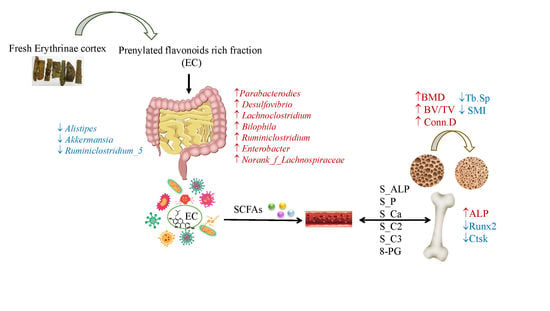
Prenylated Isoflavonoids-Rich Extract of Erythrinae Cortex Exerted Bone Protective Effects by Modulating Gut Microbial Compositions and Metabolites in Ovariectomized Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Herbal Collection and Preparation
2.2. Prenylated Isoflavonoids’ Identification in EC Extract Using Ultra-Performance Liquid Chromatography Orbitrap-Mass Spectrometry (UPLC-Obitrap-MS) Analysis
2.3. Animal and Treatments
2.4. Chemistries in Serum and Urine
2.5. Micro-Computed Tomography (Micro-CT) Analysis of Bone Properties
2.6. Real-Time Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
2.7. Fecal DNA Extraction and PCR Amplification
2.8. 16S rRNA Sequencing and Bioinformatics Analysis
2.9. Short Chain Fatty Acid (SCFA) Measurements
2.10. Statistical Analysis
3. Results
3.1. The Prenylated Isoflavonoids Identified in EC Extract
3.2. EC Exerted Protective Effects on Ovariectomy-Induced Bone Loss
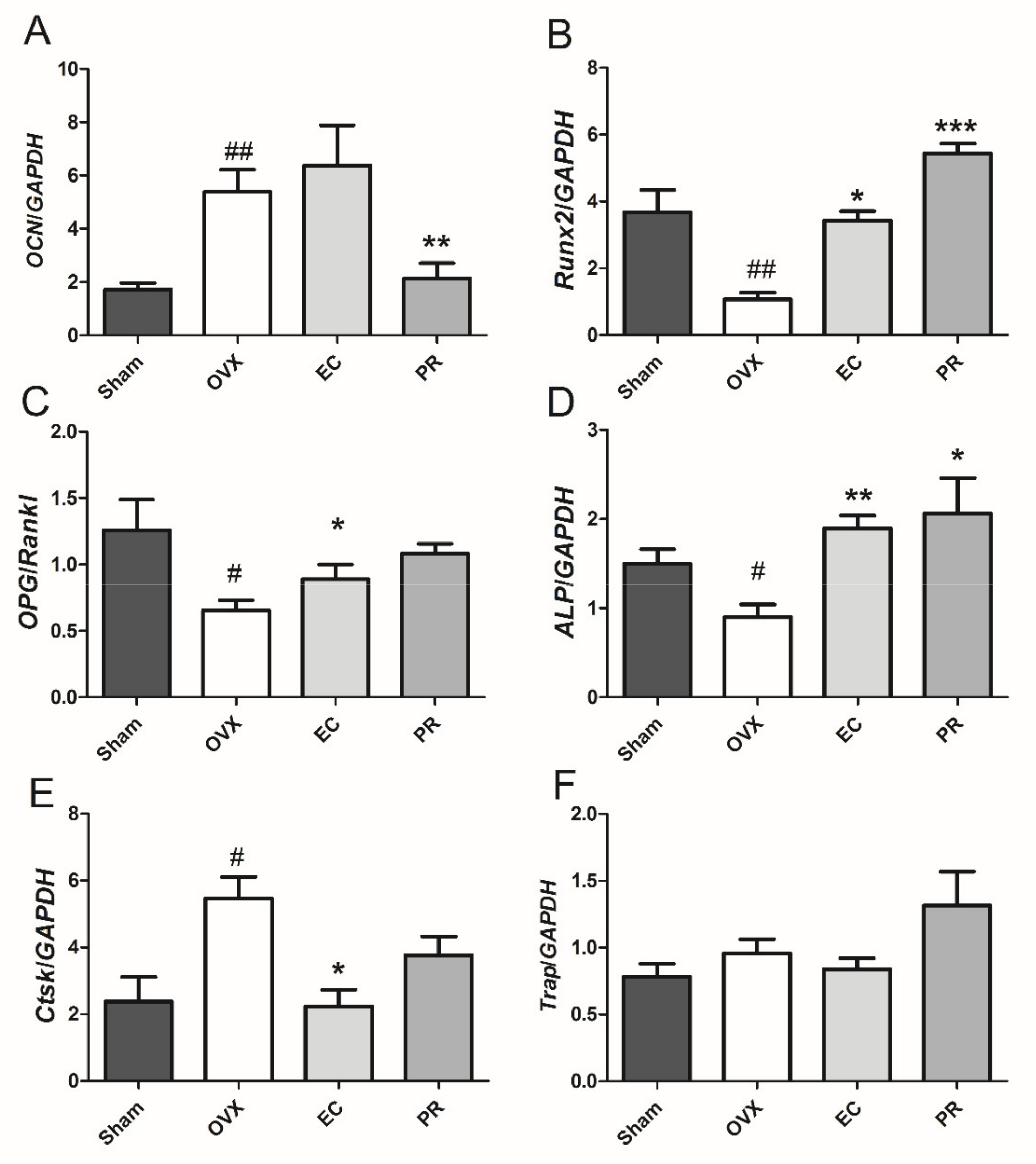
3.3. Effects of EC Treatment on Regulating Osteoblast-Specific and Osteoclast-Specific mRNA Expressions
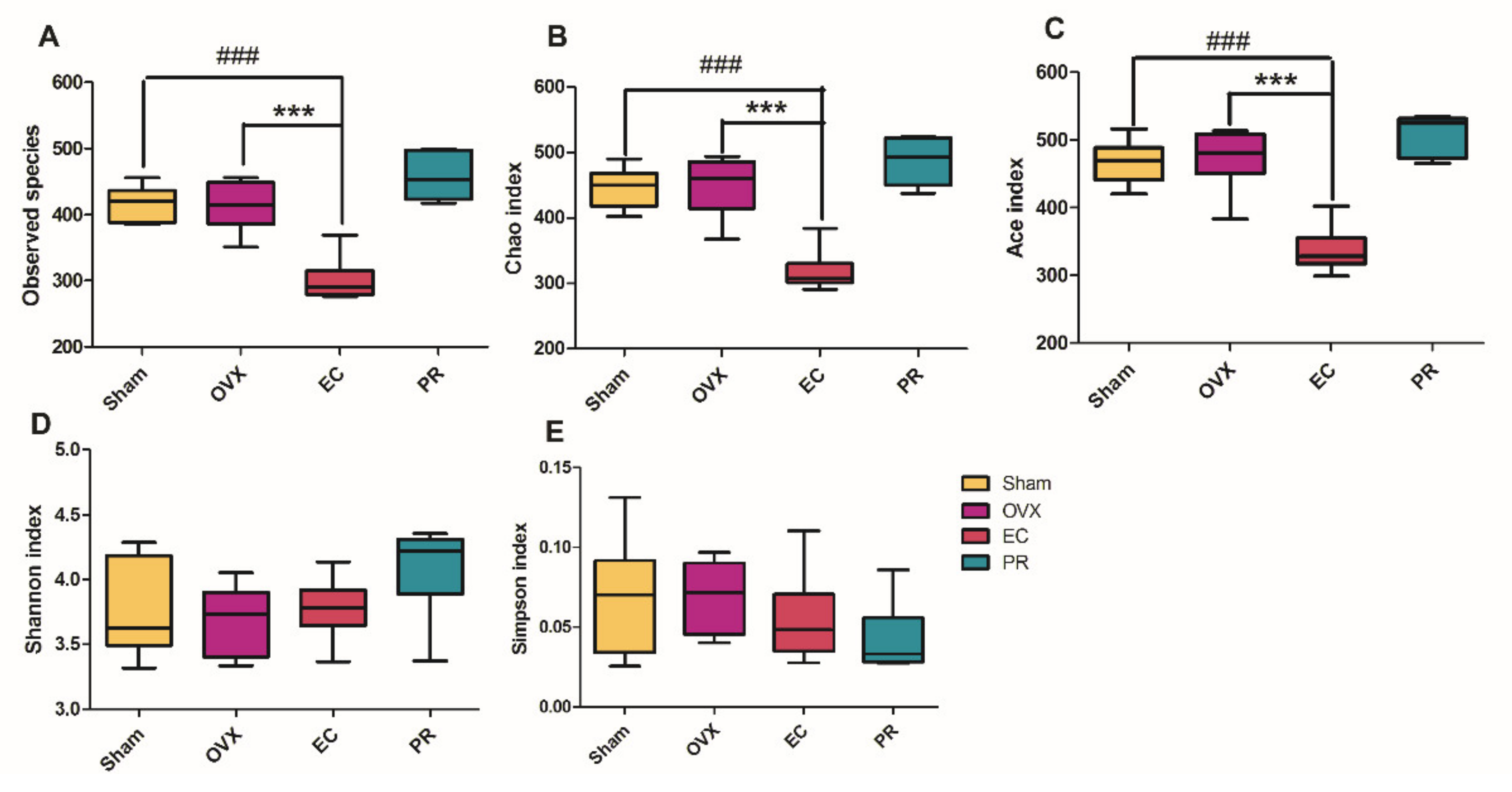
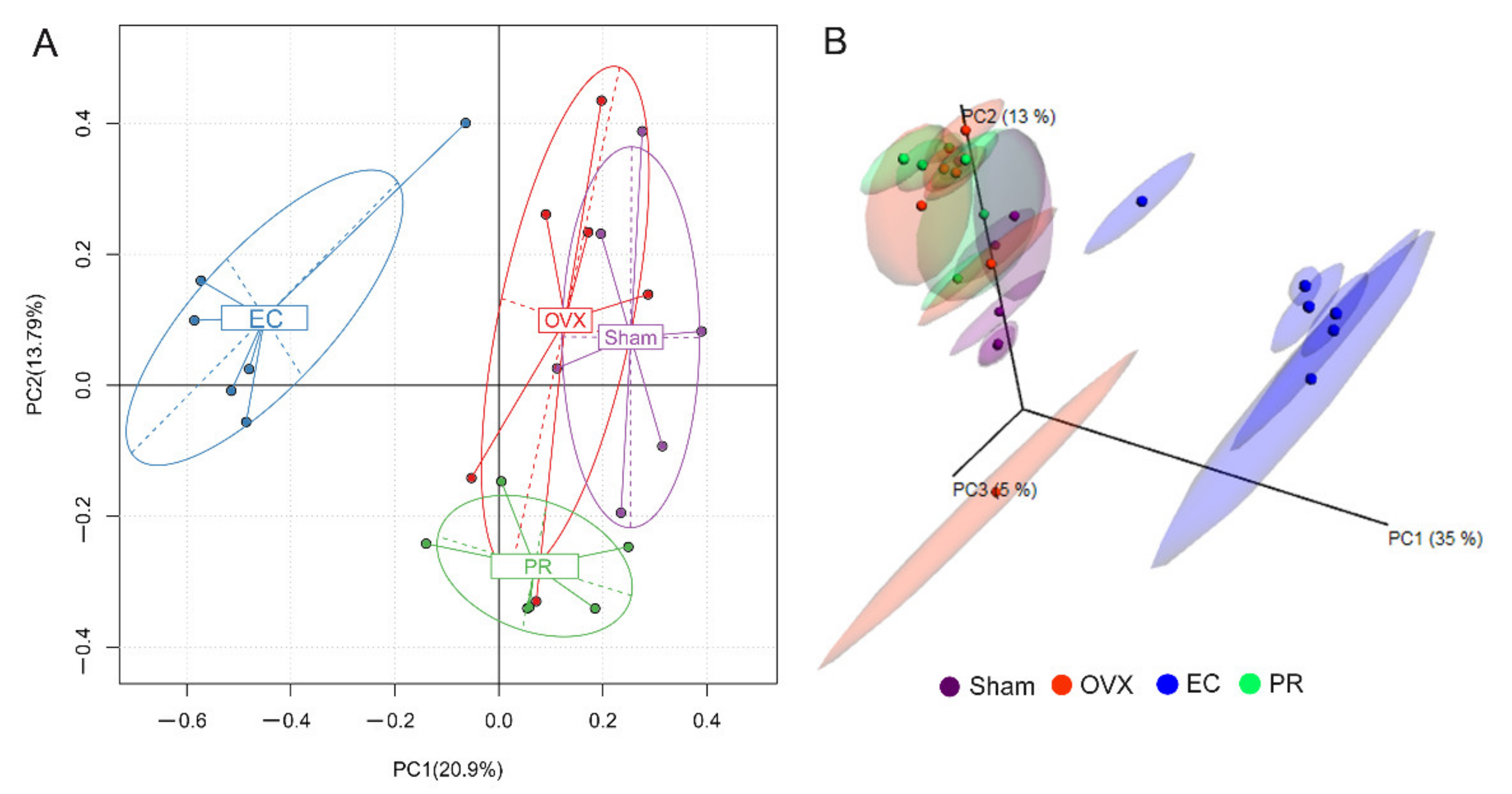
3.4. Effects of EC Extract on the Gut Microbiota Structure in Ovariectomized Rats
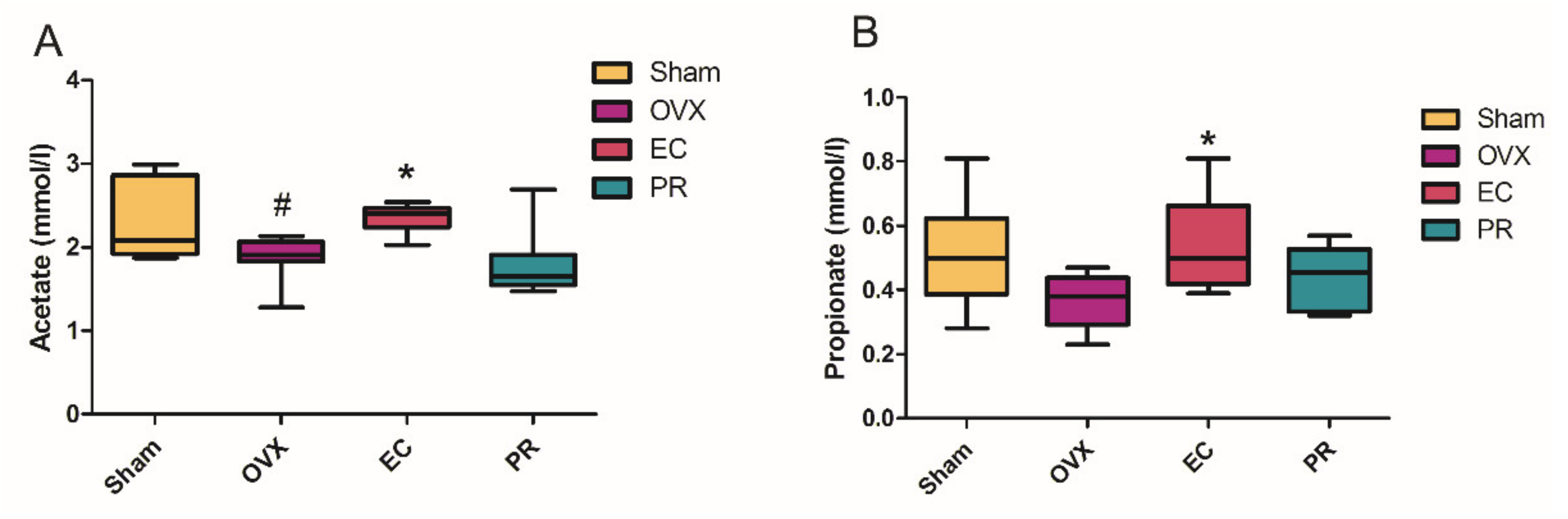
3.5. The Contents of SCFA in Serum and Its Actions on Bone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Cotts, K.G.; Cifu, A.S. Treatment of osteoporosis. JAMA 2018, 319, 1040–1041. [Google Scholar] [CrossRef]
- Carolyn, B. Osteoporosis: Staying strong. Nature 2017, 550, S15. [Google Scholar]
- Stavre, Z.; Upchurch, K.; Kay, J.; Gravallese, E. Differential effects of inflammation on bone and response to biologics in rheumatoid arthritis and spondyloarthritis. Curr. Rheumatol. Rep. 2016, 18, 1–13. [Google Scholar] [CrossRef]
- Gérard, K.; Mathieu, F. The contribution of bone to whole-organism physiology. Nature 2012, 481, 314. [Google Scholar]
- Solomon, C.G.; Black, D.M.; Rosen, C.J. Postmenopausal osteoporosis. NEJM 2016, 374, 254–262. [Google Scholar]
- Watts, N.B.; Manson, J.E. Osteoporosis and fracture risk evaluation and management: Shared decision making in clinical practice. JAMA 2017, 317, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, P.; Cooper, C. Osteoporosis. Lancet 2006, 367, 2010–2018. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.-W.; Zeng, G.-F.; Zong, S.-H.; Zhang, Z.-Y.; Zou, B.; Fang, Y.; Lu, L.; Xiao, D.-Q. Systematic review and meta-analysis of the bone protective effect of phytoestrogens on osteoporosis in ovariectomized rats. Nutr. Res. 2014, 34, 467. [Google Scholar] [CrossRef]
- Chiang, S.-S.; Pan, T.-M. Beneficial effects of phytoestrogens and their metabolites produced by intestinal microflora on bone health. Appl. Microbiol. Biotechnol. 2013, 97, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, K.; Vance, T.M.; Vance, T.M.; Chun, O.K.; Chun, O.K. Estimated intake and major food sources of flavonoids among US adults: Changes between 1999–2002 and 2007–2010 in NHANES. Eur. J. Nutr. 2016, 55, 833–843. [Google Scholar] [CrossRef]
- Tai, T.; Tsai, K.; Tu, S.; Wu, J.; Chang, C.; Chen, C.; Shaw, N.; Peng, H.; Wang, S.; Wu, C. The effect of soy isoflavone on bone mineral density in postmenopausal Taiwanese women with bone loss: A 2-year randomized double-blind placebo-controlled study. Osteoporos. Int. 2012, 23, 1571–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nash, L.A.; Ward, W.E. Tea and bone health: Findings from human studies, potential mechanisms, and identification of knowledge gaps. Crit. Rev. Food Sci. Nutr. 2017, 57, 1603–1617. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, D.; Yang, D.; Zhen, W.; Zhang, J.; Peng, S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos. Int. 2018, 29, 535–544. [Google Scholar] [CrossRef]
- Ming, L.-G.; Lv, X.; Ma, X.-N.; Ge, B.-F.; Zhen, P.; Song, P.; Zhou, J.; Ma, H.-P.; Xian, C.J.; Chen, K.-M. The prenyl group contributes to activities of phytoestrogen 8-prenynaringenin in enhancing bone formation and inhibiting bone resorption in vitro. Endocrinology 2013, 154, 1202–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Emmanuel, M.; Wong, M.-S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; González-Arceo, M.; Fernández-Quintela, A.; Eseberri, I.; Trepiana, J.; Portillo, M.P. Scientific evidence supporting the beneficial effects of isoflavones on human health. Nutrients 2020, 12, 3853. [Google Scholar] [CrossRef]
- Kumar, A.; Lingadurai, S.; Jain, A.; Barman, N.R. Erythrina variegata Linn: A review on morphology, phytochemistry, and pharmacological aspects. Pharmacogn. Rev. 2010, 4, 147–152. [Google Scholar]
- Song, L.R. Dictionary of Modern Medicine, 1st ed.; Song, L.R., Hong, X., Ding, X.L., Zang, Z.Y., Eds.; People’s Mecial Publishing House: Beijing, China, 2001; p. 1796. [Google Scholar]
- Zhang, Y.; Li, Q.; Li, X.; Wan, H.Y.; Wong, M.-S. Erythrina variegata extract exerts osteoprotective effects by suppression of the process of bone resorption. Br. J. Nutr. 2010, 104, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, X.L.; Lai, W.P.; Chen, B.; Chow, H.K.; Wu, C.F.; Wang, N.L.; Yao, X.S.; Wong, M.S. Anti-osteoporotic effect of Erythrina variegata L. in ovariectomized rats. J. Ethnopharmacol. 2007, 109, 165–169. [Google Scholar] [CrossRef]
- Li, X.; Wang, N.; Wong Man, S.; Albert, S.C.C.; Yao, X. Four new isoflavonoids from the stem bark of Erythrina variegata. Chem. Pharm. Bull. 2006, 54, 570–573. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.-L.; Yao, X.-S.; Wong, M.-S. Osteogenic activities of genistein derivatives were influenced by the presence of prenyl group at ring a. Arch. Pharm. Res. 2008, 31, 1534–1539. [Google Scholar] [CrossRef]
- Wallace, T.C.; Guarner, F.; Madsen, K.; Cabana, M.D.; Gibson, G.; Hentges, E.; Sanders, M.E. Human gut microbiota and its relationship to health and disease. Nutr. Rev. 2011, 69, 392. [Google Scholar] [CrossRef]
- Lyu, M.; Wang, Y.-f.; Fan, G.-w.; Wang, X.-y.; Xu, S.-y.; Zhu, Y. Balancing herbal medicine and functional food for prevention and treatment of cardiometabolic diseases through modulating gut microbiota. Front. Microbiol. 2017, 8, 2146. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Chen, L.; Xue, B.; Liu, Q.; Ou, S.; Wang, Y.; Peng, X. Different flavonoids can shape unique gut microbiota profile in vitro. J. Food. Sci. 2016, 81, H2273–H2279. [Google Scholar] [CrossRef]
- Woting, A.; Blaut, M. The intestinal microbiota in metabolic disease. Nutrients 2016, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.H.; Dai, Y.; Wan, H.Y.; Wong, M.S.; Yao, X.S. Bone-protective effects of bioactive fractions and ingredients in Sambucus williamsii HANCE. Br. J. Nutr. 2011, 106, 1802–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.C.; Shing, L.K.; Kit, L.H.; Ying, W.H.; Kwan, H.C.; Zhang, Y.; Sau, W.M. Er-Xian decoction exerts estrogen-like osteoprotective effects in vivo and in vitro. Am. J. Chin. Med. 2014, 42, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-H.; Lu, L.; Poon, C.C.-W.; Chan, C.-O.; Wang, L.-J.; Zhu, Y.-X.; Zhou, L.-P.; Cao, S.; Yu, W.-X.; Wong, K.Y.; et al. The lignan-rich fraction from Sambucus Williamsii Hance ameliorates dyslipidemia and insulin resistance and modulates gut microbiota composition in ovariectomized rats. Biomed. Pharmacother. 2021, 137, 111372. [Google Scholar] [CrossRef]
- Li, J.; Chassaing, B.; Tyagi, A.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.; Weitzmann, M.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef] [Green Version]
- Nima, M.-N.; Younes, G.; Mohammad Hossein, D.; Pedram, T.; Farhad, K.; Ahmad, G. Supportive role of probiotic strains in protecting rats from ovariectomy-induced cortical bone loss. Probiotics Antimicro. 2019, 11, 1145–1154. [Google Scholar]
- Wang, J.H.; Wang, Y.Y.; Gao, W.J.; Wang, B.; Zhao, H.P.; Zeng, Y.H.; Ji, Y.H.; Hao, D.J. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. Peer J. 2017, 5, e3450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Yu, P.; Gui, X.; Wang, Y.; Xue, C.; Wang, J. Siaglycoprotein isolated from the eggs of Carassius Auratus prvents bone loss: An efffect associated with the regulation of gut microbiota in ovariectomized rats. Food Funct. 2016, 7, 4764–4771. [Google Scholar] [CrossRef]
- Jin, G.; Asou, Y.; Ishiyama, K.; Okawa, A.; Kanno, T.; Niwano, Y. Proanthocyanidin-rich grape seed extract modulates intestinal microbiota in ovariectomized mice. J. Food Sci. 2018, 83, 1149–1152. [Google Scholar] [CrossRef]
- Li, C.; Huang, Q.; Yang, R.; Dai, Y.; Zeng, Y.; Tao, L.; Li, X.; Zeng, J.; Wang, Q. Gut microbiota composition and bone mineral loss—epidemiologic evidence from individuals in Wuhan, China. Osteoporos. Int. 2019, 30, 1003–1013. [Google Scholar] [CrossRef]
- Romero Barco, C.M.; Manrique Arija, S.; Rodríguez Pérez, M. Biochemical markers in osteoporosis: Usefulness in clinical practice. Reumatol. Clín. Engl. Ed. 2012, 8, 149–152. [Google Scholar] [CrossRef]
- Feng, W.; Ao, H.; Peng, C. Gut microbiota, short-chain fatty acids, and herbal medicines. Front. Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef]
- Corrêa, R.O.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.; Rao, S.T.; Cheng, Y.Z.; Zhuo, X.Y.; Deng, C.H.; Xu, N.N.; Zhang, H.; Yang, L. Microbial osteoporosis: The interplay between the gut microbiota and bone via host metabolism and immunity. Microbiology 2018, 8, e810. [Google Scholar] [CrossRef] [Green Version]
- Hiroshi, T. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.; Jia, L.; Mo, L.; Yuan, S.; Zheng, X.; He, J.; Chen, V.; Guo, Q.; Zheng, L.; Yuan, Q.; et al. Berberine ameliorates periodontal bone loss by regulating gut microbiota. J. Dent. Res. 2019, 98, 107–116. [Google Scholar] [CrossRef]
- Kleesen, B.; Stoof, G.; Proll, J.; Schmiedl, D.; Noack, J.; Blaut, M. Feeding resistant starch affects fecal and cecal microflora and short-chain fatty acids in rats. J. Anim. Sci. 1997, 9, 2453–2462. [Google Scholar] [CrossRef] [Green Version]
- Beers-Schreurs, H.M.G.v.; Nabuurs, M.J.A.; Vellenga, L.; Kalsbeek-van-der Valk, H.J.; Wensing, T.; Breukink, H.J. Weaning and the weanling diet influence the villous height and crypt depth in the small intestine of pigs and alter the concentrations of short-chain fatty acids in the large intestine and blood. J. Nutr. 1998, 128, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Emma, A.-V. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Li, S.; You, J.; Wang, Z.; Liu, Y.; Wang, B.; Du, M.; Zou, T. Curcumin alleviates high-fat diet-induced hepatic steatosis and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2021, 143, 110270. [Google Scholar] [CrossRef] [PubMed]



| Primer | Accession No. | Sequence (5′-3′) | Tm (°C) |
|---|---|---|---|
| Runx2 | NM_001278484 | F: TAACGGTCTTCACAAATCCTC | 56 |
| R: GGCGGTCCAGAGAACAAACTA | |||
| ALP | NM_013059 | F: GTGGTGGACGGTGAACGGGAGAA | 53 |
| R: ATGGACGCCGTGAAGCAGGTGAG | |||
| OCN | NM_013414 | F: GCAGCTTCAGCTTTGGCTACTCT | 56 |
| R: CAACCGTTCCTCATCTGGACTTTA | |||
| OPG | NM_012870 | F: ACAATGAACAAGTGGCTGTGCTG | 55 |
| R: CGGTTTCTGGGTCATAATGCAAG | |||
| Rankl | NM_057149 | F: GCAGCATCGCTCTGTTCCTGTA | 55 |
| R: GCATGAGTCAGGTAGTGCTTCTGTG | |||
| Ctsk | NM_031560 | F: CGCCACGGCAAAGGCAGCTAA | 64 |
| R: CGGGTCCTACCCGAGCCACT | |||
| Trap | NM_001270889 | F: ACCGCCTACCTGTGTGGGCA | 62 |
| R: CCATGAAGTTGCCGGCCCCA | |||
| GAPDH | NM_017008 | F: CAAGTTCAACGGCACAGTCAAGG | 51 |
| R: ACATACTCAGCACCAGCATCACC |
| Sham | OVX | EC | PR | ||
|---|---|---|---|---|---|
| Tibia | BMD (mgHA/cm3) | 360.0 ± 11.8 | 118.3 ± 6.3 ### | 179.5 ± 17.1 * | 293.2 ± 14.2 *** |
| Tb.N (mm−1) | 3.93 ± 0.20 | 1.18 ± 0.02 ### | 1.67 ± 0.21 | 3.11 ± 0.19 *** | |
| Tb.Sp (mm) | 0.22 ± 0.01 | 0.87 ± 0.02 ### | 0.68 ± 0.07 * | 0.31 ± 0.02 *** | |
| Tb.Th (mm) | 0.133 ± 0.004 | 0.099 ± 0.002 ### | 0.106 ± 0.001 | 0.120 ± 0.003 * | |
| BV/TV (%) | 0.42 ± 0.02 | 0.08 ± 0.01 ### | 0.15 ± 0.02 * | 0.31 ± 0.02 *** | |
| Conn. D (mm−3) | 60.9 ± 3.6 | 8.6 ± 0.8 ### | 22.6 ± 4.4 * | 45.7 ± 3.9 *** | |
| SMI | 0.54 ± 0.22 | 2.47 ± 0.07 ### | 1.99 ± 0.08 ** | 1.28 ± 0.17 *** | |
| Femur | BMD (mgHA/cm3) | 341.6 ± 15.9 | 112.7 ± 5.5 ### | 164.5 ± 10.0 * | 265.9 ± 16.7 *** |
| Tb.N (mm−1) | 3.28 ± 0.33 | 1.31 ± 0.06 ### | 1.46 ± 0.12 | 2.23 ± 0.17 * | |
| Tb.Sp (mm) | 0.33 ± 0.05 | 0.82 ± 0.05 ### | 0.73 ± 0.07 | 0.51 ± 0.05 ** | |
| Tb.Th (mm) | 0.147 ± 0.005 | 0.105 ± 0.003 ### | 0.111 ± 0.002 | 0.123 ± 0.003 | |
| BV/TV (%) | 0.40 ± 0.03 | 0.07 ± 0.01 ### | 0.15 ± 0.01 * | 0.28 ± 0.03 *** | |
| Conn. D (mm−3) | 44.7 ± 3.7 | 9.0 ± 1.3 ### | 21.4 ± 3.2 * | 40.2 ± 3.5 *** | |
| SMI | 0.29 ± 0.28 | 2.40 ± 0.07 ### | 1.72 ± 0.10 | 0.93 ± 0.23 *** | |
| Lumbar Vertabra | BMD (mgHA/cm3) | 385.1 ± 12.9 | 214.2 ± 9.6 ### | 271.5 ± 15.8 * | 338.3 ± 13.8 *** |
| Tb.N (mm−1) | 3.61 ± 0.14 | 2.25 ± 0.14 ### | 2.82 ± 0.19 | 3.33 ± 0.10 *** | |
| Tb.Sp (mm) | 0.24 ± 0.02 | 0.44 ± 0.03 ### | 0.34 ± 0.03 * | 0.27 ± 0.01 *** | |
| Tb.Th (mm) | 0.14 ± 0.02 | 0.10 ± 0.01 | 0.11 ± 0.02 | 0.11 ± 0.02 | |
| BV/TV (%) | 0.46 ± 0.02 | 0.17 ± 0.01 ### | 0.27 ± 0.03 * | 0.39 ± 0.02 *** | |
| Conn. D (mm−3) | 34.1 ± 2.33 | 17.6 ± 1.89 ### | 30.3 ± 3.8 * | 33.9 ± 1.38 *** | |
| Weight change (%) | 16.2 ± 1.6 | 27.6 ± 2.1 ### | 26.2 ± 1.7 | 22.5 ± 1.8 | |
| Uterus index (UI, mg/g) | 1.87 ± 0.07 | 0.33 ± 0.02 ### | 0.30 ± 0.01 | 0.91 ± 0.06 *** | |
| Serum Ca (mmol/L) | 2.71 ± 0.02 | 2.50 ± 0.01 ### | 2.53 ± 0.02 | 2.59 ± 0.03 * | |
| Serum P (mmol/L) | 1.10 ± 0.04 | 1.30 ± 0.04 | 1.29 ± 0.06 | 1.18 ± 0.08 | |
| Serum ALP (ng/mL) | 47.7 ± 6.3 | 107.6 ± 12.7 ### | 74.6 ± 3.5 * | 70.3 ± 6.0 * | |
| Urine Ca/Cr | 0.071 ± 0.010 | 0.158 ± 0.048 | 0.029 ± 0.005 ** | 0.078 ± 0.025 | |
| Sham | OVX | EC | PR | |
|---|---|---|---|---|
| OTU | 722 | 739 | 565 | 756 |
| Phylum | 18 | 17 | 14 | 18 |
| Class | 31 | 29 | 24 | 30 |
| Order | 51 | 48 | 36 | 47 |
| Family | 80 | 80 | 62 | 75 |
| Genus | 174 | 175 | 147 | 166 |
| Species | 295 | 302 | 252 | 300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.-H.; Yu, X.; Yang, C.; Chan, C.-O.; Lu, L.; Cao, S.; Wan, S.-W.; Lan, Z.-J.; Mok, D.K.-W.; Chen, S.; et al. Prenylated Isoflavonoids-Rich Extract of Erythrinae Cortex Exerted Bone Protective Effects by Modulating Gut Microbial Compositions and Metabolites in Ovariectomized Rats. Nutrients 2021, 13, 2943. https://doi.org/10.3390/nu13092943
Xiao H-H, Yu X, Yang C, Chan C-O, Lu L, Cao S, Wan S-W, Lan Z-J, Mok DK-W, Chen S, et al. Prenylated Isoflavonoids-Rich Extract of Erythrinae Cortex Exerted Bone Protective Effects by Modulating Gut Microbial Compositions and Metabolites in Ovariectomized Rats. Nutrients. 2021; 13(9):2943. https://doi.org/10.3390/nu13092943
Chicago/Turabian StyleXiao, Hui-Hui, Xueli Yu, Chen Yang, Chi-On Chan, Lu Lu, Sisi Cao, Siu-Wai Wan, Ze-Jun Lan, Daniel Kam-Wah Mok, Sheng Chen, and et al. 2021. "Prenylated Isoflavonoids-Rich Extract of Erythrinae Cortex Exerted Bone Protective Effects by Modulating Gut Microbial Compositions and Metabolites in Ovariectomized Rats" Nutrients 13, no. 9: 2943. https://doi.org/10.3390/nu13092943
APA StyleXiao, H.-H., Yu, X., Yang, C., Chan, C.-O., Lu, L., Cao, S., Wan, S.-W., Lan, Z.-J., Mok, D. K.-W., Chen, S., & Wong, M. (2021). Prenylated Isoflavonoids-Rich Extract of Erythrinae Cortex Exerted Bone Protective Effects by Modulating Gut Microbial Compositions and Metabolites in Ovariectomized Rats. Nutrients, 13(9), 2943. https://doi.org/10.3390/nu13092943