aging Cells. Cells 2020, 9, 1308. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, D.L.; Palmer, K.; Marengoni, A.; Marzetti, E.; Lattanzio, F.; Roller-Wirnsberger, R.; Samaniego, L.L.; Rodríguez-Mañas, L.; Bernabei, R.; Onder, G. Frailty and Multimorbidity: A Systematic Review and Meta-analysis. J. Gerontol. Ser. A 2018, 74, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.Y.; Kuha, J.; Murphy, M. Multidimensional predictors of physical frailty in older people: Identifying how and for whom they exert their effects. Biogerontology 2017, 18, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Bukman, A.J.; Ronteltap, A.; Lebrun, M. Interpersonal determinants of eating behaviours in Dutch older adults living independently: A qualitative study. BMC Nutr. 2020, 6, 55. [Google Scholar] [CrossRef]
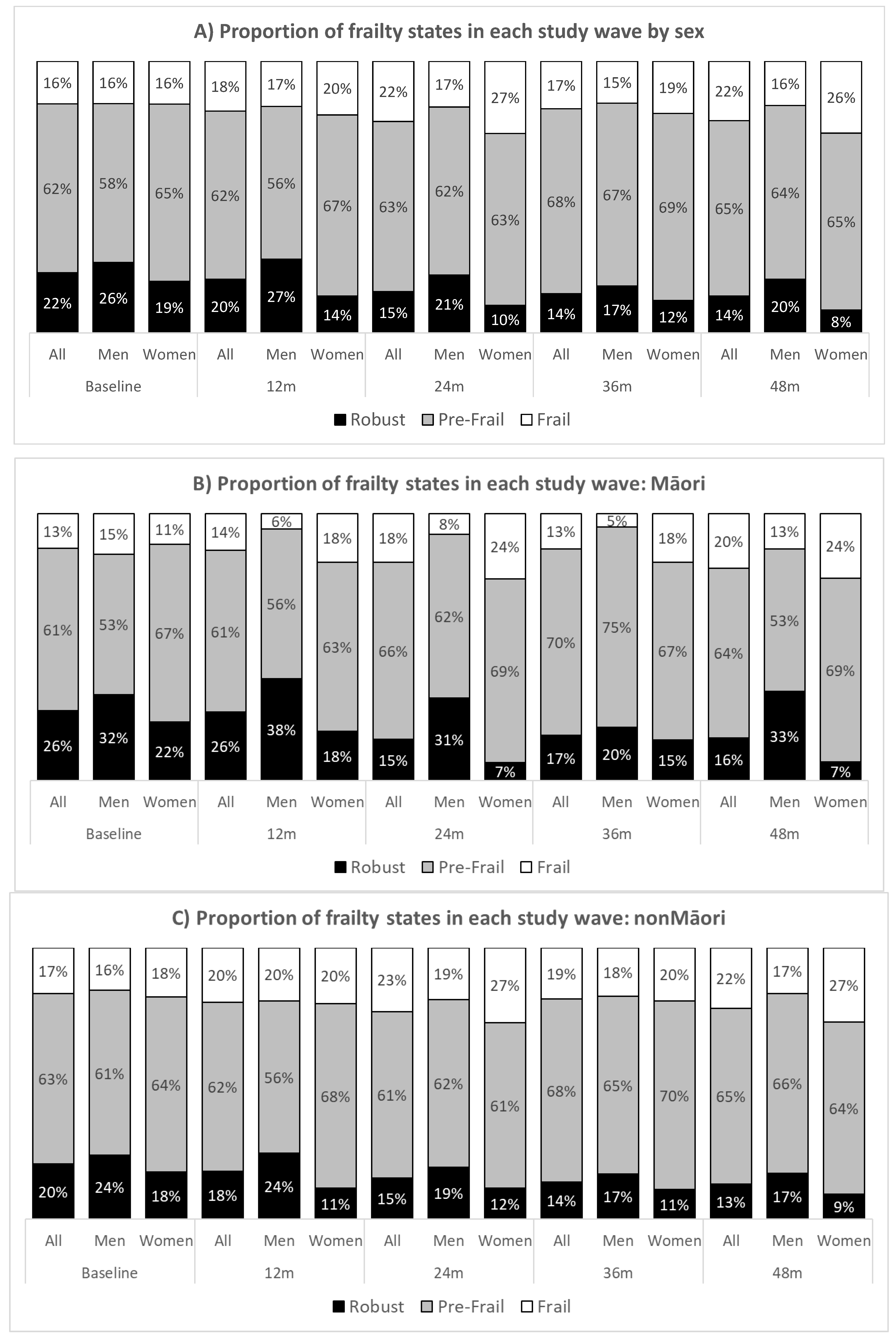
| Robust Fried Score = 0 | Pre-Frai Fried Score = 1–2 | Frail Fried Score = 3–5 | Total | p * | |
|---|---|---|---|---|---|
| n | 102 (22%) | 285 (62%) | 72 (16%) | 459 | |
| Women, n (%) | 47 (46.1) | 161 (56.5) | 39 (54.2) | 247 (53.8) | 0.194 |
| Age, (years) | 85.1 (2.0) | 85.4 (1.7) | 85.7 (1.7) | 85.4 (1.8) | 0.067 |
| Māori, n (%) | 38 (37.3) | 89 (31.2) | 19 (26.4) | 146 (31.8) | 0.299 |
| Education, n (%) | 0.956 | ||||
| Primary or no schooling | 17 (16.8) | 62 (21.8) | 19 (26.4) | 98 (21.4) | |
| Secondary school, no qualification | 37 (36.6) | 101 (35.6) | 23 (31.9) | 161 (35.2) | |
| Secondary school, qualification | 22 (21.8) | 59 (20.8) | 15 (20.8) | 96 (21.0) | |
| Trade, occupational | 9 (8.9) | 24 (8.5) | 6 (8.3) | 39 (8.5) | |
| Tertiary qualification | 16 (15.8) | 38 (13.4) | 9 (12.5) | 63 (13.8 | |
| Co-morbidity | 2.4 (2.0) | 2.8 (1.7) | 4.1 (2.4) | 2.9 (2.0) | <0.001 |
| Body weight, (kg) | 73.4 (11.1) | 70.7 (13.1) | 71.2 (16.3) | 71.4 (13.3) | 0.219 |
| BMI, (kg/m2) | 27.7 (4.0) | 27.1 (4.3) | 27.3 (6.4) | 27.3 (4.7) | 0.555 |
| Energy intake, (MJ/d) | 7.5 (2.4) | 7.1 (2.2) | 6.8 (2.4) | 7.2 (2.3) | 0.141 |
| Protein intake, (g/d) | 71.1 (24.1) | 67.3 (21.9) | 64.1 (25.7) | 67.7 (23.1) | 0.134 |
| Men | 80.8 (25.2) | 74.5 (23.2) | 75.6 (28.3) | 76.3 (25.6) | 0.288 |
| Women | 59.8 (17.0) | 61.8 (19.1) | 54.4 (18.7) | 60.3 (18.8) | 0.086 |
| Protein intake, (g/kg BW/d) | 0.99 (0.37) | 0.97 (0.31) | 0.93 (0.38) | 0.97 (0.34) | 0.508 |
| Men | 1.08 (0.39) | 0.97 (0.32) | 1.02 (0.43) | 1.01 (0.36) | 0.177 |
| Women | 0.89 (0.32) | 0.97 (0.31) | 0.86 (0.32) | 0.93 (0.31) | 0.071 |
| ≥0.8 g/kg BW/d, n (%) | 66 (64.7) | 198 (69.5) | 44 (61.1) | 308 (67.1) | 0.339 |
| Men | 42 (76.4) | 89 (71.8) | 24 (72.7) | 155 (73.1) | 0.814 |
| Women | 24 (51.1) | 109 (67.7) | 20 (51.3) | 153 (61.9) | 0.039 |
| ≥1.0 g/kg BW/d, n (%) | 42 (41.2) | 118 (41.4) | 26 (36.1) | 186 (40.5) | 0.708 |
| Men | 28 (50.9) | 49 (39.5) | 15 (45.5) | 92 (43.4) | 0.353 |
| Women | 14 (29.8) | 69 (42.9) | 11 (28.2) | 94 (38.1) | 0.103 |
| Carbohydrate intake, (g/d) | 194 (58.6) | 187 (55.0) | 178 (60.0) | 187 (56.7) | 0.203 |
| Fat intake, (g/d) | 75.5 (32.7) | 71.4 (31.9) | 69.7 (37.1) | 72.1 (33.0) | 0.455 |
| Misreporters, n (%) | 30 (29.4) | 86 (30.2) | 26 (36.1) | 142 (30.9) | 0.580 |
| To | Robust | Pre-Frail | Frail | Dead | |
|---|---|---|---|---|---|
| From | |||||
| Robust | 95 | 124 | 4 | 20 | |
| Pre-frail | 84 | 501 | 117 | 87 | |
| Frail | 4 | 74 | 96 | 63 |
| Increase of 10 g/day | Increase of 1 g/kg BW/day | ≥0.8 g/kg BW/day | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Robust → Pre-Frail (n = 124) | ||||||
| Model 1 | 0.95 | 0.85–1.06 | 0.49 | 0.25–0.96 | 0.74 | 0.47–1.17 |
| Model 2 | 0.94 | 0.84–1.06 | 0.50 | 0.25–1.02 | 0.75 | 0.47–1.21 |
| Model 3 | 0.85 | 0.69- 1.05 | 0.28 | 0.08–0.91 | 0.74 | 0.43–1.25 |
| Pre-Frail → Robust (n = 84) | ||||||
| Model 1 | 0.92 | 0.82–1.04 | 0.49 | 0.22–1.09 | 0.60 | 0.33–1.10 |
| Model 2 | 0.92 | 0.81–1.03 | 0.47 | 0.21–1.05 | 0.61 | 0.33–1.13 |
| Model 3 | 0.81 | 0.65–1.01 | 0.24 | 0.06–0.93 | 0.58 | 0.30–1.14 |
| Pre-Frail → Frail (n = 117) | ||||||
| Model 1 | 1.00 | 0.91–1.09 | 1.02 | 0.57–1.80 | 0.71 | 0.47–1.07 |
| Model 2 | 1.00 | 0.91–1.11 | 1.19 | 0.66–2.15 | 0.72 | 0.46–1.13 |
| Model 3 | 1.03 | 0.87–1.22 | 1.59 | 0.72–3.55 | 0.65 | 0.39–1.09 |
| Frail → Pre-Frail (n = 74) | ||||||
| Model 1 | 1.06 | 0.95–1.19 | 1.59 | 0.76–3.32 | 0.92 | 0.49–1.72 |
| Model 2 | 1.06 | 0.94–1.19 | 1.63 | 0.76–3.52 | 0.77 | 0.39–1.55 |
| Model 3 | 1.04 | 0.87–1.24 | 1.78 | 0.63–5.05 | 0.66 | 0.30–1.43 |
| Pre-Frail → Dead (n = 87) | ||||||
| Model 1 | 0.85 | 0.68–1.06 | 0.29 | 0.05–0.92 | 0.72 | 0.30–1.70 |
| Model 2 | 0.85 | 0.68–1.06 | 0.19 | 0.04–0.80 | 0.63 | 0.27–1.47 |
| Model 3 | 0.98 | 0.65–1.49 | 0.22 | 0.03–1.71 | 1.16 | 0.39–3.47 |
| Frail → Dead (n = 63) | ||||||
| Model 1 | 1.07 | 0.97–1.19 | 1.96 | 1.05–3.66 | 0.93 | 0.56–1.56 |
| Model 2 | 1.07 | 0.96–1.19 | 2.01 | 1.09–3.73 | 0.99 | 0.59–1.67 |
| Model 3 | 1.03 | 0.87–1.22 | 2.04 | 0.94–4.45 | 0.76 | 0.42–1.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teh, R.; Mendonça, N.; Muru-Lanning, M.; MacDonell, S.; Robinson, L.; Kerse, N. Dietary Protein Intake and Transition between Frailty States in Octogenarians Living in New Zealand. Nutrients 2021, 13, 2843. https://doi.org/10.3390/nu13082843
Teh R, Mendonça N, Muru-Lanning M, MacDonell S, Robinson L, Kerse N. Dietary Protein Intake and Transition between Frailty States in Octogenarians Living in New Zealand. Nutrients. 2021; 13(8):2843. https://doi.org/10.3390/nu13082843
Chicago/Turabian StyleTeh, Ruth, Nuno Mendonça, Marama Muru-Lanning, Sue MacDonell, Louise Robinson, and Ngaire Kerse. 2021. "Dietary Protein Intake and Transition between Frailty States in Octogenarians Living in New Zealand" Nutrients 13, no. 8: 2843. https://doi.org/10.3390/nu13082843
APA StyleTeh, R., Mendonça, N., Muru-Lanning, M., MacDonell, S., Robinson, L., & Kerse, N. (2021). Dietary Protein Intake and Transition between Frailty States in Octogenarians Living in New Zealand. Nutrients, 13(8), 2843. https://doi.org/10.3390/nu13082843








