Dietary Selenium Deficiency Partially Mimics the Metabolic Effects of Arsenic
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Intraperitoneal Glucose Tolerance Test (IPGTT)
2.3. Arginine Stimulation Test
2.4. Blood/Plasma Assays
2.5. Sacrifice and Serum Collection
2.6. Metals Analysis
2.7. Data Analysis and Statistics
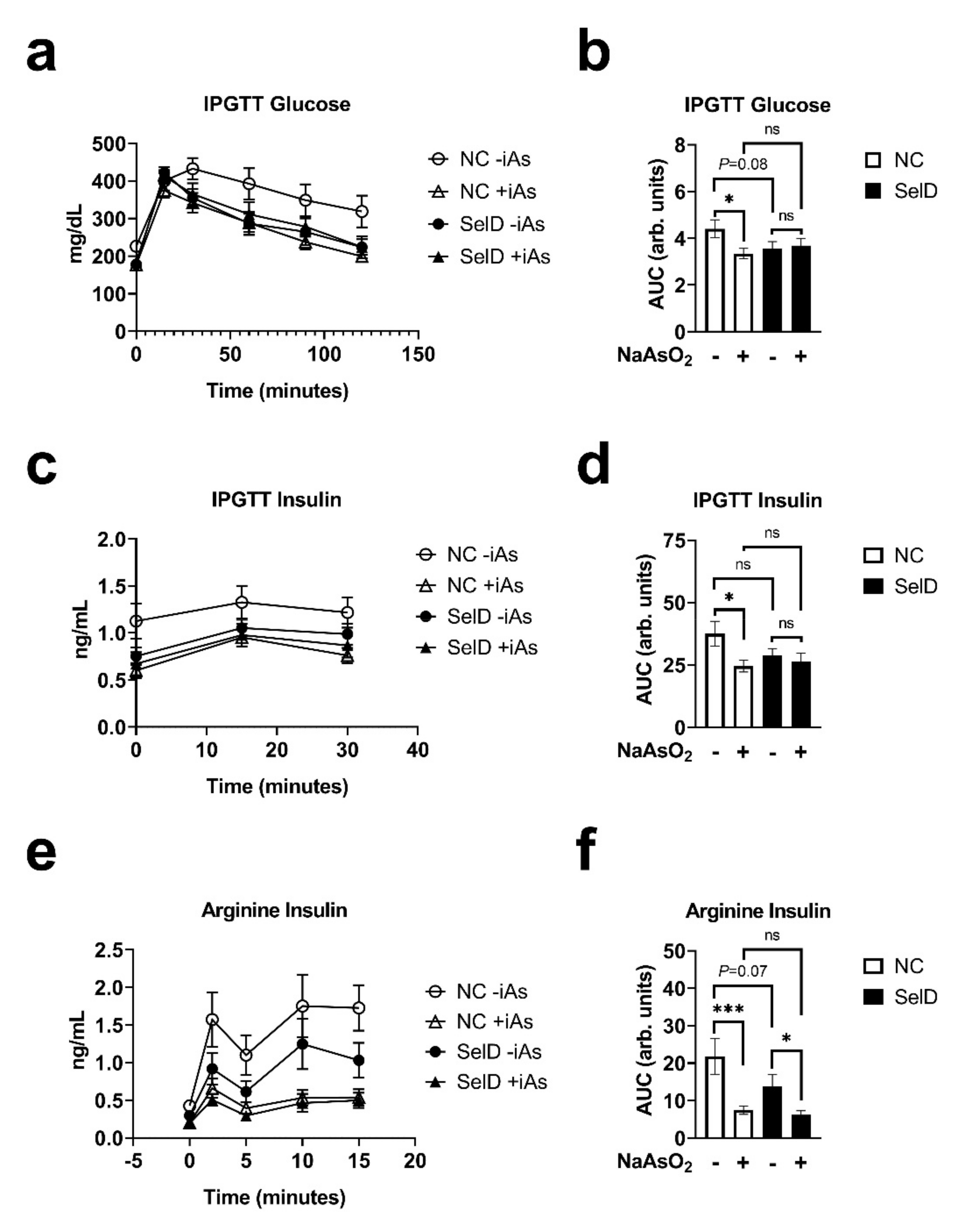
3. Results
3.1. Hepatic Metals Concentrations
3.2. Fasting Measures of Glucose Homeostasis
3.3. Body Measurements
3.4. Glucose Tolerance
3.5. Effects on Insulin Dynamics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravenscroft, P.; Brammer, H.; Richards, K. Arsenic Pollution: A Global Synthesis; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Argos, M.; Kalra, T.; Rathouz, P.J.; Chen, Y.; Pierce, B.; Parvez, F.; Islam, T.; Ahmed, A.; Rakibuz-Zaman, M.; Hasan, R.; et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): A prospective cohort study. Lancet 2010, 376, 252–258. [Google Scholar] [CrossRef]
- Tsuda, T.; Babazono, A.; Yamamoto, E.; Kurumatani, N.; Mino, Y.; Ogawa, T.; Kishi, Y.; Aoyama, H. Ingested Arsenic and Internal Cancer: A Historical Cohort Study Followed for 33 Years. Am. J. Epidemiol. 1995, 141, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Karagas, M.R. Arsenic-related mortality in Bangladesh. Lancet 2010, 376, 213–214. [Google Scholar] [CrossRef][Green Version]
- Wang, S.-L.; Chiou, J.-M.; Chen, C.-J.; Tseng, C.-H.; Chou, W.-L.; Wang, C.-C.; Wu, T.-N.; Chang, L.W. Prevalence of non-insulin-dependent diabetes mellitus and related vascular diseases in southwestern arseniasis-endemic and nonendemic areas in Taiwan. Environ. Health Perspect. 2003, 111, 155–159. [Google Scholar] [CrossRef]
- Lai, M.-S.; Hsueh, Y.-M.; Chen, C.-J.; Shyu, M.-P.; Chen, S.Y.; Kuo, T.-L.; Wu, M.-M.; Tai, T.-Y. Ingested Inorganic Arsenic and Prevalence of Diabetes Mellitus. Am. J. Epidemiol. 1994, 139, 484–492. [Google Scholar] [CrossRef]
- Islam, R.; Khan, I.; Hassan, S.M.N.; McEvoy, M.; D’Este, C.; Attia, J.; Peel, R.; Sultana, M.; Akter, S.; Milton, A.H. Association between type 2 diabetes and chronic arsenic exposure in drinking water: A cross sectional study in Bangladesh. Environ. Health 2012, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-K.; Kim, Y. Association of Diabetes Mellitus with a Combination of Vitamin D Deficiency and Arsenic Exposure in the Korean General Population: Analysis of 2008–2009 Korean National Health and Nutrition Examination Survey Data. Ann. Occup. Environ. Med. 2013, 25, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xie, Z.; Lin, Y.; Zhang, D. Association of inorganic arsenic exposure with type 2 diabetes mellitus: A meta-analysis. J. Epidemiol. Community Health 2013, 68, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.-C.; Seow, W.J.; Kile, M.L.; Hoffman, E.B.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Mostofa, G.; Lu, Q.; Christiani, D.C. Association of Low to Moderate Levels of Arsenic Exposure with Risk of Type 2 Diabetes in Bangladesh. Am. J. Epidemiol. 2013, 178, 1563–1570. [Google Scholar] [CrossRef]
- Feng, W.; Cui, X.; Liu, B.; Liu, C.; Xiao, Y.; Lu, W.; Guo, H.; He, M.; Zhang, X.; Yuan, J.; et al. Association of Urinary Metal Profiles with Altered Glucose Levels and Diabetes Risk: A Population-Based Study in China. PLoS ONE 2015, 10, e0123742. [Google Scholar] [CrossRef]
- World Health Organization. 10 Chemicals of Public Health Concern; World Health Organization: Geneva, Switzerland, 2020; Volume 2021. [Google Scholar]
- International Diabetes Federation. Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Paul, D.S.; Hernández-Zavala, A.; Walton, F.S.; Adair, B.M.; Dědina, J.; Matoušek, T.; Stýblo, M. Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: Development of a mouse model for arsenic-induced diabetes. Toxicol. Appl. Pharmacol. 2007, 222, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Maull, E.A.; Ahsan, H.; Edwards, J.; Longnecker, M.; Navas-Acien, A.; Pi, J.; Silbergeld, E.K.; Styblo, M.; Tseng, C.-H.; Thayer, K.A.; et al. Evaluation of the Association between Arsenic and Diabetes: A National Toxicology Program Workshop Review. Environ. Health Perspect. 2012, 120, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Carmean, C.M.; Kirkley, A.G.; Landeche, M.; Ye, H.; Chellan, B.; Aldirawi, H.; Roberts, A.A.; Parsons, P.J.; Sargis, R.M. Arsenic Exposure Decreases Adiposity During High-Fat Feeding. Obesity 2020, 28, 932–941. [Google Scholar] [CrossRef]
- Liu, S.; Guo, X.; Wu, B.; Yu, H.; Zhang, X.; Li, M. Arsenic induces diabetic effects through beta-cell dysfunction and increased gluconeogenesis in mice. Sci. Rep. 2015, 4, 6894. [Google Scholar] [CrossRef]
- Carmean, C.M.; Yokoi, N.; Takahashi, H.; Oduori, O.S.; Kang, C.; Kanagawa, A.; Kirkley, A.G.; Han, G.; Landeche, M.; Hidaka, S.; et al. Arsenic modifies serotonin metabolism through glucuronidation in pancreatic β-cells. Am. J. Physiol. Metab. 2019, 316, E464–E474. [Google Scholar] [CrossRef]
- Carmean, C.M.; Seino, S. Braving the Element: Pancreatic β-Cell Dysfunction and Adaptation in Response to Arsenic Exposure. Front. Endocrinol. 2019, 10, 344. [Google Scholar] [CrossRef]
- Li, Y.Y.; Douillet, C.; Huang, M.; Beck, R.; Sumner, S.J.; Styblo, M. Exposure to inorganic arsenic and its methylated metabolites alters metabolomics profiles in INS-1 832/13 insulinoma cells and isolated pancreatic islets. Arch. Toxicol. 2020, 94, 1955–1972. [Google Scholar] [CrossRef]
- Dover, E.N.; Patel, N.Y.; Stýblo, M. Impact of in vitro heavy metal exposure on pancreatic β-cell function. Toxicol. Lett. 2018, 299, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.C.; Douillet, C.; Dover, E.N.; Zhang, C.; Beck, R.; Tejan-Sie, A.; Krupenko, S.A.; Stýblo, M. Metabolic Phenotype of Wild-Type andAs3mt-Knockout C57BL/6J Mice Exposed to Inorganic Arsenic: The Role of Dietary Fat and Folate Intake. Environ. Health Perspect. 2018, 126, 127003. [Google Scholar] [CrossRef]
- Paul, D.S.; Walton, F.S.; Saunders, R.J.; Stýblo, M. Characterization of the Impaired Glucose Homeostasis Produced in C57BL/6 Mice by Chronic Exposure to Arsenic and High-Fat Diet. Environ. Health Perspect. 2011, 119, 1104–1109. [Google Scholar] [CrossRef]
- Zhang, C.; Fennel, E.M.J.; Douillet, C.; Stýblo, M. Exposures to arsenite and methylarsonite produce insulin resistance and impair insulin-dependent glycogen metabolism in hepatocytes. Arch. Toxicol. 2017, 91, 3811–3821. [Google Scholar] [CrossRef] [PubMed]
- Douillet, C.; Huang, M.C.; Saunders, R.J.; Dover, E.N.; Zhang, C.; Stýblo, M. Knockout of arsenic (+3 oxidation state) methyltransferase is associated with adverse metabolic phenotype in mice: The role of sex and arsenic exposure. Arch. Toxicol. 2016, 91, 2617–2627. [Google Scholar] [CrossRef]
- Huang, M.C.; Douillet, C.; Dover, E.N.; Stýblo, M. Prenatal arsenic exposure and dietary folate and methylcobalamin supplementation alter the metabolic phenotype of C57BL/6J mice in a sex-specific manner. Arch. Toxicol. 2018, 92, 1925–1937. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; de la Vega, M.R.; Harder, B.; Castro-Portuguez, R.; Rodrigues, S.; Wong, P.K.; Chapman, E.; Zhang, D.D. Low-level arsenic causes proteotoxic stress and not oxidative stress. Toxicol. Appl. Pharmacol. 2018, 341, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, M.M.; Bourguignon, N.S.; Bizzozzero, M.; Rodriguez, D.; Ventura, C.; Cocca, C.; Libertun, C.; Lux-Lantos, V.A. Arsenite in drinking water produces glucose intolerance in pregnant rats and their female offspring. Food Chem. Toxicol. 2017, 100, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Villaseñor, A.; Cruz, L.; Cebrián, A.; Hernández-Ramírez, R.U.; Hiriart, M.; García-Vargas, G.; Bassol, S.; Sordo, M.; Gandolfi, A.J.; Klimecki, W.T.; et al. Arsenic Exposure and Calpain-10 Polymorphisms Impair the Function of Pancreatic Beta-Cells in Humans: A Pilot Study of Risk Factors for T2DM. PLoS ONE 2013, 8, e51642. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.; Xue, Y.; Zhuang, Z.; Qian, S.; Zhou, W.; Li, X.; Qian, J.; Ding, G.; Sun, Z. Non-monotonic dose-response effects of arsenic on glucose metabolism. Toxicol. Appl. Pharmacol. 2019, 377, 114605. [Google Scholar] [CrossRef]
- Levander, O.A. Metabolic interrelationships between arsenic and selenium. Environ. Health Perspect. 1977, 19, 159–164. [Google Scholar] [CrossRef]
- Moxon, A.L. The effect of arsenic on the toxicity of seleniferous grains. Science 1938, 88, 81. [Google Scholar] [CrossRef]
- Gailer, J.; George, G.; Pickering, I.; Prince, R.; Ringwald, S.C.; Pemberton, J.E.; Glass, R.S.; Younis, H.S.; Deyoung, D.W.; Aposhian, H.V. A Metabolic Link between Arsenite and Selenite: The Seleno-bis(S-glutathionyl) Arsinium Ion. J. Am. Chem. Soc. 2000, 122, 4637–4639. [Google Scholar] [CrossRef]
- Zeidooni, L.; Ahangarpour, A.; Samimi, A.; Alboghobeish, S.; Khorsandi, L.S.; Moradi, M. Chronic exposure to arsenic and high fat diet additively induced cardiotoxicity in male mice. Res. Pharm. Sci. 2018, 13, 47–56. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Alboghobeish, S.; Rezaei, M.; Khodayar, M.J.; Oroojan, A.A.; Zainvand, M. Evaluation of Diabetogenic Mechanism of High Fat Diet in Combination with Arsenic Exposure in Male Mice. Iran. J. Pharm. Res. 2018, 17, 164–183. [Google Scholar] [PubMed]
- Robertson, R.P.; Raymond, R.H.; Lee, D.S.; Calle, R.A.; Ghosh, A.; Savage, P.J.; Shankar, S.S.; Vassileva, M.T.; Weir, G.C.; Fryburg, D.A.; et al. Arginine is preferred to glucagon for stimulation testing of β-cell function. Am. J. Physiol. Metab. 2014, 307, E720–E727. [Google Scholar] [CrossRef]
- Krohn, R.M.; Lemaire, M.; Silva, L.F.N.; Lemarié, C.; Bolt, A.; Mann, K.K.; Smits, J. High-selenium lentil diet protects against arsenic-induced atherosclerosis in a mouse model. J. Nutr. Biochem. 2016, 27, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Stępnik, M.; Stetkiewicz, J.; Krajnow, A.; Domeradzka, K.; Gradecka-Meesters, D.; Arkusz, J.; Stańczyk, M.; Palus, J.; Dziubałtowska, E.; Sobala, W.; et al. Carcinogenic effect of arsenate in C57BL/6J/Han mice and its modulation by different dietary selenium status. Ecotoxicol. Environ. Saf. 2009, 72, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sosa, M.; Montalvo, E.A.G.; Del Razo, L.M.; Vega, L. Effect of Selenomethionine Supplementation in Food on the Excretion and Toxicity of Arsenic Exposure in Female Mice. Biol. Trace Elem. Res. 2013, 156, 279–287. [Google Scholar] [CrossRef]
- Palus, J.; Lewińska, D.; Dziubałtowska, E.; Wasowicz, W.; Gromadzinska, J.; Rydzynski, K.; Stańczyk, M.; Arkusz, J.; Trzcinka-Ochocka, M.; Stępnik, M. Genotoxic Effects in C57Bl/6J Mice Chronically Exposed to Arsenate in Drinking Water and Modulation of the Effects by Low-Selenium Diet. J. Toxicol. Environ. Health Part A 2006, 69, 1843–1860. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Holladay, S.D.; Wolf, D.C.; Ahmed, S.A.; Robertson, J.L. Reproductive and Developmental Toxicity of Arsenic in Rodents: A Review. Int. J. Toxicol. 2006, 25, 319–331. [Google Scholar] [CrossRef] [PubMed]
- George, C.M.; Gamble, M.; Slavkovich, V.; Levy, D.; Ahmed, A.; Ahsan, H.; Graziano, J. A cross-sectional study of the impact of blood selenium on blood and urinary arsenic concentrations in Bangladesh. Environ. Health 2013, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hall, M.; Graziano, J.H.; Slavkovich, V.; Van Geen, A.; Parvez, F.; Ahsan, H. A Prospective Study of Blood Selenium Levels and the Risk of Arsenic-Related Premalignant Skin Lesions. Cancer Epidemiol. Biomark. Prev. 2007, 16, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Parvez, F.; Wasserman, G.A.; Factor-Litvak, P.; Liu, X.; Slavkovich, V.; Siddique, A.B.; Sultana, R.; Sultana, R.; Islam, T.; Levy, D.; et al. Arsenic Exposure and Motor Function among Children in Bangladesh. Environ. Health Perspect. 2011, 119, 1665–1670. [Google Scholar] [CrossRef]
- Kirkley, A.G.; Carmean, C.M.; Ruiz, D.; Ye, H.; Regnier, S.M.; Poudel, A.; Hara, M.; Kamau, W.S.; Johnson, D.N.; Roberts, A.A.; et al. Arsenic exposure induces glucose intolerance and alters global energy metabolism. Am. J. Physiol. Integr. Comp. Physiol. 2018, 314, R294–R303. [Google Scholar] [CrossRef]
- Vega, J.A.I.; Soto, C.A.; Sanchez-Peña, L.C.; De Vizcaya-Ruiz, A.; Del Razo, L.M. Diabetogenic effects and pancreatic oxidative damage in rats subchronically exposed to arsenite. Toxicol. Lett. 2006, 160, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chi, L.; Lai, Y.; Hsiao, Y.C.; Ru, H.; Lu, K. The gut microbiome and arsenic-induced disease-iAs metabolism in mice. Curr. Environ. Health Rep. 2021, 8, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Wu, T.-L.; Zeng, H.; Cheng, W.-H. Dietary Selenium Requirement for the Prevention of Glucose Intolerance and Insulin Resistance in Middle-Aged Mice. J. Nutr. 2021, 151, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.; Lee, B.C.; Handy, D.; Loscalzo, J.; Hatfield, D.L.; Gladyshev, V.N. Both Maximal Expression of Selenoproteins and Selenoprotein Deficiency Can Promote Development of Type 2 Diabetes-Like Phenotype in Mice. Antioxid. Redox Signal. 2011, 14, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Seale, L.A.; Hashimoto, A.C.; Kurokawa, S.; Gilman, C.L.; Seyedali, A.; Bellinger, F.P.; Raman, A.V.; Berry, M.J. Disruption of the Selenocysteine Lyase-Mediated Selenium Recycling Pathway Leads to Metabolic Syndrome in Mice. Mol. Cell. Biol. 2012, 32, 4141–4154. [Google Scholar] [CrossRef]
- Pepper, M.P.; Vatamaniuk, M.; Yan, X.; Roneker, C.A.; Lei, X.G. Impacts of Dietary Selenium Deficiency on Metabolic Phenotypes of Diet-Restricted GPX1-Overexpressing Mice. Antioxid. Redox Signal. 2011, 14, 383–390. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, K.; Lei, X.G. Selenium and diabetes—Evidence from animal studies. Free Radic. Biol. Med. 2013, 65, 1548–1556. [Google Scholar] [CrossRef]
- Hu, W.; Zhao, C.; Hu, H.; Yin, S. Food Sources of Selenium and Its Relationship with Chronic Diseases. Nutrients 2021, 13, 1739. [Google Scholar] [CrossRef]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021, 8, 292. [Google Scholar] [CrossRef] [PubMed]

| Test | Time Point | +iAs vs. −iAs (NC Diet) | +iAs vs. −iAs (SelD Diet) | +SelD vs. NC (−iAs) | +SelD vs. −NC (+iAs) |
|---|---|---|---|---|---|
| Body Mass | 0 weeks | 0.05 | |||
| 1 weeks | 0.09 | * | |||
| 2 weeks | * | 0.06 | |||
| 3 weeks | * | * | |||
| 4 weeks | ** | * | |||
| 5 weeks | * | ** | * | ||
| 6 weeks | * | ** | 0.08 | * | |
| 7 weeks | * | ** | * | ||
| IPGTT Glucose | 0 min | *** | *** | ||
| 15 min | |||||
| 30 min | * | 0.08 | |||
| 60 min | 0.07 | * | |||
| 90 min | * | 0.07 | |||
| 120 min | ** | * | |||
| IPGTT Insulin | 0 min | ** | * | ||
| 15 min | * | ||||
| 30 min | * | ||||
| Arginine Stimulation | 0 min | ** | 0.07 | ||
| 2 min | ** | * | |||
| 5 min | ** | * | |||
| 10 min | ** | * | |||
| 15 min | *** | * | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmean, C.M.; Mimoto, M.; Landeche, M.; Ruiz, D.; Chellan, B.; Zhao, L.; Schulz, M.C.; Dumitrescu, A.M.; Sargis, R.M. Dietary Selenium Deficiency Partially Mimics the Metabolic Effects of Arsenic. Nutrients 2021, 13, 2894. https://doi.org/10.3390/nu13082894
Carmean CM, Mimoto M, Landeche M, Ruiz D, Chellan B, Zhao L, Schulz MC, Dumitrescu AM, Sargis RM. Dietary Selenium Deficiency Partially Mimics the Metabolic Effects of Arsenic. Nutrients. 2021; 13(8):2894. https://doi.org/10.3390/nu13082894
Chicago/Turabian StyleCarmean, Christopher M., Mizuho Mimoto, Michael Landeche, Daniel Ruiz, Bijoy Chellan, Lidan Zhao, Margaret C. Schulz, Alexandra M. Dumitrescu, and Robert M. Sargis. 2021. "Dietary Selenium Deficiency Partially Mimics the Metabolic Effects of Arsenic" Nutrients 13, no. 8: 2894. https://doi.org/10.3390/nu13082894
APA StyleCarmean, C. M., Mimoto, M., Landeche, M., Ruiz, D., Chellan, B., Zhao, L., Schulz, M. C., Dumitrescu, A. M., & Sargis, R. M. (2021). Dietary Selenium Deficiency Partially Mimics the Metabolic Effects of Arsenic. Nutrients, 13(8), 2894. https://doi.org/10.3390/nu13082894





