Nutritional Imbalances in Adult Celiac Patients Following a Gluten-Free Diet
Abstract
:1. Introduction
2. Materials and Methods
3. GFD in CD Treatment
3.1. Newly Diagnosed Patients: Recovery of Previous Nutritional Deficiencies
3.2. Adherence to the GFD
3.3. Nutritional Composition of the GFD
3.3.1. Macronutrient Intake
- (a)
- Fats
- (b)
- Carbohydrates
- (c)
- Fiber
- (d)
- Proteins
3.3.2. Micronutrient Intake
- (a)
- Vitamins
- (b)
- Minerals
3.3.3. Differences between Men and Women
3.4. Comparison with the Healthy General Population
3.4.1. Macronutrient Comparison
3.4.2. Micronutrient Comparison
3.5. Dietary Guidelines for a Balanced Diet
- The most remarkable guideline would be to improve the diet by promoting a greater consumption of plant-based foods, such as fruits, vegetables, legumes, nuts and naturally gluten-free whole grain cereals and pseudocereals followed by reducing GFP consumption.
- Although we can consider that protein intakes are sufficient, we will have to make sure they understand the importance of protein sources by promoting the intake of high-quality protein rich foods, which will be also related to the intake of high-quality fats, and thus a better dietary lipid profile [55].
- Naturally gluten-free foods rich in micronutrients are proposed before recommending fortified foods or supplements [55]. However, it could be interesting to combine the two options, with the aim of achieving a faster recovery from some vitamin and mineral deficiencies, thus, once suitable levels have been recovered, it could be enough to follow an appropriate GFD.
- Another possible improvement to consider is the one mentioned by González et al. who claim a fortification of the GFP, knowing in advance which are the nutrients that are most needed in the GFD [18]. This, could help to improve micronutrient deficiencies, but on the other hand, the macronutrient content should be corrected (reducing fats for example) and thus total balance could be achieved. In fact, it is noteworthy that the food industry is making great progress in developing healthier GFPs, which is a great challenge that has a direct impact on the health of the patients.
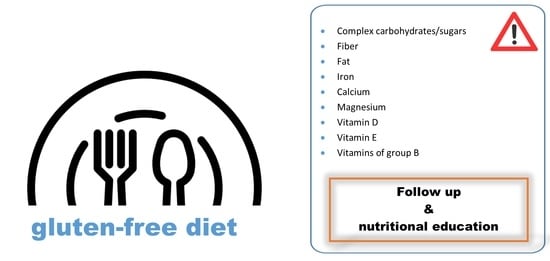
- The deficiencies in iron, calcium and vitamin D are noteworthy, in relation to their involvement in pathologies such as anemia or osteoporosis, which are more prevalent among the celiac population. Thus, it is recommended to take care of their intake through their food sources, such as legumes, cereals and dairy products [55].
- Similarly, to overcome deficiencies observed in B group vitamins, involved, at least in part, in the higher prevalence of CVD in CD, dietary treatment is especially important. Folate is usually given more importance; its consumption should be promoted through vegetables, pulses and pseudocereals. Moreover, this micronutrient is one of those proposed for food fortification and liable to be obtained through supplementation in high risk cases [18,36,44].
- It is common to find nutrient deficiencies in GFD, but this does not mean that it has to be normalized. Thus, Bascuñan et al. claim that although adherence to GFD may seem enough, patients with celiac disease should be continuously supervised to prevent some usual deficiencies, and to ensure that they continue having sufficient adherence to the GFD [25].
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lebwohl, B.; Rubio-Tapia, A. Epidemiology, Presentation, and Diagnosis of Celiac Disease. Gastroenterology 2020, 160, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836. [Google Scholar] [CrossRef] [Green Version]
- Di Nardo, G.; Villa, M.P.; Conti, L.; Ranucci, G.; Pacchiarotti, C.; Principessa, L.; Raucci, U.; Parisi, P. Nutritional deficiencies in children with celiac disease resulting from a gluten-free diet: A systematic review. Nutrients 2019, 11, 1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustalahti, K.; Catassi, C.; Reunanen, A.; Fabiani, E.; Heier, M.; McMillan, S.; Murray, L.; Metzger, M.H.; Gasparin, M.; Bravi, E.; et al. The prevalence of celiac disease in Europe: Results of a centralized, international mass screening project. Ann. Med. 2010, 42, 587–595. [Google Scholar] [CrossRef]
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.E.A.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac disease. Nat. Rev. Dis. Prim. 2019, 5, 3. [Google Scholar] [CrossRef]
- Kikut, J.; Konecka, N.; Szczuko, M. Quantitative assessment of nutrition and nutritional status of patients with celiac disease aged 13–18. Rocz. Panstw. Zakl. Hig. 2019, 70, 359–367. [Google Scholar] [CrossRef]
- Itzlinger, A.; Branchi, F.; Elli, L.; Schumann, M. Gluten-free diet in celiac disease—Forever and for all? Nutrients 2018, 10, 1796. [Google Scholar] [CrossRef] [Green Version]
- Hujoel, I.A.; Reilly, N.R.; Rubio-Tapia, A. Celiac Disease: Clinical Features and Diagnosis. Gastroenterol. Clin. N. Am. 2019, 48, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Abadie, V.; Sollid, L.M.; Barreiro, L.B.; Jabri, B. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu. Rev. Immunol. 2011, 29, 493–525. [Google Scholar] [CrossRef] [Green Version]
- Sollid, L.M. Coeliac disease: Dissecting a complex inflammatory disorder. Nat. Rev. Immunol. 2002, 2, 647–655. [Google Scholar] [CrossRef]
- Fernández, C.B.; Varela-Moreiras, G.; Úbeda, N.; Alonso-Aperte, E. Nutritional status in Spanish children and adolescents with celiac disease on a gluten free diet compared to non-celiac disease controls. Nutrients 2019, 11, 2329. [Google Scholar] [CrossRef] [Green Version]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef] [Green Version]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.R.; Hadjivassiliou, M.; Holdoway, A.; Van Heel, D.A.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British society of gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef]
- Cichewicz, A.B.; Mearns, E.S.; Taylor, A.; Boulanger, T.; Gerber, M.; Leffler, D.A.; Drahos, J.; Sanders, D.S.; Thomas Craig, K.J.; Lebwohl, B. Diagnosis and Treatment Patterns in Celiac Disease. Dig. Dis. Sci. 2019, 64, 2095–2106. [Google Scholar] [CrossRef]
- Szakács, Z.; Mátrai, P.; Hegyi, P.; Szabó, I.; Vincze, Á.; Balaskó, M.; Mosdósi, B.; Sarlós, P.; Simon, M.; Márta, K.; et al. Younger age at diagnosis predisposes to mucosal recovery in celiac disease on a gluten-free diet: A meta-analysis. PLoS ONE 2017, 12, e0187526. [Google Scholar] [CrossRef] [PubMed]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- González, T.; Larretxi, I.; Vitoria, J.C.; Castaño, L.; Simón, E.; Churruca, I.; Navarro, V.; Lasa, A. Celiac male’s gluten-free diet profile: Comparison to that of the control population and celiac women. Nutrients 2018, 10, 1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churruca, I.; Miranda, J.; Lasa, A.; Bustamante, M.; Larretxi, I.; Simon, E. Analysis of body composition and food habits of Spanish celiac women. Nutrients 2015, 7, 5515–5531. [Google Scholar] [CrossRef] [Green Version]
- Miranda, J.; Lasa, A.; Bustamante, M.A.; Churruca, I.; Simon, E. Nutritional Differences Between a Gluten-free Diet and a Diet Containing Equivalent Products with Gluten. Plant Food. Hum. Nutr. 2014, 69, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Lerma, J.; Crespo-Escobar, P.; Martínez-Barona, S.; Fornés-Ferrer, V.; Donat, E.; Ribes-Koninckx, C. Differences in the macronutrient and dietary fibre profile of gluten-free products as compared to their gluten-containing counterparts. Eur. J. Clin. Nutr. 2019, 73, 930–936. [Google Scholar] [CrossRef]
- Larretxi, I.; Simon, E.; Benjumea, L.; Miranda, J.; Bustamante, M.A.; Lasa, A.; Eizaguirre, F.J.; Churruca, I. Gluten-free-rendered products contribute to imbalanced diets in children and adolescents with celiac disease. Eur. J. Nutr. 2019, 58, 775–783. [Google Scholar] [CrossRef]
- Simón, E.; Larretxi, I.; Churrica, I.; Lasa, A.; Bustamante, M.Á.; Navarro, V.; Fernandez-Gil, M.d.P.; Miranda, J. Nutritional and Analytical Approaches of Gluten-Free Diet in Celiac Disease, 1st ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Annibale, B.; Severi, C.; Chistolini, A.; Antonelli, G.; Lahner, E.; Marcheggiano, A.; Iannoni, C.; Monarca, B.; Delle Fave, G. Efficacy of Gluten-Free Diet Alone on Recovery from Iron Deficiency Anemia in Adult Celiac Patients. Am. J. Gastroenterol. 2001, 96, 132–137. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Elli, L.; Pellegrini, N.; Scricciolo, A.; Lombardo, V.; Doneda, L.; Vecchi, M.; Scarpa, C.; Araya, M.; Roncoroni, L. Impact of FODMAP content restrictions on the quality of diet for patients with celiac disease on a gluten-free diet. Nutrients 2019, 11, 2220. [Google Scholar] [CrossRef] [Green Version]
- Low, M.S.Y.; Speedy, J.; Styles, C.E.; De-Regil, L.M.; Pasricha, S.R. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database Syst. Rev. 2016, 4, 1465–1858. [Google Scholar] [CrossRef]
- Sategna-Guidetti, C.; Grosso, S.B.; Grosso, S.; Mengozzi, G.; Aimo, G.; Zaccaria, T.; Di Stefano, M.; Isaia, G.C. The effects of 1-year gluten withdrawal on bone mass, bone metabolism and nutritional status in newly-diagnosed adult coeliac disease patients. Aliment. Pharmacol. Ther. 2000, 14, 35–43. [Google Scholar] [CrossRef]
- Zanchetta, M.B.; Longobardi, V.; Costa, F.; Longarini, G.; Mazure, R.M.; Moreno, M.L.; Vázquez, H.; Silveira, F.; Niveloni, S.; Smecuol, E.; et al. Impaired Bone Microarchitecture Improves After One Year On Gluten-Free Diet: A Prospective Longitudinal HRpQCT Study in Women With Celiac Disease. J. Bone Miner. Res. 2017, 32, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Stenson, W.F.; Newberry, R.; Lorenz, R.; Baldus, C.; Civitelli, R. Increased Prevalence of Celiac Disease and Need for Routine Screening Among Patients With Osteoporosis. JAMA Intern. Med. 2005, 165, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Zanchetta, M.B.; Longobardi, V.; Bai, J.C. Bone and Celiac Disease. Curr. Osteoporos. Rep. 2016, 14, 43–48. [Google Scholar] [CrossRef]
- Lanzini, A.; Lanzarotto, F.; Villanacci, V.; Mora, A.; Bertolazzi, S.; Turini, D.; Carella, G.; Malagoli, A.; Ferrante, G.; Cesana, B.M.; et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment. Pharmacol. Ther. 2009, 29, 1299–1308. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Giorgetti, G.M.; Elisei, W.; Inchingolo, C.D.; Monardo, E.; Aiello, F. Endoscopic and histological findings in the duodenum of adults with celiac disease before and after changigng to a gluten-free diet: A 2-year prospective study. Endoscopy 2006, 38, 702–707. [Google Scholar] [CrossRef]
- Moreno, M.D.L.; Cebolla, Á.; Munõz-Suano, A.; Carrillo-Carrion, C.; Comino, I.; Pizarro, Á.; León, F.; Rodríguez-Herrera, A.; Sousa, C. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017, 66, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Kurppa, K.; Lauronen, O.; Collin, P.; Ukkola, A.; Laurila, K.; Huhtala, H.; Mäki, M.; Kaukinen, K. Factors associated with dietary adherence in celiac disease: A nationwide study. Digestion 2013, 86, 309–314. [Google Scholar] [CrossRef]
- Hopman, E.G.D.; Cessie, L.; Mary, B.; Von Blomberg, E.; Mearin, M.L. Nutritional Management of the Gluten-Free Diet in Young People with Celiac Disease in The Netherlands; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; Volume 43. [Google Scholar]
- Nachman, F.; Mauriño, E.; Vázquez, H.; Sfoggia, C.; Gonzalez, A.; Gonzalez, V.; del Campo, M.P.; Smecuol, E.; Niveloni, S.; Sugai, E.; et al. Quality of life in celiac disease patients. Prospective analysis on the importance of clinical severity at diagnosis and the impact of treatment. Dig. Liver Dis. 2009, 41, 15–25. [Google Scholar] [CrossRef]
- Ukkola, A.; Mäki, M.; Kurppa, K.; Collin, P.; Huhtala, H.; Kekkonen, L.; Kaukinen, K. Diet Improves Perception of Health and Well-being in Symptomatic, but Not Asymptomatic, Patients With Celiac Disease. Clin. Gastroenterol. Hepatol. 2011, 9, 118–123. [Google Scholar] [CrossRef]
- Branchi, F.; Tomba, C.; Ferretti, F.; Norsa, L.; Roncoroni, L.; Bardella, M.T.; Conte, D.; Elli, L. Celiac Disease and Drug-Based Therapies: Inquiry into Patients Demands. Digestion 2016, 93, 160–166. [Google Scholar] [CrossRef]
- Rodrigo, L.; Pérez-Martínez, I.; Lauret-Braña, E.; Suárez-González, A. Descriptive Study of the Different Tools Used to Evaluate the Adherence to a Gluten-Free Diet in Celiac Disease Patients. Nutrients 2018, 10, 1777. [Google Scholar] [CrossRef] [Green Version]
- Tuire, I.; Marja-Leena, L.; Teea, S.; Katri, H.; Jukka, P.; Päivi, S.; Heini, H.; Markku, M.; Pekka, C.; Katri, K. Persistent duodenal intraepithelial lymphocytosis despite a long-term strict gluten-free diet in celiac disease. Am. J. Gastroenterol. 2012, 107, 1563–1569. [Google Scholar] [CrossRef]
- Kaukinen, K. Updates on systemic consequences of coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 87–88. [Google Scholar] [CrossRef]
- Hallert, C.; Grant, C.; Grehn, S.; Grännö, C.; Hultén, S.; Midhagen, G.; Ström, M.; Svensson, H.; Valdimarsson, T. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment. Pharmacol. Ther. 2002, 16, 1333–1339. [Google Scholar] [CrossRef]
- Wild, D.; Robins, G.G.; Burley, V.J.; Howdle, P.D. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment. Pharmacol. Ther. 2010, 32, 573–581. [Google Scholar] [CrossRef]
- Martin, J.; Geisel, T.; Maresch, C.; Krieger, K.; Stein, J. Inadequate nutrient intake in patients with celiac disease: Results from a German dietary survey. Digestion 2013, 87, 240–246. [Google Scholar] [CrossRef]
- Thompson, T.; Dennis, M.; Higgins, L.A.; Lee, A.R.; Sharrett, M.K. Gluten-free diet survey: Are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J. Hum. Nutr. Diet. 2005, 18, 163–169. [Google Scholar] [CrossRef]
- Jamieson, J.A.; Neufeld, A. Food sources of energy and nutrients among Canadian adults following a gluten-free diet. Peer J. 2020, 8, e9590. [Google Scholar] [CrossRef]
- Ballestero-Fernández, C.; Varela-Moreiras, G.; Úbeda, N.; Alonso-Aperte, E. Nutritional Status in Spanish Adults with Celiac Disease Following a Long-Term Gluten-Free Diet Is Similar to Non-Celiac. Nutrients 2021, 13, 1626. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Babio, N.; Martínez-González, M.A.; Corella, D.; Ros, E.; Martín-Peláez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef] [Green Version]
- Penagini, F.; Dilillo, D.; Meneghin, F.; Mameli, C.; Fabiano, V.; Zuccotti, G.V. Gluten-free diet in children: An approach to a nutritionally adequate and balanced diet. Nutrients 2013, 5, 4553–4565. [Google Scholar] [CrossRef] [Green Version]
- Gołąbek, K.D.; Regulska-Ilow, B. Dietary support in insulin resistance: An overview of current scientific reports. Adv. Clin. Exp. Med. 2019, 28, 1577–1585. [Google Scholar] [CrossRef]
- Lebwohl, B.; Green, P.H.R.; Söderling, J.; Roelstraete, B.; Ludvigsson, J.F. Association between Celiac Disease and Mortality Risk in a Swedish Population. JAMA J. Am. Med. Assoc. 2020, 323, 1277–1285. [Google Scholar] [CrossRef]
- Blanco-Rojo, R.; Sandoval-Insausti, H.; López-Garcia, E.; Graciani, A.; Ordovás, J.M.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Consumption of Ultra-Processed Foods and Mortality: A National Prospective Cohort in Spain. Mayo Clin. Proc. 2019, 94, 2178–2188. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Núñez, B.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef]
- Moreiras, O.; Carbajal, Á.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos—Guía de Prácticas; Pirámide: Madrid, Spain, 2018; Volume 19. [Google Scholar]
- Matos Segura, M.E.; Rosell, C.M. Chemical Composition and Starch Digestibility of Different Gluten-free Breads. Plant Food. Hum. Nutr. 2011, 66, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xian, T.; Jia, X.; Zhang, L.; Liu, L.; Man, F.; Zhang, X.; Zhang, J.; Pan, Q.; Guo, L. A cross-sectional study on the associations of insulin resistance with sex hormone, abnormal lipid metabolism in T2DM and IGT patients. Medicine 2017, 96, e7378. [Google Scholar] [CrossRef]
- Laurikka, P.; Lindfors, K.; Oittinen, M.; Huhtala, H.; Salmi, T.; Lähdeaho, M.L.; Ilus, T.; Mäki, M.; Kaukinen, K.; Kurppa, K. Dietary Factors and Mucosal Immune Response in Celiac Disease Patients Having Persistent Symptoms Despite a Gluten-free Diet. J. Clin. Gastroenterol. 2019, 53, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Nguyen, L.H.; Song, M.; Jovani, M.; Liu, P.H.; Cao, Y.; Tam, I.; Wu, K.; Giovannucci, E.L.; Strate, L.L.; et al. Intake of Dietary Fiber, Fruits, and Vegetables and Risk of Diverticulitis. Am. J. Gastroenterol. 2019, 114, 1531–1538. [Google Scholar] [CrossRef]
- Dreher, M.L. Whole fruits and fruit fiber emerging health effects. Nutrients 2018, 10, 1833. [Google Scholar] [CrossRef] [Green Version]
- Spiller, R.C. Changing views on diverticular disease: Impact of aging, obesity, diet, and microbiota. Neurogastroenterol. Motil. 2015, 27, 305–312. [Google Scholar] [CrossRef]
- Lionetti, E.; Antonucci, N.; Marinelli, M.; Bartolomei, B.; Franceschini, E.; Gatti, S.; Catassi, G.N.; Verma, A.K.; Monachesi, C.; Catassi, C. Nutritional status, dietary intake, and adherence to the mediterranean diet of children with celiac disease on a gluten-free diet: A case-control prospective study. Nutrients 2020, 12, 143. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-garach, A.; García-fontana, B.; Muñoz-torres, M. Nutrients and dietary patterns related to osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef]
- Hallert, C.; Svensson, M.; Tholstrup, J.; Hultberg, B. Clinical trial: B vitamins improve health in patients with coeliac disease living on a gluten-free diet. Aliment. Pharmacol. Ther. 2009, 29, 811–816. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Caruso, R.; Pallone, F.; Stasi, E.; Romeo, S.; Monteleone, G. Appropriate nutrient supplementation in celiac disease. Ann. Med. 2013, 45, 522–531. [Google Scholar] [CrossRef]
- Larretxi, I.; Txurruka, I.; Navarro, V.; Lasa, A.; Bustamante, M.Á.; Fernández-Gil, M.D.P.; Simón, E.; Miranda, J. Micronutrient analysis of gluten-free products: Their low content is not involved in gluten-free diet imbalance in a cohort of celiac children and adolescent. Foods 2019, 8, 321. [Google Scholar] [CrossRef] [Green Version]
- Government of Canada. Food and Drug Regulations. 2019. Available online: https://laws-lois.justice.gc.ca/eng/regulations/C.R.C.,_c._870/ (accessed on 30 July 2019).
- Questions and Answers on FDA’s Fortification Policy-Guidance for Industry. Guidance for Industry. Available online: www.fda.gov/media/94563/download (accessed on 15 July 2019).
- Gładyś, K.; Dardzińska, J.; Guzek, M.; Adrych, K.; Kochan, Z.; Małgorzewicz, S. Expanded Role of a Dietitian in Monitoring a Gluten-Free Diet in Patients with Celiac Disease: Implications for Clinical Practice. Nutrients 2021, 13, 1859. [Google Scholar] [CrossRef]
| Author | Sample Size (n) GFD Duration | Type of Study | Country | Biochemical Data | Anthropometric Parameters |
|---|---|---|---|---|---|
| Zanchetta et al. (2017) [28] | −n = 26 (women) −1 year GFD | Observational, longitudinal cohort study | Argentina | Low vitamin D Normal Ca, Hb, PTH | Low bone microarchitectural parameters |
| Annibale et al. (2001) [24] | −n = 20 −GFD: 6, 12 and 24 months | Italy | Low Fe, low Ferr, low Hb Anaemia Normal Glu, TG, proteins, Alb | ||
| Sategna-Guidetti et al. (2000) [27] | −n = 86 −1 year GFD | Italy | Low vitamin D Normal Ca, P, Alb, PA, Hb, Fe, Ferr, Trans, Fol | BMI = 20.85 kg/m2 |
| Author | Sample GFD Duration Adherence | Type of Study | Country | Macronutrient Intake | Micronutrient Intake |
|---|---|---|---|---|---|
| González et al. (2018) [18] | n = 42 men, 31.5 y ± 11.9 ≥1 year GFD ND | Observational, transversal cohort study | Spain | High fat, specially SFA High protein, Low CHO, Low fiber, High cholesterol. | Low vitamin D and E, folate, iodine, and magnesium. |
| Churruca et al. (2015) [19] | n = 54 women, 34 y ± 13 Median duration of GFD = 10 years ND | Observational, transversal cohort study | Spain | Low energy intake, Low CHO, Low fiber, High fat. | Low vitamin D and E, Folate, Calcium, Iron, Magnesium, Iodine, Potassium and Selenium. |
| Bascuñán et al. (2019) [25] | n = 46 (43 women), 41.1 y ± 10.1 ≥1 year GFD Strict adherence 100% of participants | Randomized double bind controlled study | Italy | High fat, low CHO. | Low vitamin D, vitamin E, folate, thiamine (B1), calcium, iron, zinc, sodium and potassium. |
| Hopman et al. (2006) [36] | n = 132 (87 women), 16.6 y ± 4.4 Median duration of GFD = 9.6 years Strict adherence 75% of participants | Observational, transversal cohort study | Netherlands | High saturated fat, Low fiber. | Low Iron and Calcium. |
| Hallert et al. (2002) [43] | n = 30 (18 women), 55 y −10 years GFD Strict adherence 100% of participants | Observational, longitudinal cohort study | Sweden | Low folate. | |
| Wild et al. (2010) [44] | n = 93 62 women, 53 y ± 13; 31 men, 56 y ± 15 ≥6 months GFD (mean duration: 8 y) ND | Observational, longitudinal cohort study | UK | Low fiber, high sugar. | Low vitamin D, folate, calcium, iron, zinc, magnesium and manganese. |
| Martin et al. (2013) [45] | n = 73 (55 women), 18–80 y Median duration of GFD = 7.5 years ND | Observational, transversal cohort study | Germany | Low CHO, Low fiber. | Low vitamin B1, B2, B6, Folate, magnesium and iron. |
| Thompson et al. (2005) [46] | n = 47 (39 women), 51 y ± 11 Median duration of GFD = 5.3 years Strict adherence 100% of participants | Observational, transversal cohort study | USA | Low fiber. | Low iron and calcium. |
| Jamieson et al. (2020) [47] | n = 35 (29 women), 47 y ± 11.5 Median duration of GFD = 6.7 years ND | Observational, transversal cohort study | Canada | Low CHO, high fat, low fiber. | Low iron, calcium and vitamin C. |
| Ballestero-Fernández et al. (2021) [48] | n = 64 43 women, 39.17 y ± 10.62; 21 men, 38.58 y ± 9.61 ≥1 year GFD ND | Observational, transversal case-control study | Spain | Low CHO, PUFA and fiber high protein, fat and sugars. | Low folate, vitamin E, vitamin D, iodine, calcium, zinc, magnesium. Low iron in women |
| Celiac Adults | Celiac Children | |
|---|---|---|
| Fat | High fat and SFA intakes | = |
| Carbohydrates | Low complex carbohydrate intake, but high simple sugar intake | = |
| Fiber | Low fiber intake | = |
| Vitamins | Low intakes of Vitamin D, E and B group vitamins (B1, B2, B6, B9) | Low intakes of Vitamin D and B group vitamins (B1, B2, B6, B9) |
| Minerals | Low intakes of iron, calcium, magnesium, zinc, iodine, potassium, selenium and manganese. | Low intakes of iron, calcium, magnesium and zinc. |
| Women | Men | |
|---|---|---|
| Macronutrients | Unbalanced distribution and intake | = |
| Fiber | Lower fiber intake | Low fiber intake |
| Vitamins | Various deficiencies | = |
| Minerals | Lower Fe, Ca, I and K intakes | Lower Mg intake |
| Healthy Population | Celiac Patients | |
|---|---|---|
| Energy | = | = |
| Fat | High fat intake | Higher fat and SFA intake |
| Proteins | = | = |
| Carbohydrates | Low carbohydrate intake | Lower complex carbohydrate intake, higher simple sugar intake |
| Fiber | Low intake | Lower fiber intake |
| Vitamins | Low folate intake | Lower vitamin E and B group vitamins intakes. Lower folate intake and higher tHcy serum levels. |
| Minerals | Lower magnesium, selenium, iron and zinc intake |
| Guideline for a Secure and Balanced GFD | Result in the Dietary Profile |
|---|---|
| Promote consumption of plant-based foods, such as fruits, vegetables, legumes, nuts and naturally gluten-free whole grain cereals and pseudocereals (quinoa, amaranth, etc.) | ↓ fats, specially saturated ↓ sugars ↑ complex carbohydrates ↑ fiber ↑ vitamins (folate, riboflavin, vitamin C and E, etc.) ↑ minerals |
| Reduce GFP consumption. | |
| Fortify naturally gluten-free foods in micronutrients | ↑ vitamins ↑ minerals |
| Fortify GFP in micronutrients and balance their macronutrient content | Helpful in the macronutrient and micronutrient balance achievement |
| Increase dairy products, as well as legumes and cereals | ↑ iron, calcium and vitamin D |
| Increase vegetables, pulses and pseudocereals | ↑ B group vitamins |
| Continuous supervision of celiac patients going on a GFD | ↑ adherence to the diet |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardo, A.; Churruca, I.; Lasa, A.; Navarro, V.; Vázquez-Polo, M.; Perez-Junkera, G.; Larretxi, I. Nutritional Imbalances in Adult Celiac Patients Following a Gluten-Free Diet. Nutrients 2021, 13, 2877. https://doi.org/10.3390/nu13082877
Cardo A, Churruca I, Lasa A, Navarro V, Vázquez-Polo M, Perez-Junkera G, Larretxi I. Nutritional Imbalances in Adult Celiac Patients Following a Gluten-Free Diet. Nutrients. 2021; 13(8):2877. https://doi.org/10.3390/nu13082877
Chicago/Turabian StyleCardo, Aner, Itziar Churruca, Arrate Lasa, Virginia Navarro, Maialen Vázquez-Polo, Gesala Perez-Junkera, and Idoia Larretxi. 2021. "Nutritional Imbalances in Adult Celiac Patients Following a Gluten-Free Diet" Nutrients 13, no. 8: 2877. https://doi.org/10.3390/nu13082877
APA StyleCardo, A., Churruca, I., Lasa, A., Navarro, V., Vázquez-Polo, M., Perez-Junkera, G., & Larretxi, I. (2021). Nutritional Imbalances in Adult Celiac Patients Following a Gluten-Free Diet. Nutrients, 13(8), 2877. https://doi.org/10.3390/nu13082877