Vitamin D Receptor Protects against Radiation-Induced Intestinal Injury in Mice via Inhibition of Intestinal Crypt Stem/Progenitor Cell Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and VD Treatment
2.2. Cell Apoptosis Assay
2.3. Cell Immunofluorescence
2.4. Animals and IR
2.5. Histology, Immunohistochemistry and Immunofluorescence
2.6. RNA Isolation and Real-Time Quantitative PCR
2.7. Western Blotting (WB)
2.8. TUNEL Assay
2.9. Isolation of Crypt Stem/Progenitor Cells
2.10. siRNA Transfection
2.11. Statistical Analysis
3. Results
3.1. VD/VDR Attenuates IR-Induced DNA Damage and Apoptosis in IEC-6 Cells
3.2. VDR Is Highly Expressed in Intestinal Crypts and Significantly Upregulated in Response to IR
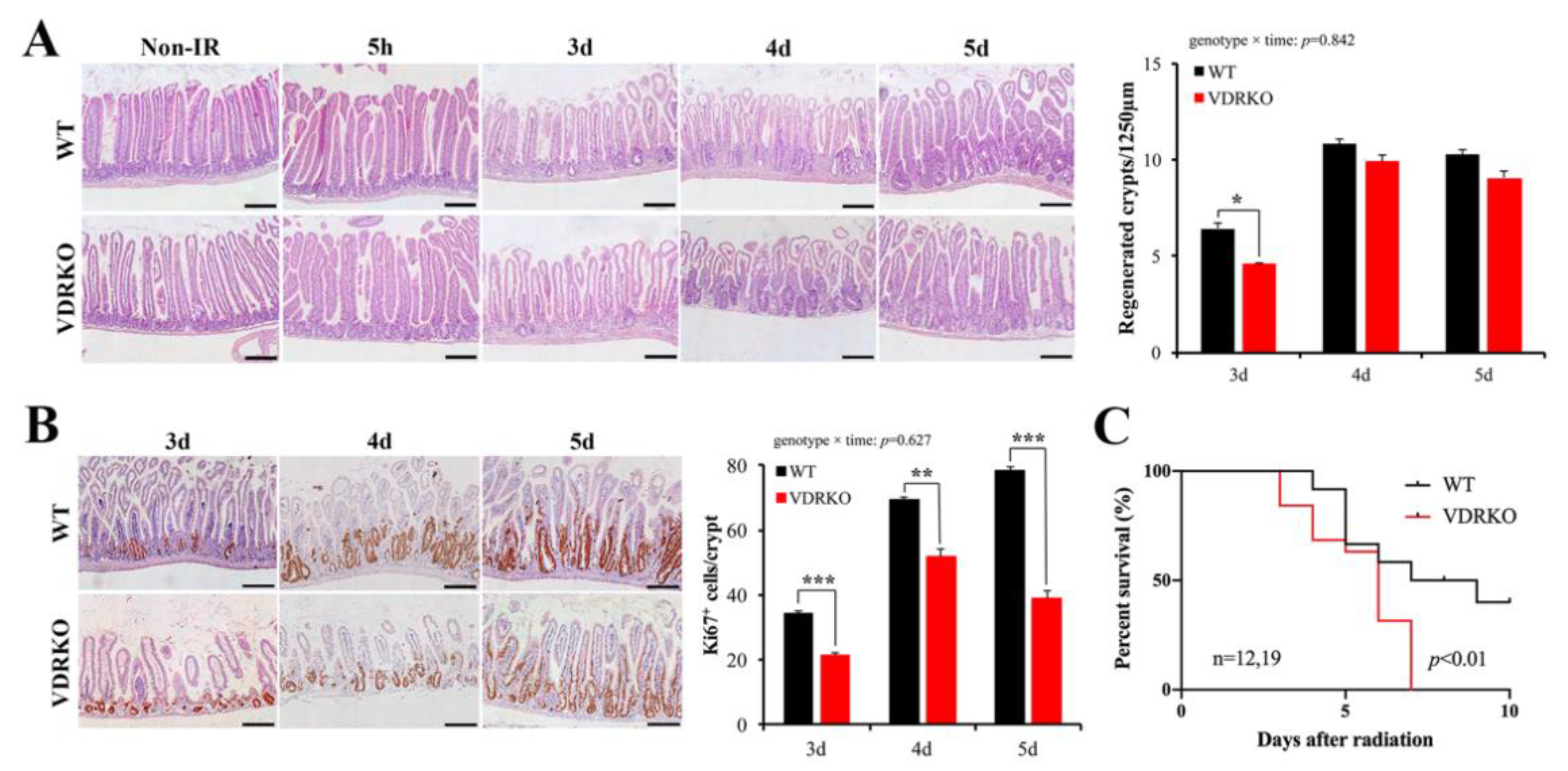
3.3. VDR Deficiency Impairs Intestinal Structure and Crypt Stem/Progenitor Cell Proliferation
3.4. VDR Deficiency Suppressed Intestinal Epithelial Regeneration Following IR
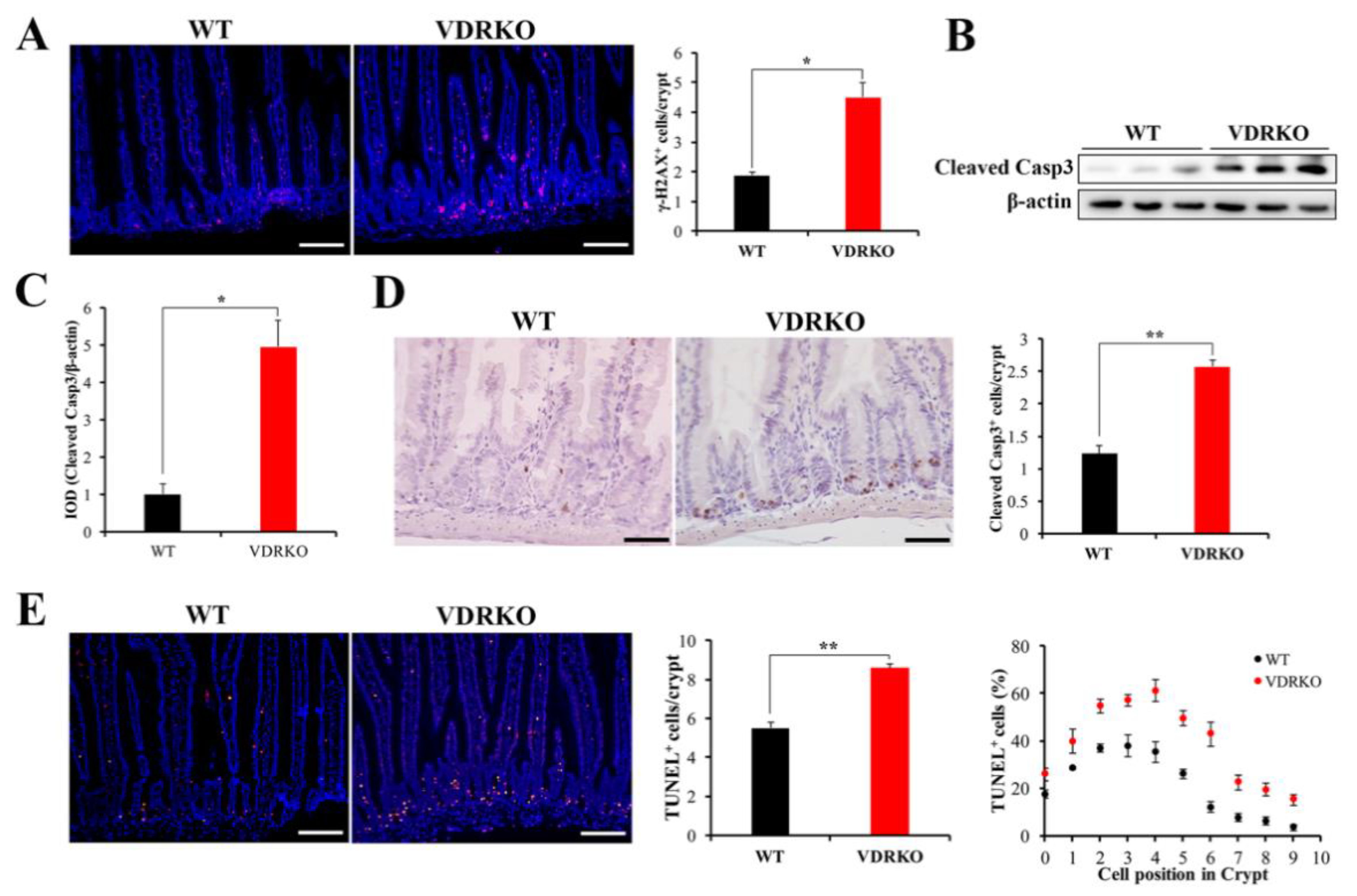
3.5. VDR Deficiency Aggravates IR-Induced Crypt Stem/Progenitor Cell Apoptosis
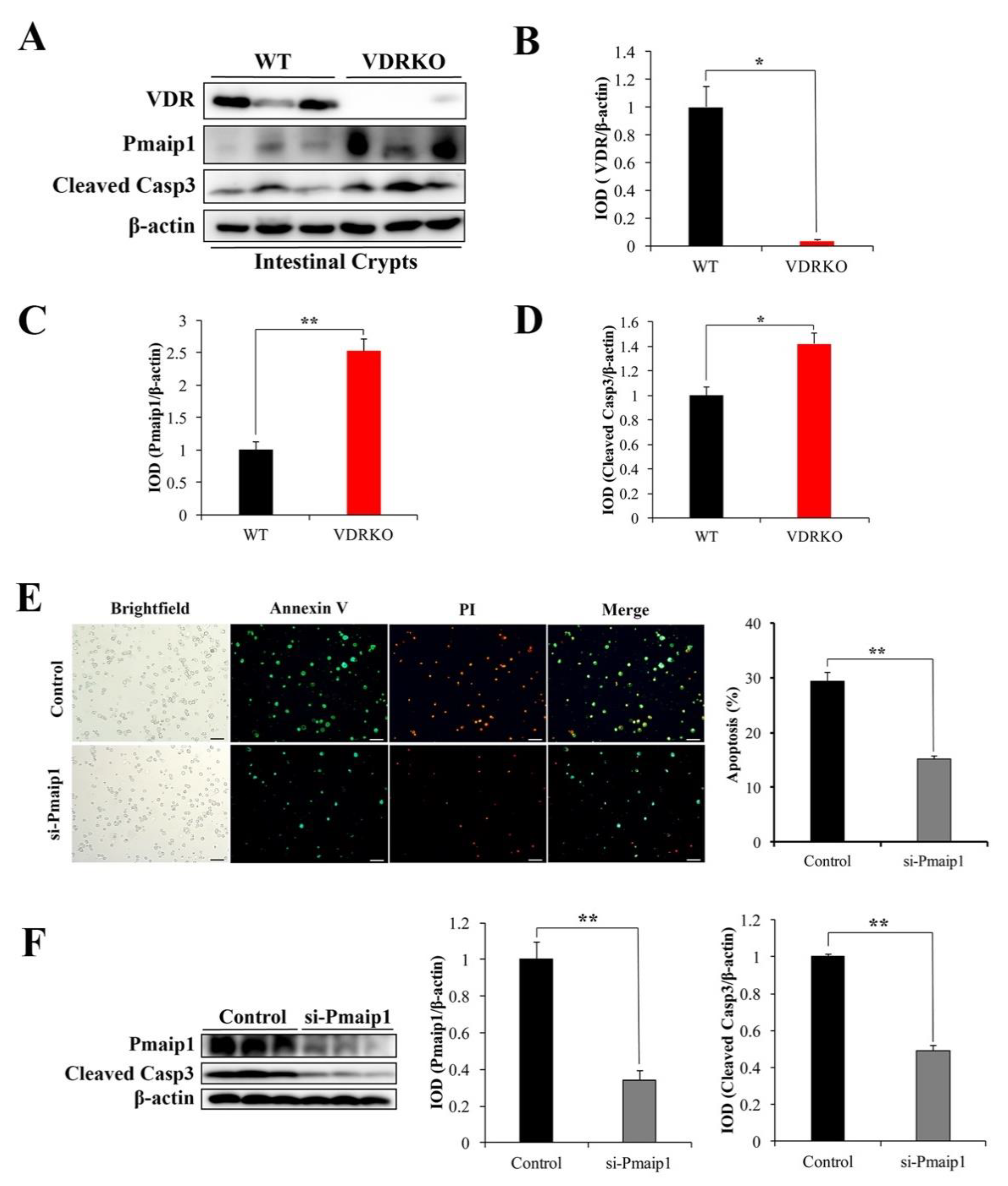
3.6. VDR Suppresses IR-Induced Crypt Stem/Progenitor Cell Apoptosis via Pmaip1-Mediated Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hauer-Jensen, M.; Denham, J.W.; Andreyev, H.J.N. Radiation enteropathy–pathogenesis, treatment and prevention. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.P. The protection afforded by vitamin against low radiation damage. Int. J. Low Radiat. 2008, 5, 368–394. [Google Scholar] [CrossRef]
- Castro-Eguiluz, D.; Leyva-Islas, J.A.; Luvian-Morales, J.; Martínez-Roque, V.; Sánchez-López, M.; Trejo-Durán, G.; Jiménez-Lima, R.; Leyva-Rendón, F.J. Nutrient Recommendations for Cancer Patients Treated with Pelvic Radiotherapy, with or without Comorbidities. Rev. Investig. Clin. 2018, 70, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Nuszkiewicz, J.; Woz niak, A.; Szewczyk-Golec, K. Ionizing radiation as a source of oxidative stress-the protective role of melatonin and vitamin D. Int. J. Mol. Sci. 2020, 21, 5804. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Snyder, L.; Arora, J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh-Moghaddam, A.; Gholamrezaei, A.; Hemati, S. Vitamin D deficiency is associated with the severity of radiation-induced proctitis in cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Xiang, J.; Zhou, P. Vitamin D, gut microbiota, and radiation-related resistance: A love-hate triangle. J. Exp. Clin. Cancer Res. 2019, 38, 493. [Google Scholar] [CrossRef] [Green Version]
- Gil, A.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and novel actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.J.; Hsieh, C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef]
- Barbáchano, A.; Fernández-Barral, A.; Ferrer-Mayorga, G.; Costales-Carrera, A.; Larriba, M.J.; Muñoz, A. The endocrine vitamin D system in the gut. Mol. Cell. Endocrinol. 2017, 453, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Lawrie, W.T.; Song, D.S.; Abrams, R.A.; Kafonek, D.R.; Boitnott, J.K.; Deweese, T.L. Acute and late radiotherapy toxicity in patients with inflammatory bowel disease. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 455–459. [Google Scholar] [CrossRef]
- Wada, K.; Tanaka, H.; Maeda, K.; Inoue, T.; Noda, E.; Amano, R.; Kubo, N.; Muguruma, K.; Yamada, N.; Yashiro, M.; et al. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol. Rep. 2009, 22, 1021–1025. [Google Scholar] [PubMed] [Green Version]
- Froicu, M.; Weaver, V.; Wynn, T.A.; McDowell, M.A.; Welsh, J.E.; Cantorna, M.T. A Crucial Role for the Vitamin D Receptor in Experimental Inflammatory Bowel Diseases. Mol. Endocrinol. 2003, 17, 2386–2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.O.; Claud, E.C.; Chen, D.; Chang, E.B.; Carmeliet, G.; et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 2015, 64, 1082–1094. [Google Scholar] [CrossRef]
- He, L.; Liu, T.; Shi, Y.; Tian, F.; Hu, H.; Deb, D.K.; Chen, Y.; Bissonnette, M.; Li, Y.C. Gut Epithelial Vitamin D Receptor Regulates Microbiota-Dependent Mucosal Inflammation by Suppressing Intestinal Epithelial Cell Apoptosis. Endocrinology 2018, 159, 967–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Chen, Y.; Golan, M.A.; Annunziata, M.L.; Du, J.; Dougherty, U.; Kong, J.; Musch, M.; Huang, Y.; Pekow, J.; et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J. Clin. Investig. 2013, 123, 3983–3996. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.J.M.; Lo, Y.H.; Mah, A.T.; Kuo, C.J. The Intestinal Stem Cell Niche: Homeostasis and Adaptations. Trends Cell Biol. 2018, 28, 1062–1078. [Google Scholar] [CrossRef]
- Potten, C.S. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat. Res. 2004, 161, 123–136. [Google Scholar] [CrossRef]
- Hua, G.; Thin, T.H.; Feldman, R.; Haimovitz–Friedman, A.; Clevers, H.; Fuks, Z.; Kolesnick, R. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology 2012, 143, 1266–1276. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Tian, H.; Jiang, J.; Yang, Y.; Tan, S.; Lin, X.; Liu, H.; Wu, B. β-Arrestin-2 modulates radiation-induced intestinal crypt progenitor/stem cell injury. Cell Death Differ. 2016, 23, 1529–1541. [Google Scholar] [CrossRef] [Green Version]
- Peregrina, K.; Houston, M.; Daroqui, C.; Dhima, E.; Sellers, R.S.; Augenlicht, L.H. Vitamin D is a determinant of mouse intestinal lgr5 stem cell functions. Carcinogenesis 2015, 36, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Peregrina, K.; Houston, M.; Augenlicht, L.H. Vitamin D and the nutritional environment in functions of intestinal stem cells: Implications for tumorigenesis and prevention. J. Steroid Biochem. 2020, 198, 105556. [Google Scholar] [CrossRef]
- Li, Y.C.; Chen, Y.; Du, J. Critical roles of intestinal epithelial vitamin D receptor signaling in controlling gut mucosal inflammation. J. Steroid Biochem. 2015, 148, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, M.; Schewe, M.; Sacchetti, A.; Feijtel, D.; van de Geer, W.S.; Teeuwssen, M.; Sleddens, H.F.; Joosten, R.; van Royen, M.E.; van de Werken, H.J.G.; et al. Paneth Cells Respond to Inflammation and Contribute to Tissue Regeneration by Acquiring Stem-like Features through SCF/c-Kit Signaling. Cell Rep. 2018, 24, 2312–2328. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Liu, T.J.; Shi, Y.Y.; Zhao, Q. Vitamin D/VDR signaling pathway ameliorates 2,4,6-trinitrobenzene sulfonic acid-induced colitis by inhibiting intestinal epithelial apoptosis. Int. J. Mol. Med. 2015, 35, 1213–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühne, H.; Hause, G.; Grundmann, S.M.; Schutkowski, A.; Brandsch, C.; Stangl, G.I. Vitamin D receptor knockout mice exhibit elongated intestinal microvilli and increased ezrin expression. Nutr. Res. 2016, 36, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lin, Y.; Sheng, X.; Xu, J.; Hou, X.; Li, Y.; Zhang, H.; Guo, H.; Yu, Z.; Ren, F. Arachidonic acid promotes intestinal regeneration by activating WNT signaling. Stem Cell Rep. 2020, 15, 374–388. [Google Scholar] [CrossRef]
- Mah, L.J.; El-Osta, A.; Karagiannis, T.C. γH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 2013, 154, 274–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracz, A.D.; Ramalingam, S.; Magness, S.T. Sox9 expression marks a subset of CD24-expressing small intestine epithelial stem cells that form organoids in vitro. AJP-Gastrointest. Liver Physiol. 2010, 298, G590–G600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oda, E.; Ohki, R.; Murasawa, H.; Nemoto, J.; Shibue, T.; Yamashita, T.; Tokino, T.; Taniguchi, T.; Tanaka, N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 2000, 288, 1053–1058. [Google Scholar] [CrossRef]
- Shibue, T.; Takeda, K.; Oda, E.; Tanaka, H.; Murasawa, H.; Takaoka, A.; Morishita, Y.; Akira, S.; Taniguchi, T.; Tanaka, N. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 2003, 17, 2233–2238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaroni, A.; Wands, J.; Trelstad, R.L.; Isselbacher, K.J. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J. Cell Biol. 1979, 80, 248–265. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Shim, S.; Park, S.; Kim, H.; Lee, S.J.; Kim, M.J.; Jang, W.S.; Kim, Y.; Jang, H. Ghrelin reverts intestinal stem cell loss associated with radiation-induced enteropathy by activating notch signaling. Phytomedicine 2021, 81, 153424. [Google Scholar] [CrossRef]
- Peña-Villalobos, I.; Casanova-Maldonado, I.; Lois, P.; Sabat, P.; Palma, V. Adaptive physiological and morphological adjustments mediated by intestinal stem cells in response to food availability in mice. Front. Physiol. 2019, 9, 1821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yantiss, R.K.; Nam, H.; Chin, Y.; Zhou, X.K.; Scherl, E.J.; Bosworth, B.P.; Subbaramaiah, K.; Dannenberg, A.J.; Benezra, R. ID1 is a Functional Marker for Intestinal Stem and Progenitor Cells Required for Normal Response to Injury. Stem Cell Rep. 2014, 3, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, S.; Nivarthi, H.; Mayhew, C.N.; Lo, Y.H.; Noah, T.K.; Vallance, J.; Rülicke, T.; Müller, M.; Jegga, A.G.; Tang, W.; et al. Activated STAT5 Confers Resistance to Intestinal Injury by Increasing Intestinal Stem Cell Proliferation and Regeneration. Stem Cell Rep. 2015, 4, 209–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, R.; Zhang, Y.G.; Xia, Y.; Sun, J. Imbalance of autophagy and apoptosis in intestinal epithelium lacking the vitamin D receptor. FASEB J. 2019, 33, 11845–11856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves-Pérez, A.; Yilmaz, M.; Perna, C.; de la Rose, S.; Djouder, N. URI is required to maintain intestinal architecture during ionizing radiation. Science 2019, 364, eaaq1165. [Google Scholar] [CrossRef]
- Yousefi, M.; Li, N.; Nakauka-Ddamba, A.; Wang, S.; Davidow, K.; Schoenberger, J.; Yu, Z.; Jensen, S.T.; Kharas, M.G.; Lengner, C.J. Msi RNA-binding proteins control reserve intestinal stem cell quiescence. J. Cell Biol. 2016, 215, 401–413. [Google Scholar] [CrossRef]
- Ploner, C.; Kofler, R.; Villunger, A. Noxa: At the tip of the balance between life and death. Oncogene 2008, 27, S84–S92. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; An, Y.M.; Choi, W.H.; Kim, J.M.; Cho, S.; Yoo, B.R.; Kang, J.W.; Lee, Y.S.; Lee, Y.J.; Cho, J. Pro-apoptotic Noxa is involved in ablative focal irradiation-induced lung injury. J. Cell. Mol. Med. 2017, 21, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Kobeissy, H.; Hage-Sleiman, R.; Dakdouk, Z.; Kozhaya, L.; Dbaibo, G. Crosstalk between Noxa, Bcl-2, and ceramide in mediating p53-dependent apoptosis in Molt-4 human T-cell leukemia. Mol. Cell. Biochem. 2020, 475, 215–226. [Google Scholar] [CrossRef] [PubMed]



| Gene | Sense (5′-3′) | Antisense (5′-3′) |
|---|---|---|
| Vdr | GAATGTGCCTCGGATCTGTGG | ATGCGGCAATCTCCATTGAAG |
| Gapdh | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Lin, Y.; Luo, Y.; Wang, Y.; Lu, Y.; Li, Y.; Guo, H. Vitamin D Receptor Protects against Radiation-Induced Intestinal Injury in Mice via Inhibition of Intestinal Crypt Stem/Progenitor Cell Apoptosis. Nutrients 2021, 13, 2910. https://doi.org/10.3390/nu13092910
Li W, Lin Y, Luo Y, Wang Y, Lu Y, Li Y, Guo H. Vitamin D Receptor Protects against Radiation-Induced Intestinal Injury in Mice via Inhibition of Intestinal Crypt Stem/Progenitor Cell Apoptosis. Nutrients. 2021; 13(9):2910. https://doi.org/10.3390/nu13092910
Chicago/Turabian StyleLi, Wusun, Yingying Lin, Yujia Luo, Yuqi Wang, Yao Lu, Yixuan Li, and Huiyuan Guo. 2021. "Vitamin D Receptor Protects against Radiation-Induced Intestinal Injury in Mice via Inhibition of Intestinal Crypt Stem/Progenitor Cell Apoptosis" Nutrients 13, no. 9: 2910. https://doi.org/10.3390/nu13092910
APA StyleLi, W., Lin, Y., Luo, Y., Wang, Y., Lu, Y., Li, Y., & Guo, H. (2021). Vitamin D Receptor Protects against Radiation-Induced Intestinal Injury in Mice via Inhibition of Intestinal Crypt Stem/Progenitor Cell Apoptosis. Nutrients, 13(9), 2910. https://doi.org/10.3390/nu13092910






