Urinary Tartaric Acid, a Biomarker of Wine Intake, Correlates with Lower Total and LDL Cholesterol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anthropometric, Dietary, and Physical Activity Assessments
2.3. Clinical Measurements
2.4. Tartaric Acid Determination
2.4.1. Reagents and Standards
2.4.2. Sample Preparation
2.4.3. LC–ESI-MS/MS Analysis
2.5. Statistical Analyses
3. Results
3.1. Study Population
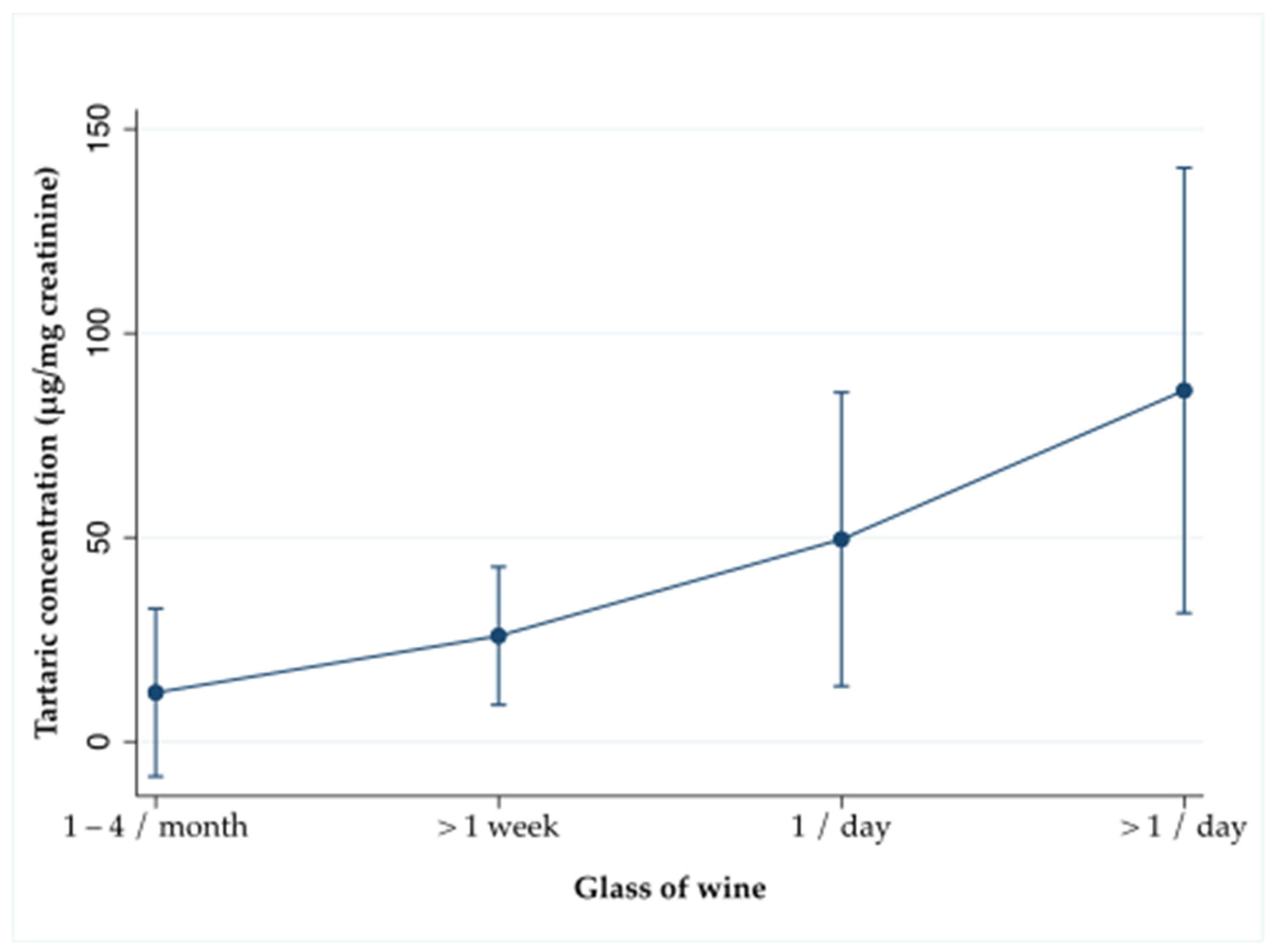
3.2. Tartaric Acid as a Biomarker of Wine Consumption
3.3. Anthropometric Measurements and Urinary Tartaric Acid
3.4. Biochemical and Clinical Measurements and Urinary Tartaric Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Greendale, G.; Crawford, S.L.; Avis, N.E.; Brooks, M.M.; Thurston, R.C.; Karvonen-Gutierrez, C.; Waetjen, L.E.; Matthews, K. The menopause transition and women’s health at midlife: A progress report from the Study of Women’s Health across the Nation (SWAN). Menopause 2019, 26, 1213–1227. [Google Scholar] [CrossRef]
- Matthews, K.A.; Meilahn, E.; Kuller, L.H.; Kelsey, S.F.; Cagguila, A.W.; Wing, R.R. Menopause and the Risk Factors of Coronary Heart Disease. N. Engl. J. Med. 1974, 306, 802–805. [Google Scholar]
- Matthews, K.A.; El Khoudary, S.R.; Brooks, M.M.; Derby, C.A.; Harlow, S.D.; Barinas-Mitchell, E.J.; Thurston, R.C. Lipid Changes around the Final Menstrual Period Predict Carotid Subclinical Disease in Postmenopausal Women. Stroke 2017, 1, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Bonithon-kopp, C.; Scarabin, P.Y.; Darne, B.; Malmejac, A.; Guize, L. Menopause-related changes in lipoproteins and some other cardiovascular risk factors. Int. J. Epidemiol. 1990, 19, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Crawford, S.L.; Chae, C.U.; Everson-Rose, S.A.; Sowers, M.F.; Sternfeld, B.; Sutton-Tyrrell, K. Are Changes in Cardiovascular Disease Risk Factors in Midlife Women Due to Chronological Aging or to the Menopausal Transition? J. Am. Coll. Cardiol. 2009, 54, 2366–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greendale, G.A.; Sternfeld, B.; Huang, M.H.; Han, W.; Karvonen-Gutierrez, C.; Ruppert, K.; Cauley, J.A.; Finkelstein, J.S.; Jiang, S.F.; Karlamangla, A.S. Changes in body composition and weight during the menopause transition. JCI Insight 2019, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M.J.; Hu, F.B.; Manson, J.E.; Rimm, E.B.; Willett, W.C. Primary Prevention of Coronary Heart Disease in Women Through Diet and Lifestyle. N. Engl. J. Med. 2000, 343, 16. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; English, D.R.; Itsiopoulos, C.; O’Dea, K.; Giles, G.G. Does a Mediterranean diet reduce the mortality risk associated with diabetes: Evidence from the Melbourne Collaborative Cohort Study. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 733–739. [Google Scholar] [CrossRef]
- Marcos, A.; Serra-Majem, L.; Pérez-Jiménez, F.J.; Pascual, V.; Tinahones, F.J.; Estruch, R. Moderate consumption of beer and its effects on cardiovascular and metabolic health: An updated review of recent scientific evidence. Nutrients 2021, 13, 879. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and Policy Prioritites for CVD, diabetes and obesity—a comprehensive review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health: A Critical Review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274. [Google Scholar] [CrossRef] [Green Version]
- Chiva-Blanch, G.; Arranz, S.; Lamuela-Raventos, R.M.; Estruch, R. Effects of wine, alcohol and polyphenols on cardiovascular disease risk factors: Evidences from human studies. Alcohol Alcohol. 2013, 48, 270–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Bulló, M.; Salas-Salvadó, J.; Corella, D.; Fitó, M.; Gea, A.; Gómez-Gracia, E.; Lapetra, J.; et al. Moderate red wine consumption is associated with a lower prevalence of the metabolic syndrome in the PREDIMED population. Br. J. Nutr. 2015, 113, S121–S130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costanzo, S.; Di Castelnuovo, A.; Donati, M.B.; Iacoviello, L.; De Gaetano, G. Wine, beer or spirit drinking in relation to fatal and non-fatal cardiovascular events: A meta-analysis. Eur. J. Epidemiol. 2011, 26, 833–850. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, V.E.; Dietrich, A.M.; Estabrooks, P.A.; Savla, J.; Serrano, E.; Davy, B.M. Dietary biomarkers: Advances, limitations and future directions. Nutr. J. 2012, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Velioglu, Y.S. Food acids: Organic acids, volatile organic acids, and phenolic acids. In Advances in Food Biochemistry; CRC Press: Boca Raton, FL, USA, 2009; p. 522. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Organic Acids in Wine. In Handbook of Enology; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 1–49. [Google Scholar]
- Regueiro, J.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Estruch, R.; Lamuela-Raventós, R.M. Urinary tartaric acid as a potential biomarker for the dietary assessment of moderate wine consumption: A randomised controlled trial. Br. J. Nutr. 2014, 111, 1680–1685. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, A.J.; Willis, N.D.; Wilson, T.; Zubair, H.; Chambers, E.; Garcia-Perez, I.; Xie, L.; Tailliart, K.; Beckmann, M.; Mathers, J.C.; et al. Addressing the pitfalls when designing intervention studies to discover and validate biomarkers of habitual dietary intake. Metabolomics 2019, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Martínez-González, M.Á.; Corella, D.; Salas-salvadó, J.; Ros, E.; Covas, M.I.; Fiol, M.; Wärnberg, J.; Arós, F.; Ruíz-Gutiérrez, V.; Lamuela-Raventós, R.M.; et al. Cohort profile: Design and methods of the PREDIMED study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Martn-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [Green Version]
- Elosua, R.; Garcia, M.; Aguilar, A.; Molina, L.; Covas, M.-I.; Marrugat, J. Validation of the Minnesota Leisure Time Spanish Women. Med. Sci. Sport. Exerc. 2000, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruíz-Gutiérrez, V.; Covas, M.-I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-Style Diet on Cardiovascular Risk FactorsA Randomized Trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Estruch, R.; Lamuela-Raventós, R. Development of a LC-ESI-MS/MS approach for the rapid quantification of main wine organic acids in human urine. J. Agric. Food Chem. 2013, 61, 6763–6768. [Google Scholar] [CrossRef] [PubMed]
- Medina-Remón, A.; Barrionuevo-González, A.; Zamora-Ros, R.; Andres-Lacueva, C.; Estruch, R.; Martínez-González, M.Á.; Diez-Espino, J.; Lamuela-Raventos, R.M. Rapid Folin-Ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake. Anal. Chim. Acta 2009, 634, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M.; Hsieh, S.D. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int. J. Food Sci. Nutr. 2005, 56, 303–307. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- WHO. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Care, D.; Suppl, S.S. Classification and diagnosis of diabetes: Standards of medical care in Diabetesd2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.R. Methodologic and statistical considerations regarding use of biomarkers of nutritional exposure in epidemiology. J. Nutr. 2003, 133, 881–887. [Google Scholar] [CrossRef] [Green Version]
- Esteban-Fernández, A.; Ibañez, C.; Simó, C.; Bartolomé, B.; Moreno-Arribas, M.V. An Ultrahigh-Performance Liquid Chromatography-Time-of-Flight Mass Spectrometry Metabolomic Approach to Studying the Impact of Moderate Red-Wine Consumption on Urinary Metabolome. J. Proteome Res. 2018, 17, 1624–1635. [Google Scholar] [CrossRef]
- Romero-Pérez, A.I.; Lamuela-Raventós, R.M.; Waterhouse, A.L.; De La Torre-Boronat, M.C. Levels of cis- and trans-Resveratrol and Their Glucosides in White and Rosé Vitis vinifera Wines from Spain. J. Agric. Food Chem. 1996, 44, 2124–2128. [Google Scholar] [CrossRef]
- Lukasiewicz, E.; Mennen, L.I.; Bertrais, S.; Arnault, N.; Preziosi, P.; Galan, P.; Hercberg, S. Alcohol intake in relation to body mass index and waist-to-hip ratio: The importance of type of alcoholic beverage. Public Health Nutr. 2005, 8, 315–320. [Google Scholar] [CrossRef]
- Tolstrup, J.S.; Halkjær, J.; Heitmann, B.L.; Tjønneland, A.M.; Overvad, K.; Sørensen, T.I.A.; Grønbæk, M.N. Alcohol drinking frequency in relation to subsequent changes in waist circumference. Am. J. Clin. Nutr. 2008, 87, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Alcácera, M.A.; Marques-Lopes, I.; Fajó-Pascual, M.; Puzo, J.; Pérez, J.B.; Bes-Rastrollo, M.; Martínez-González, M.Á. Lifestyle factors associated with BMI in a Spanish graduate population: The SUN study. Obes. Facts 2008, 1, 80–87. [Google Scholar] [CrossRef]
- French, M.T.; Norton, E.C.; Fang, H.; Maclean, J.C. Alcohol consumption and body weight. Health Econ. 2010, 19, 814–832. [Google Scholar] [CrossRef] [PubMed]
- Ambikairajah, A.; Walsh, E.; Cherbuin, N. Lipid profile differences during menopause: A review with meta-analysis. Menopause 2019, 26, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Rifler, J.P.; Lorcerie, F.; Durand, P.; Delmas, D.; Ragot, K.; Limagne, E.; Mazué, F.; Riedinger, J.M.; D’Athis, P.; Hudelot, B.; et al. A moderate red wine intake improves blood lipid parameters and erythrocytes membrane fluidity in post myocardial infarct patients. Mol. Nutr. Food Res. 2012, 56, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Taborsky, M.; Ostadal, P.; Adam, T.; Moravec, O.; Gloger, V.; Schee, A.; Skala, T. Red or white wine consumption effect on atherosclerosis in healthy individuals (In Vino Veritas study). Taborsky. Clin. Study 2017, 118, 292–298. [Google Scholar] [CrossRef]
- Apostolidou, C.; Adamopoulos, K.; Lymperaki, E.; Iliadis, S.; Papapreponis, P.; Kourtidou-Papadeli, C. Cardiovascular risk and benefits from antioxidant dietary intervention with red wine in asymptomatic hypercholesterolemics. Clin. Nutr. ESPEN 2015, 10, e224–e233. [Google Scholar] [CrossRef]
- Droste, D.W.; Iliescu, C.; Vaillant, M.; Gantenbein, M.; De Bremaeker, N.; Lieunard, C.; Velez, T.; Meyer, M.; Guth, T.; Kuemmerle, A.; et al. A daily glass of red wine associated with lifestyle changes independently improves blood lipids in patients with carotid arteriosclerosis: Results from a randomized controlled trial. Nutr. J. 2013, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kechagias, S.; Zanjani, S.; Gjellan, S.; Leinhard, O.D.; Kihlberg, J.; Smedby, Ö.; Johansson, L.; Kullberg, J.; Ahlström, H.; Lindström, T.; et al. Effects of moderate red wine consumption on liver fat and blood lipids: A prospective randomized study. Ann. Med. 2011, 43, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Di Minno, G.; Ritieni, A. Red wine consumption and cardiovascular health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamora-Ros, R.; Urpi-Sarda, M.; Lamuela-Raventós, R.M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Arós, F.; Fitó, M.; Lapetra, J.; Estruch, R.; Andres-Lacueva, C. High urinary levels of resveratrol metabolites are associated with a reduction in the prevalence of cardiovascular risk factors in high-risk patients. Pharmacol. Res. 2012, 65, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Choleva, M.; Antonopoulou, S.; Demopoulos, C.A. Wine and its metabolic effects. A comprehensive review of clinical trials. Metabolism 2018, 83, 102–119. [Google Scholar] [CrossRef]
- Covas, M.I.; Gambert, P.; Fitó, M.; de la Torre, R. Wine and oxidative stress: Up-to-date evidence of the effects of moderate wine consumption on oxidative damage in humans. Atherosclerosis 2010, 208, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Argyrou, C.; Vlachogianni, I.; Stamatakis, G.; Demopoulos, C.A.; Antonopoulou, S.; Fragopoulou, E. Postprandial effects of wine consumption on Platelet Activating Factor metabolic enzymes. Prostaglandins Other Lipid Mediat. 2017, 130, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Argyrou, C.; Detopoulou, M.; Tsitsou, S.; Seremeti, S.; Yannakoulia, M.; Antonopoulou, S.; Kolovou, G.; Kalogeropoulos, P. The effect of moderate wine consumption on cytokine secretion by peripheral blood mononuclear cells: A randomized clinical study in coronary heart disease patients. Cytokine 2021, 146, 155629. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.P.; Roggerio, A.; Goes, M.F.S.; Avakian, S.D.; Leal, D.P.; Maranhão, R.C.; Strunz, C.M.C. Serum concentrations and gene expression of sirtuin 1 in healthy and slightly overweight subjects after caloric restriction or resveratrol supplementation: A randomized trial. Int. J. Cardiol. 2017, 227, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Stünkel, W.; Campbell, R.M. Sirtuin 1 (SIRT1): The misunderstood HDAC. J. Biomol. Screen. 2011, 16, 1153–1169. [Google Scholar] [CrossRef] [Green Version]
| General Characteristics | |
|---|---|
| Age, years | 66.9 ± 0.4 |
| Type 2 diabetes, n (%) | 93 (42.08) |
| Hypertension, n (%) | 193 (87.34) |
| Hypercholesterolemia, n (%) | 169 (76.55) |
| Medication use, n (%) | |
| ACE inhibitors | 64 (28.96) |
| Diuretics | 58 (26.24) |
| Statins | 90 (40.72) |
| Other lipid-lowering agents | 14 (6.33) |
| Insulin | 10 (4.52) |
| Oral hypoglycemic agents | 53 (23.98) |
| Antiplatelet therapy | 46 (20.81) |
| Current smoker, n (%) | 20 (9.05) |
| Leisure-time physical activity, MET·min/week | 186.5 ± 10.9 |
| Educational level, n (%) | |
| Low | 182 (82.35) |
| Medium | 24 (10.86) |
| High | 15 (6.79) |
| Wine consumption, n (%) | 101 (45.70) |
| Urinary tartaric acid, μg/mg creatinine | 28.47 + 4.03 |
| Daily wine consumers, n (%) | 81 (40.4) |
| Anthropometric measurements, mean + SD | |
| Weight, kg | 72.9 ± 0.7 |
| BMI, kg/m2 | 30.3 ± 0.28 |
| WC, cm | 97.5 ± 0.7 |
| WtHR | 63.0 ± 0.5 |
| Biochemical measurements, mean + SD | |
| Total cholesterol, mg/dL | 221.0 ± 2.7 |
| LDL cholesterol, mg/dL | 136.5 ± 2.4 |
| HDL cholesterol, mg/dL | 57.7 ± 1.0 |
| Triglycerides, mg/dL | 134.0 ± 5.2 |
| Glucose, mg/dL | 118.1 ± 2.4 |
| Blood pressure, mean + SD | |
| Systolic, mm Hg | 148.7 ± 1.2 |
| Diastolic, mm Hg | 83.8 ± 0.6 |
| Dietary intake, mean + SD | |
| Total energy, kcal/day | 2161 ± 33 |
| Carbohydrate, % of energy | 42.0 ± 0.5 |
| Protein, % of energy | 16.8 ± 0.2 |
| Fat, % of energy | 39.8 ± 0.5 |
| β (95% IC) | p-Value | ||
|---|---|---|---|
| BMI, kg/m2 | Model 1 | <−0.01 (−0.01, 0.01) | 0.519 |
| Model 2 | <−0.01 (−0.01, 0.01) | 0.426 | |
| Model 3 | <−0.01 (−0.01, 0.01) | 0.973 | |
| WC, cm | Model 1 | 0.56 (−0.25, 1.38) | 0.173 |
| Model 2 | 0.61 (−0.04, 1.26) | 0.064 | |
| Model 3 | 0.70 (−0.12, 1.51) | 0.087 | |
| Weight, kg | Model 1 | <−0.01 (−0.01, 0.01) | 0.770 |
| Model 2 | <−0.01 (−0.01, 0.01) | 0.766 | |
| Model 3 | <0.01 (−0.01, 0.01) | 0.886 | |
| WtHR | Model 1 | 0.29 (−0.25, 0.82) | 0.291 |
| Model 2 | 0.32 (−0.17, 0.81) | 0.175 | |
| Model 3 | 0.41 (−0.14, 0.96) | 0.128 |
| β (95% CI) | p-Value | ||
|---|---|---|---|
| Total cholesterol, mg/dL | Model 1 | −3.32 (−6.53, −0.10) | 0.043 |
| Model 2 | −3.24 (−5.78, −0.72) | 0.017 | |
| Model 3 | −3.13 (−5.54, −0.71) | 0.016 | |
| LDL cholesterol, mg/dL | Model 1 | −3.44 (−6.34, −0.53) | 0.021 |
| Model 2 | −3.43 (−5.86, −1.00) | 0.010 | |
| Model 3 | −3.03 (−5.62, −0.42) | 0.027 | |
| HDL cholesterol, mg/dL | Model 1 | <−0.01 (−0.02, 0.02) | 0.689 |
| Model 2 | −0.01 (−0.03, 0.01) | 0.220 | |
| Model 3 | <−0.01 (−0.02, 0.01) | 0.633 | |
| Triglycerides, mg/dL | Model 1 | 0.01 (−0.04, 0.05) | 0.739 |
| Model 2 | 0.02 (−0.03, 0.07) | 0.422 | |
| Model 3 | 0.02 (−0.04, 0.07) | 0.525 | |
| Glucose, mg/dL | Model 1 | 0.02 (<−0.01, 0.04) | 0.092 |
| Model 2 | 0.03 (−0.01, 0.07) | 0.119 | |
| Model 3 | 0.02 (−0.01, 0.06) | 0.180 | |
| Systolic BP, mmHg | Model 1 | −0.34 (−1.79, 1.11) | 0.647 |
| Model 2 | −0.09 (−1.53, 1.71) | 0.904 | |
| Model 3 | 0.36 (−1.27, 1.99) | 0.633 | |
| Diastolic BP, mmHg | Model 1 | 0.05 (−0.71, 0.81) | 0.893 |
| Model 2 | 0.24 (−0.47, 0.95) | 0.472 | |
| Model 3 | 0.21 (−0.47, 0.89) | 0.502 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-López, I.; Parilli-Moser, I.; Arancibia-Riveros, C.; Tresserra-Rimbau, A.; Martínez-González, M.A.; Ortega-Azorín, C.; Salas-Salvadó, J.; Castañer, O.; Lapetra, J.; Arós, F.; et al. Urinary Tartaric Acid, a Biomarker of Wine Intake, Correlates with Lower Total and LDL Cholesterol. Nutrients 2021, 13, 2883. https://doi.org/10.3390/nu13082883
Domínguez-López I, Parilli-Moser I, Arancibia-Riveros C, Tresserra-Rimbau A, Martínez-González MA, Ortega-Azorín C, Salas-Salvadó J, Castañer O, Lapetra J, Arós F, et al. Urinary Tartaric Acid, a Biomarker of Wine Intake, Correlates with Lower Total and LDL Cholesterol. Nutrients. 2021; 13(8):2883. https://doi.org/10.3390/nu13082883
Chicago/Turabian StyleDomínguez-López, Inés, Isabella Parilli-Moser, Camila Arancibia-Riveros, Anna Tresserra-Rimbau, Miguel Angel Martínez-González, Carolina Ortega-Azorín, Jordi Salas-Salvadó, Olga Castañer, José Lapetra, Fernando Arós, and et al. 2021. "Urinary Tartaric Acid, a Biomarker of Wine Intake, Correlates with Lower Total and LDL Cholesterol" Nutrients 13, no. 8: 2883. https://doi.org/10.3390/nu13082883
APA StyleDomínguez-López, I., Parilli-Moser, I., Arancibia-Riveros, C., Tresserra-Rimbau, A., Martínez-González, M. A., Ortega-Azorín, C., Salas-Salvadó, J., Castañer, O., Lapetra, J., Arós, F., Fiol, M., Serra-Majem, L., Pintó, X., Gómez-Gracia, E., Ros, E., Lamuela-Raventós, R. M., & Estruch, R. (2021). Urinary Tartaric Acid, a Biomarker of Wine Intake, Correlates with Lower Total and LDL Cholesterol. Nutrients, 13(8), 2883. https://doi.org/10.3390/nu13082883