Effects of L-Citrulline Supplementation and Aerobic Training on Vascular Function in Individuals with Obesity across the Lifespan
Abstract
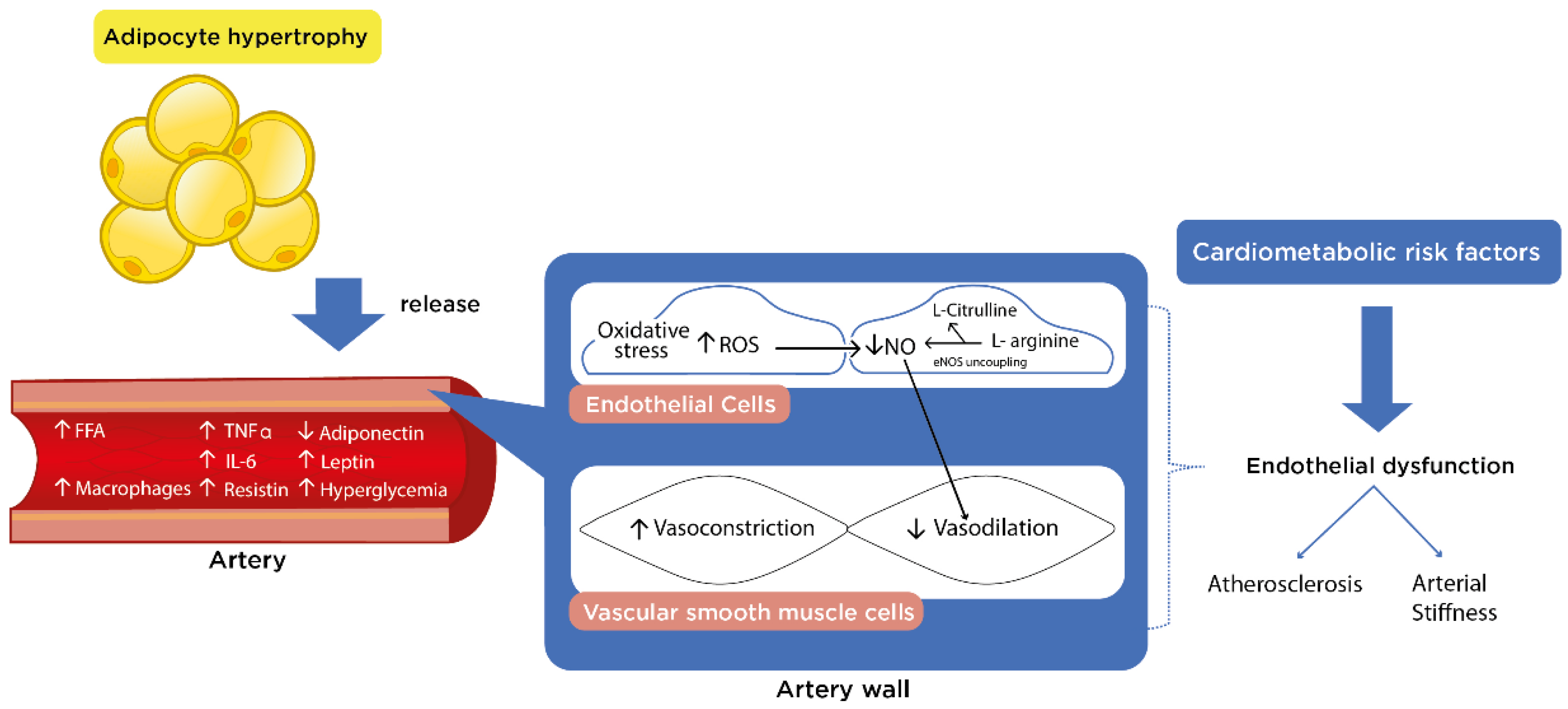
1. Introduction
2. Endothelial Function
Endothelial-Mediated Vasodilation
3. Vascular Function and Structure in Individuals with Obesity
3.1. Carotid-Intima Media Thickness
3.2. Arterial Stiffness
4. Effects of L-Citrulline Supplementation on Vascular and Metabolic Parameters in Obesity
| Articles | Total Sample | Intervention Group | Supplementation Characteristics | SBP (mmHg) | NOx | PWV (m/s) | Glucose (mg/dL) | Triglycerides (mg/dL) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Age | Dose | Duration | ||||||||
| [23] | n = 41 | 14 women | 58 ± 4 years | 6 g/day | 8 weeks | B | 137 ± 13 | 28.2 ± 7.3 | NM | NM | NM |
| A | 130 ± 15 * | 35.2 ± 9.5 * | |||||||||
| [109] | n = 16 | 16 men | 24 ± 2 (SE) | 6 g/day | 2 weeks | B | 123 ± 3 | NM | 11.8 a | NM | NM |
| A | 121 ± 3 | 11.2 a | |||||||||
| [24] | n = 23 | 12 women | 58 ± 1 years | 6 g/day | 8 weeks | B | 138 ± 4 | NM | NM | NM | NM |
| A | 131 ± 5 *# | ||||||||||
| [25] | n = 40 | 14 women | 58 ± 1 years | 6 g/ day | 8 weeks | B | 137 ± 4 | NM | 11.5 ± 0.4 b 10.01 ± 0.2 c 14.1 ± 0.5 a | NM | NM |
| A | NR | 11.3 ± 0.5 b 9.6 ± 0.2 c* 13.2 ± 0.5 a* | |||||||||
| [110] | n = 41 | 41 adults | 18–66 years | 15g/day | 2 weeks | B | 130 (126–134) | NM | NM | 88 (84–92) | 101 (77–126) |
| C | −1.6 (−6.3–3.1) | NR | NR | ||||||||
5. Effects of the Aerobic Training in Children and Adolescents with Obesity on Vascular and Metabolic Parameters
6. Effects of L-Citrulline Supplementation and Exercise Training in Individuals with Obesity on Vascular and Metabolic Parameters
| Article | Total Sample | Intervention Groups | Exercise Characteristics | L-Citrulline Dose | SBP (mmHg) | aSBP (mmHg) | Aix (%) | NOx (µmol/L) | PWV (m/s) | WC (cm) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Age | Training | Session | Frequency/ Duration | ||||||||||
| [23] | n = 41 | 13 women | 58 ± 3 years | WBVT: dynamic leg exercises | 1–5 sets (30–60 s) | 3 days/week For 8 weeks | 6 g/day | B | 140 ± 9 | 133 ± 9 | 43.5 ± 10.2 | 28.2 ± 14.9 | NM | NM |
| A | 132 ± 9 * | 123± 9 * | 33.3 ± 8.3 * | 38.3 ± 19.6 * | NM | |||||||||
| [25] | n = 41 | 13 women | 58 ± 1 years | WBVT: dynamic leg exercises | 1–5 sets (30–60 s) | 3 days/week For 8 weeks | 6 g/day | B | 140 ± 3 | NM | NM | NM | 11.7 ± 0.3 b 14.7 ± 0.4 a 10.4 ± 0.3 c | NM |
| A | NR | 10.8± 0.3 b* 13.4 ± 0.4 a* 9.8 ± 0.2 c* | NM | |||||||||||
| [138] | n = 56 | 26 adults | 65.7 ± 4.2 years | HIIT | 30 min | 3 days/week For 12 weeks | 10 g/day | B | NM | NM | NM | NM | NM | 107 ± 11 |
| A | 104 ± 11 * | |||||||||||||
| [136] | n = 44 | 23 adults | 67.6 ± 5.01 years | HIIT | 30 min | 3 days/week For 12 weeks | 10 g/day | B | NM | NM | NM | NM | NM | 98.3 ± 10.2 |
| A | 95.9 ± 10.9 * | |||||||||||||
7. Conclusions and Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 June 2021).
- Deal, B.J.; Huffman, M.D.; Binns, H.; Stone, N.J. Perspective: Childhood obesity requires new strategies for prevention. Adv. Nutr. 2020, 11, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Bramlage, P.; Pittrow, D.; Wittchen, H.-U.; Kirch, W.; Boehler, S.; Lehnert, H.; Hoefler, M.; Unger, T.; Sharma, A.M. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am. J. Hypertens. 2004, 17, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, Y.; Yu, J.; Zha, M.; Zhu, Y.; Rahimi, K.; Rudan, I. Global prevalence of hypertension in children: A systematic review and meta-analysis. JAMA Pediatr. 2019, 173, 1154–1163. [Google Scholar] [CrossRef]
- Hinnouho, G.-M.; Czernichow, S.; Dugravot, A.; Batty, G.; Kivimaki, M.; Singh-Manoux, A. Metabolically healthy obesity and risk of mortality: Does the definition of metabolic health matter? Diabetes Care 2013, 36, 2294–2300. [Google Scholar] [CrossRef]
- Kramer, C.K.; Zinman, B.; Retnakaran, R. Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysis. Ann. Intern. Med. 2013, 159, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Song, Y.; Chen, Y.; Hui, R.; Zhang, W. Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Int. J. Cardiol. 2013, 168, 4761–4768. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; López-Bermejo, A.; Caserta, C.A.; Medeiros, C.C.M.; Kollias, A.; Bassols, J.; Romeo, E.L.; Ramos, T.D.A.; Stergiou, G.S.; Yang, L.; et al. Metabolically healthy obesity and high carotid intima-media thickness in children and adolescents: International childhood vascular structure evaluation consortium. Diabetes Care 2018, 42, 119–125. [Google Scholar] [CrossRef]
- Al-Hamad, D.; Raman, V. Metabolic syndrome in children and adolescents. Transl. Pediatr. 2017, 6, 397–407. [Google Scholar] [CrossRef]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J. Pediatric obesity—Assessment, treatment, and prevention: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kelly, A.S. Review of childhood obesity: From epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S.; et al. The metabolic syndrome in children and adolescents? An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
- Sun, S.S.; Grave, G.D.; Siervogel, R.M.; Pickoff, A.A.; Arslanian, S.S.; Daniels, S.R. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics 2007, 119, 237–246. [Google Scholar] [CrossRef]
- Sun, S.S.; Liang, R.; Huang, T.T.-K.; Daniels, S.R.; Arslanian, S.; Liu, K.; Grave, G.D.; Siervogel, R.M. Childhood obesity predicts adult metabolic syndrome: The Fels longitudinal study. J. Pediatr. 2008, 152, 191–200.e1. [Google Scholar] [CrossRef]
- Khoury, M.; Urbina, E.M. Cardiac and vascular target organ damage in pediatric hypertension. Front. Pediatr. 2018, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Magge, S.; Goodman, E.; Armstrong, S.C.; Committee on Nutrition; Section on Endocrinology; Section on Obesity. The metabolic syndrome in children and adolescents: Shifting the focus to cardiometabolic risk factor clustering. Pediatrics 2017, 140, e20171603. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-E.; Zhang, C.-L.; Zhen, Q. Metabolic syndrome in children (Review). Exp. Ther. Med. 2016, 12, 2390–2394. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.M.; Srinivasan, S.R.; Xu, J.-H.; Chen, W.; Berenson, G.S. Fasting plasma glucose levels within the normoglycemic range in childhood as a predictor of prediabetes and type 2 diabetes in adulthood: The Bogalusa heart study. Arch. Pediatr. Adolesc. Med. 2010, 164, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial dysfunction in obesity-induced inflammation: Molecular mechanisms and clinical implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef]
- Čolak, E.; Pap, D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J. Med. Biochem. 2021, 40, 1–9. [Google Scholar] [CrossRef]
- Breuillard, C.; Cynober, L.; Moinard, C. Citrulline and nitrogen homeostasis: An overview. Amino Acids 2015, 47, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Sakurada, M.; Watanabe, F.; Yamasaki, T.; Doi, H.; Ezaki, H.; Morishita, K.; Miyakex, T. Effects of oral L-Citrulline supplementation on Lipoprotein oxidation and Endothelial dysfunction in humans with Vasospastic Angina. Immunol. Endocr. Metab. Agents Med. Chem. 2013, 13, 214–220. [Google Scholar] [CrossRef]
- Wong, A.; Alvarez-Alvarado, S.; Jaime, S.J.; Kinsey, A.W.; Spicer, M.T.; Madzima, T.A.; Figueroa, A. Combined whole-body vibration training and L-Citrulline supplementation improves pressure wave reflection in obese postmenopausal women. Appl. Physiol. Nutr. Metab. 2016, 41, 292–297. [Google Scholar] [CrossRef]
- Wong, A.; Chernykh, O.; Figueroa, A. Chronic L-Citrulline supplementation improves cardiac sympathovagal balance in obese postmenopausal women: A preliminary report. Auton. Neurosci. Basic Clin. 2016, 198, 50–53. [Google Scholar] [CrossRef]
- Figueroa, A.; Alvarez-Alvarado, S.; Ormsbee, M.J.; Madzima, T.A.; Campbell, J.C.; Wong, A. Impact of L-Citrulline supplementation and whole-body vibration training on arterial stiffness and leg muscle function in obese postmenopausal women with high blood pressure. Exp. Gerontol. 2015, 63, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Hayashi, T.; Morita, M.; Ina, K.; Maeda, M.; Watanabe, F.; Morishita, K. Short-term effects of L-Citrulline supplementation on arterial stiffness in middle-aged men. Int. J. Cardiol. 2012, 155, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Melchior, J.-C.; Faure, C.; Paul, M.; Canoui-Poitrine, F.; Boirie, Y.; Chevenne, D.; Forasassi, C.; Guery, E.; Herbaud, S.; et al. Impact of 3-week citrulline supplementation on postprandial protein metabolism in malnourished older patients: The Ciproage randomized controlled trial. Clin. Nutr. 2019, 38, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Rey-López, J.P.; Vicente-Rodríguez, G.; Biosca, M.; Moreno, L.A. Sedentary behaviour and obesity development in children and adolescents. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Hills, A.P.; Okely, A.; Baur, L. Addressing childhood obesity through increased physical activity. Nat. Rev. Endocrinol. 2010, 6, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.R. Childhood obesity and the role of physical activity. J. R. Soc. Promot. Health 2004, 124, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.-D.; Pekas, E.J.; Scott, S.D.; Son, W.-M.; Park, S.-Y. The effects of a 12-week jump rope exercise program on abdominal adiposity, vasoactive substances, inflammation, and vascular function in adolescent girls with prehypertension. Eur. J. Appl. Physiol. 2019, 119, 577–585. [Google Scholar] [CrossRef]
- Chuensiri, N.; Suksom, D.; Tanaka, H. Effects of high-intensity intermittent training on vascular function in obese preadolescent boys. Child. Obes. 2018, 14, 41–49. [Google Scholar] [CrossRef]
- Da Silva, M.R.; Waclawovsky, G.; Perin, L.; Camboim, I.; Eibel, B.; Lehnen, A.M. Effects of high-intensity interval training on endothelial function, lipid profile, body composition and physical fitness in normal-weight and overweight-obese adolescents: A clinical trial. Physiol. Behav. 2020, 213, 112728. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Libman, I.; Hughan, K.S.; Kuk, J.L.; Barinas-Mitchell, E.; Chung, H.; Arslanian, S. Effects of exercise modality on body composition and cardiovascular disease risk factors in adolescents with obesity: A randomized clinical trial. Appl. Physiol. Nutr. Metab. 2020, 45, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.L.; Litwin, S.E.; Pollock, N.K.; Waller, J.L.; Zhu, H.; Dong, Y.; Kapuku, G.; Bhagatwala, J.; Harris, R.A.; Looney, J.; et al. Exercise effects on arterial stiffness and heart health in children with excess weight: The SMART RCT. Int. J. Obes. 2019, 44, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Kampoli, A.-M.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on Endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; De Silva, T.M.; Sobey, C.G.; Lim, K.; Drummond, G.R.; Vinh, A.; Jelinic, M. The vascular consequences of metabolic syndrome: Rodent models, endothelial dysfunction, and current therapies. Front. Pharmacol. 2020, 11, 148. [Google Scholar] [CrossRef]
- Jamwal, S.; Sharma, S. Vascular endothelium dysfunction: A conservative target in metabolic disorders. Inflamm. Res. 2018, 67, 391–405. [Google Scholar] [CrossRef]
- Schmidt, T.S.; Alp, N.J. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin. Sci. 2007, 113, 47–63. [Google Scholar] [CrossRef]
- Xia, N.; Horke, S.; Habermeier, A.; Closs, E.; Reifenberg, G.; Gericke, A.; Mikhed, Y.; Münzel, T.; Daiber, A.; Förstermann, U.; et al. Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose tissue of diet-induced obese mice. Arter. Thromb. Vasc. Biol. 2016, 36, 78–85. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, C.-B.; Liu, C.; Fan, Y.; Zhu, H.-Y.; Wu, X.-W.; Hu, L.; Li, Q.-P. Upregulation of arginase activity contributes to intracellular ROS production induced by high glucose in H9c2 cells. Int. J. Clin. Exp. Pathol. 2015, 8, 2728–2736. [Google Scholar]
- Michell, D.M.; Andrews, K.L.; Chin-Dusting, J.P.F. Endothelial dysfunction in hypertension: The role of arginase. Front. Biosci. 2011, 3, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, A.; Yao, L.; Xu, Z.; Toque, H.A.; Chen, J.; Atawia, R.T.; Fouda, A.; Bagi, Z.; Lucas, R.; Caldwell, R.; et al. Obesity-induced vascular dysfunction and arterial stiffening requires endothelial cell arginase 1. Cardiovasc. Res. 2017, 113, 1664–1676. [Google Scholar] [CrossRef]
- Meza, C.A.; La Favor, J.D.; Kim, D.-H.; Hickner, R.C. Endothelial dysfunction: Is there a Hyperglycemia-induced imbalance of NOX and NOS? Int. J. Mol. Sci. 2019, 20, 3775. [Google Scholar] [CrossRef] [PubMed]
- Fantin, F.; Giani, A.; Zoico, E.; Rossi, A.P.; Mazzali, G.; Zamboni, M. Weight loss and hypertension in obese subjects. Nutrients 2019, 11, 1667. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Fitzgerald, K.A. Inflammasome complexes: Emerging mechanisms and effector functions. Cell 2016, 165, 792–800. [Google Scholar] [CrossRef]
- Paz-Filho, G.; Wong, M.-L.; Licinio, J.; Mastronardi, C. Leptin therapy, insulin sensitivity, and glucose homeostasis. Indian J. Endocrinol. Metab. 2012, 16, 549–555. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Coleman, T.T.; Sasser, J.M.; Pittman, K.M.; Hankins, M.W.; Stec, D.E. Vascular smooth muscle-specific deletion of the leptin receptor attenuates leptin-induced alterations in vascular relaxation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R960–R967. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; Silva, A.; Wang, Z.; Hall, M.E. Obesity, kidney dysfunction and hypertension: Mechanistic links. Nat. Rev. Nephrol. 2019, 15, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.J.; Bruno, R.M.; Van Mil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.C.; Urbina, E.; Witt, S.A.; Glascock, B.J.; Kimball, T.R.; Mitsnefes, M. Flow-mediated vasodilatation of the brachial artery in children with chronic kidney disease. Pediatr. Nephrol. 2008, 23, 1297–1302. [Google Scholar] [CrossRef]
- Shah, A.S.; Urbina, E.M. Vascular and Endothelial function in youth with Type 2 Diabetes Mellitus. Curr. Diabetes Rep. 2017, 17, 36. [Google Scholar] [CrossRef]
- Cote, A.T.; Harris, K.; Panagiotopoulos, C.; Sandor, G.; Devlin, A. Childhood obesity and cardiovascular dysfunction. J. Am. Coll. Cardiol. 2013, 62, 1309–1319. [Google Scholar] [CrossRef]
- Su, Y.; Liu, X.-M.; Sun, Y.-M.; Jin, H.-B.; Fu, R.; Wang, Y.-Y.; Wu, Y.; Luan, Y. The relationship between endothelial dysfunction and oxidative stress in diabetes and prediabetes. Int. J. Clin. Pr. 2008, 62, 877–882. [Google Scholar] [CrossRef]
- Pacifico, L.; Perla, F.M.; Tromba, L.; Carbotta, G.; Lavorato, M.; Pierimarchi, P.; Chiesa, C. Carotid extra-media thickness in children: Relationships with Cardiometabolic risk factors and Endothelial function. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Miniello, V.L.; Faienza, M.F.; Scicchitano, P.; Cortese, F.; Gesualdo, M.; Zito, A.; Basile, M.; Recchia, P.; Leogrande, D.; Viola, D.; et al. Insulin resistance and endothelial function in children and adolescents. Int. J. Cardiol. 2014, 174, 343–347. [Google Scholar] [CrossRef]
- Urbina, E.M.; Bean, J.A.; Daniels, S.R.; D’Alessio, D.; Dolan, L.M. Overweight and hyperinsulinemia provide individual contributions to compromises in brachial artery distensibility in healthy adolescents and young adults: Brachial distensibility in children. J. Am. Soc. Hypertens. 2007, 1, 200–207. [Google Scholar] [CrossRef]
- Rodriguez, C.J.; Miyake, Y.; Grahame-Clarke, C.; Di Tullio, M.R.; Sciacca, R.R.; Boden-Albala, B.; Sacco, R.L.; Homma, S. Relation of plasma glucose and Endothelial function in a population-based multiethnic sample of subjects without Diabetes Mellitus. Am. J. Cardiol. 2005, 96, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Fulton, D.J.; Caldwell, R.B.; Toque, H.A. Hyperglycemia-impaired aortic vasorelaxation mediated through arginase elevation: Role of stress kinase pathways. Eur. J. Pharmacol. 2019, 844, 26–37. [Google Scholar] [CrossRef]
- Curley, S.; Gall, J.; Byrne, R.; Yvan-Charvet, L.; McGillicuddy, F.C. Metabolic inflammation in obesity—At the crossroads between fatty acid and cholesterol metabolism. Mol. Nutr. Food Res. 2020, 65, e1900482. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, E.K.; Fernandez, M.L. Impact of obesity and metabolic syndrome on immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Llewellyn, A.; Simmonds, M.C.; Owen, C.; Woolacott, N. Childhood obesity as a predictor of morbidity in adulthood: A systematic review and meta-analysis. Obes. Rev. 2015, 17, 56–67. [Google Scholar] [CrossRef]
- Ley, K.; Miller, Y.I.; Hedrick, C.C. Monocyte and macrophage dynamics during atherogenesis. Arter. Thromb. Vasc. Biol. 2011, 31, 1506–1516. [Google Scholar] [CrossRef]
- Landsberg, L.; Aronne, L.J.; Beilin, L.J.; Burke, V.; Igel, L.I.; Lloyd-Jones, D.; Sowers, J. Obesity-related hypertension: Pathogenesis, cardiovascular risk, and treatment—A position paper of the The Obesity Society and the American Society of Hypertension. Obesity 2012, 21, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Juonala, M.; Magnussen, C.; Berenson, G.S.; Venn, A.; Burns, T.L.; Sabin, M.; Srinivasan, S.R.; Daniels, S.R.; Davis, P.H.; Chen, W.; et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N. Engl. J. Med. 2011, 365, 1876–1885. [Google Scholar] [CrossRef]
- Hall, J.E.; Carmo, J.M.D.; Silva, A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Abrignani, M.G.; Lucà, F.; Favilli, S.; Benvenuto, M.; Rao, C.M.; Di Fusco, S.A.; Gabrielli, D.; Gulizia, M.M. Lifestyles and cardiovascular prevention in childhood and adolescence. Pediatr. Cardiol. 2019, 40, 1113–1125. [Google Scholar] [CrossRef]
- Theodore, R.F.; Broadbent, J.; Nagin, D.S.; Ambler, A.; Hogan, S.; Ramrakha, S.; Cutfield, W.; Williams, M.; Harrington, H.; Moffitt, T.; et al. Childhood to early-midlife systolic blood pressure trajectories: Early life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension 2015, 66, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Hanspeter, B.; Cockroft, J.R.; Deanfield, J.; Donald, A.; Ferrannini, E.; Halcox, J.; Kiowski, W.; Lüscher, T.F.; Mancia, G.; Natali, A.; et al. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension*. J. Hypertens. 2005, 23, 233–246. [Google Scholar] [CrossRef]
- Eley, V.; Christensen, R.; Guy, L.; Dodd, B. Perioperative blood pressure monitoring in patients with obesity. Anesthesia Analg. 2019, 128, 484–491. [Google Scholar] [CrossRef]
- Skilton, M.R.; Celermajer, D.S.; Cosmi, E.; Crispi, F.; Gidding, S.S.; Raitakari, O.T.; Urbina, E.M. Natural history of atherosclerosis and abdominal aortic intima-media thickness: Rationale, evidence, and best practice for detection of atherosclerosis in the young. J. Clin. Med. 2019, 8, 1201. [Google Scholar] [CrossRef]
- Cochain, C.; Zernecke, A. Macrophages in vascular inflammation and atherosclerosis. Pflügers Arch. Eur. J. Physiol. 2017, 469, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Ryder, J.R.; Northrop, E.; Rudser, K.D.; Kelly, A.S.; Gao, Z.; Khoury, P.R.; Kimball, T.R.; Dolan, L.M.; Urbina, E.M. Accelerated early vascular aging among adolescents with obesity and/or Type 2 Diabetes Mellitus. J. Am. Heart Assoc. 2020, 9, e014891. [Google Scholar] [CrossRef] [PubMed]
- Onut, R.; Balanescu, A.P.S.; Constantinescu, D.; Calmac, L.; Marinescu, M.; Dorobantu, M. Imaging Atherosclerosis by Carotid Intima-media Thickness in vivo: How to, Where and in Whom? MAEDICA J. Clin. Med. 2012, 7, 153–162. [Google Scholar]
- Skrzypczyk, P.; Pańczyk-Tomaszewska, M. Methods to evaluate arterial structure and function in children–State-of-the art knowledge. Adv. Med. Sci. 2017, 62, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.P.; Denton, E.R.E.; Robinson, J.; Macdonald, L.M.; Rymer, J.M. High resolution ultrasound assessment of the carotid artery: Its relevance in postmenopausal women and the effects of tibolone on carotid artery ultrastructure. Climacteric 1999, 2, 13–20. [Google Scholar] [CrossRef]
- Acevedo, M.; Krämer, V.; Tagle, R.; Arnaiz, P.; Corbalán, R.; Berríos, X.; Navarrete, C. Cardiovascular risk factors among young subjects with high carotid intima media thickness. Rev. Medica Chile 2012, 139, 1322. [Google Scholar] [CrossRef]
- Shah, B.N.; Chahal, N.S.; Kooner, J.S.; Senior, R. Contrast-enhanced ultrasonography vs B-mode ultrasound for visualization of intima-media thickness and detection of plaques in human carotid arteries. Echocardiography 2017, 34, 723–730. [Google Scholar] [CrossRef]
- Suárez-Cuenca, J.A.; Ruíz-Hernández, A.S.; Mendoza-Castañeda, A.A.; Domínguez-Pérez, G.A.; Hernández-Patricio, A.; Vera-Gómez, E.; De La Peña-Sosa, G.; Banderas-Lares, D.Z.; Montoya-Ramírez, J.; Blas-Azotla, R.; et al. Neutrophil-to-lymphocyte ratio and its relation with pro-inflammatory mediators, visceral adiposity and carotid intima-media thickness in population with obesity. Eur. J. Clin. Investig. 2019, 49, e13085. [Google Scholar] [CrossRef]
- Raitakari, O.T.; Juonala, M.; Kähönen, M.; Taittonen, L.; Laitinen, T.; Mäki-Torkko, N.; Järvisalo, M.J.; Uhari, M.; Jokinen, E.; Rönnemaa, T.; et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood. The cardiovascular risk in Young Finns study. JAMA 2003, 290, 2277–2283. [Google Scholar] [CrossRef]
- Stehouwer, C.D.A.; Henry, R.M.A.; Ferreira, I. Arterial stiffness in diabetes and the metabolic syndrome: A pathway to cardiovascular disease. Diabetologia 2008, 51, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Segers, P.; Rietzschel, E.R.; Chirinos, J.A. How to measure arterial stiffness in humans. Arter. Thromb. Vasc. Biol. 2020, 40, 1034–1043. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [CrossRef]
- Mitchell, G.F. Arterial stiffness and hypertension: Chicken or egg? Hypertension 2014, 64, 210–214. [Google Scholar] [CrossRef]
- Munakata, M. Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: Recent evidence and clinical applications. Curr. Hypertens. Rev. 2014, 10, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Nam, J.S.; Cho, M.H.; Yoo, J.S.; Ahn, C.W.; Jee, S.H.; Lee, H.S.; Cha, B.-S.; Kim, K.R.; Lee, H.C. Insulin resistance independently influences arterial stiffness in normoglycemic normotensive postmenopausal women. Menopause 2010, 17, 779–784. [Google Scholar] [CrossRef]
- Dangardt, F.; Osika, W.; Volkmann, R.; Gan, L.-M.; Friberg, P. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin. Physiol. Funct. Imaging 2008, 28, 287–293. [Google Scholar] [CrossRef]
- Dangardt, F.; Chen, Y.; Berggren, K.; Osika, W.; Friberg, P. Increased rate of arterial stiffening with obesity in adolescents: A five-year follow-up study. PLoS ONE 2013, 8, e57454. [Google Scholar] [CrossRef]
- Lentferink, Y.E.; Kromwijk, L.A.J.; Van Der Aa, M.P.; Knibbe, C.A.J.; Van Der Vorst, M.M.J. Increased arterial stiffness in adolescents with obesity. Glob. Pediatr. Health 2019, 6, 6. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; O’Rourke, M.F.; Safar, M.E.; Baou, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: A systematic review and meta-analysis. Eur. Heart J. 2010, 31, 1865–1871. [Google Scholar] [CrossRef]
- Nichols, W.W.; DeNardo, S.J.; Wilkinson, I.B.; McEniery, C.M.; Cockcroft, J.; O’Rourke, M.F. Effects of arterial stiffness, pulse wave velocity, and wave reflections on the central aortic pressure waveform. J. Clin. Hypertens. 2008, 10, 295–303. [Google Scholar] [CrossRef]
- McEniery, C.M.; Yasmin; Hall, I.; Qasem, A.; Wilkinson, I.B.; Cockcroft, J.R. Normal vascular aging: Differential effects on wave reflection and aortic pulse wave velocity: The Anglo-Cardiff Collaborative Trial (ACCT). J. Am. Coll. Cardiol. 2005, 46, 1753–1760. [Google Scholar] [CrossRef]
- García-Espinosa, V.; Curcio, S.; Marotta, M.; Castro, J.M.; Arana, M.; Peluso, G.; Chiesa, P.; Giachetto, G.; Bia, D.; Zócalo, Y. Changes in central aortic pressure levels, wave components and determinants associated with high peripheral blood pressure states in childhood: Analysis of hypertensive phenotype. Pediatr. Cardiol. 2016, 37, 1340–1350. [Google Scholar] [CrossRef]
- Castro, J.M.; García-Espinosa, V.; Curcio, S.; Arana, M.; Chiesa, P.; Giachetto, G.; Zócalo, Y.; Bia, D. Childhood obesity associates haemodynamic and vascular changes that result in increased central aortic pressure with augmented incident and reflected wave components, without changes in peripheral amplification. Int. J. Vasc. Med. 2016, 2016, 3129304. [Google Scholar] [CrossRef]
- Urbina, E.M.; Kieltkya, L.; Tsai, J.; Srinivasan, S.R.; Berenson, G.S. Impact of multiple cardiovascular risk factors on brachial artery distensibility in young adults: The Bogalusa heart study. Am. J. Hypertens. 2005, 18, 767–771. [Google Scholar] [CrossRef][Green Version]
- Bailey, S.J.; Blackwell, J.R.; Williams, E.; Vanhatalo, A.; Wylie, L.; Winyard, P.G.; Jones, A.M. Two weeks of watermelon juice supplementation improves nitric oxide bioavailability but not endurance exercise performance in humans. Nitric Oxide 2016, 59, 10–20. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-Citrulline and L-Arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Bénazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2007, 99, 855–862. [Google Scholar] [CrossRef]
- Al Jasmi, F.; Al Zaabi, N.; Al-Thihli, K.; Al Teneiji, A.M.; Hertecant, J.; El-Hattab, A.W. Endothelial dysfunction and the effect of arginine and citrulline supplementation in children and adolescents with mitochondrial diseases. J. Central Nerv. Syst. Dis. 2020, 12, 1179573520909377. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Emrick, L.T.; Hsu, J.W.; Chanprasert, S.; Almannai, M.; Craigen, W.J.; Jahoor, F.; Scaglia, F. Impaired nitric oxide production in children with MELAS syndrome and the effect of arginine and citrulline supplementation. Mol. Genet. Metab. 2016, 117, 407–412. [Google Scholar] [CrossRef]
- Moinard, C.; Maccario, J.; Walrand, S.; Lasserre, V.; Marc, J.; Boirie, Y.; Cynober, L. Arginine behaviour after arginine or citrulline administration in older subjects. Br. J. Nutr. 2015, 115, 399–404. [Google Scholar] [CrossRef][Green Version]
- Wijnands, K.A.; Meesters, D.M.; Van Barneveld, K.W.Y.; Visschers, R.G.J.; Briedé, J.J.; Vandendriessche, B.; Van Eijk, H.M.H.; Bessems, B.A.F.M.; Hoven, N.V.D.; Von Wintersdorff, C.J.H.; et al. Citrulline supplementation improves organ perfusion and arginine availability under conditions with enhanced arginase activity. Nutrients 2015, 7, 5217–5238. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Shatanawi, A.; Momani, M.S.; Al-Aqtash, R.; Hamdan, M.H.; Gharaibeh, M.N. L-Citrulline supplementation increases plasma nitric oxide levels and reduces arginase activity in patients with Type 2 Diabetes. Front. Pharmacol. 2020, 11, 584669. [Google Scholar] [CrossRef]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. L-Citrulline supplementation: Impact on cardiometabolic health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef]
- Figueroa, A.; Alvarez-Alvarado, S.; Jaime, S.J.; Kalfon, R. L-Citrulline supplementation attenuates blood pressure, wave reflection and arterial stiffness responses to metaboreflex and cold stress in overweight men. Br. J. Nutr. 2016, 116, 279–285. [Google Scholar] [CrossRef]
- Holguin, F.; Grasemann, H.; Sharma, S.; Winnica, D.; Wasil, K.; Smith, V.; Cruse, M.H.; Perez, N.; Coleman, E.; Scialla, T.J.; et al. L-Citrulline increases nitric oxide and improves control in obese asthmatics. JCI Insight 2019, 4, e131733. [Google Scholar] [CrossRef]
- Gonzales, J.U.; Raymond, A.; Ashley, J.; Kim, Y. Does L-Citrulline supplementation improve exercise blood flow in older adults? Exp. Physiol. 2017, 102, 1661–1671. [Google Scholar] [CrossRef]
- Figueroa, A.; Trivino, J.A.; Sanchez-Gonzalez, M.A.; Vicil, F. Oral L-Citrulline supplementation attenuates blood pressure response to cold pressor test in young men. Am. J. Hypertens. 2010, 23, 12–16. [Google Scholar] [CrossRef]
- Yang, H.-H.; Li, X.-L.; Zhang, W.-G.; Figueroa, A.; Chen, L.-H.; Qin, L.-Q. Effect of oral L-Citrulline on brachial and aortic blood pressure defined by resting status: Evidence from randomized controlled trials. Nutr. Metab. 2019, 16, 89. [Google Scholar] [CrossRef]
- Palombo, C.; Kozakova, M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vasc. Pharmacol. 2016, 77, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Eshreif, A.; Al Batran, R.; Jamieson, K.L.; Darwesh, A.M.; Gopal, K.; Greenwell, A.; Zlobine, I.; Aburasayn, H.; Eaton, F.; Mulvihill, E.E.; et al. L-Citrulline supplementation improves glucose and exercise tolerance in obese male mice. Exp. Physiol. 2020, 105, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Yoshitomi, H.; Momoo, M.; Suguro, S.; Yamagishi, Y.; Gao, M. Evaluation of the effects and mechanism of L-Citrulline on anti-obesity by appetite suppression in obese/diabetic KK-Ay mice and high-fat diet fed SD rats. Biol. Pharm. Bull. 2017, 40, 524–530. [Google Scholar] [CrossRef]
- Moinard, C.; Le Plenier, S.; Noirez, P.; Morio, B.; Bonnefont-Rousselot, D.; Kharchi, C.; Ferry, A.; Neveux, N.; Cynober, L.; Raynaud-Simon, A. Citrulline supplementation induces changes in body composition and limits age-related metabolic changes in healthy male rats. J. Nutr. 2015, 145, 1429–1437. [Google Scholar] [CrossRef]
- Jegatheesan, P.; Beutheu, S.; Ventura, G.; Sarfati, G.; Nubret, E.; Kapel, N.; Waligora-Dupriet, A.-J.; Bergheim, I.; Cynober, L.; De-Bandt, J.-P. Effect of specific amino acids on hepatic lipid metabolism in fructose-induced non-alcoholic fatty liver disease. Clin. Nutr. 2015, 35, 175–182. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, Z.; Yang, Y.; Wang, J.; Satterfield, M.C.; Meininger, C.; Bazer, F.W.; Wu, G. Nitric oxide and energy metabolism in mammals. BioFactors 2013, 39, 383–391. [Google Scholar] [CrossRef]
- Sarti, P.; Arese, M.; Forte, E.; Giuffrè, A.; Mastronicola, D. Mitochondria and Nitric Oxide: Chemistry and pathophysiology. Adv. Exp. Med. Biol. 2011, 942, 75–92. [Google Scholar] [CrossRef]
- Brown, G.C.; Borutaite, V. Nitric oxide and mitochondrial respiration in the heart. Cardiovasc. Res. 2007, 75, 283–290. [Google Scholar] [CrossRef]
- Azizi, S.; Mahdavi, R.; Mobasseri, M.; Aliasgharzadeh, S.; Abbaszadeh, F.; Ebrahimi-Mameghani, M. The impact of L-citrulline supplementation on glucose homeostasis, lipid profile, and some inflammatory factors in overweight and obese patients with type 2 diabetes: A double-blind randomized placebo-controlled trial. Phytotherapy Res. 2021, 35, 3157–3166. [Google Scholar] [CrossRef]
- Terada, T.; Boulé, N.G.; Forhan, M.; Prado, C.M.; Kenny, G.P.; Prud’Homme, D.; Ito, E.; Sigal, R.J. Cardiometabolic risk factors in type 2 diabetes with high fat and low muscle mass: At baseline and in response to exercise. Obesity 2017, 25, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Oh, S.; Park, H.Y.; Jun, J.H.; Kim, H.J. Comparisons of different indices of low muscle mass in relationship with cardiometabolic disorder. Sci. Rep. 2019, 9, 609. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.; Barreira, T.; Broyles, S.T.; Champagne, C.M.; Chaput, J.-P.; Fogelholm, M.; Hu, G.; Johnson, W.; Kuriyan, R.; Kurpad, A.; et al. Physical activity, sedentary time, and obesity in an international sample of children. Med. Sci. Sports Exerc. 2015, 47, 2062–2069. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Leblanc, A.G.; Kho, E.M.; Saunders, T.J.; Larouche, R.; Colley, R.C.; Goldfield, G.; Gorber, S.C. Systematic review of sedentary behaviour and health indicators in school-aged children and youth. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Farah, B.Q.; Christofaro, D.G.D.; Balagopal, P.B.; Cavalcante, B.R.; De Barros, M.V.G.; Ritti-Dias, R.M. Association between resting heart rate and cardiovascular risk factors in adolescents. Eur. J. Nucl. Med. Mol. Imaging 2015, 174, 1621–1628. [Google Scholar] [CrossRef]
- Redwine, K.M.; Acosta, A.A.; Poffenbarger, T.; Portman, R.J.; Samuels, J. Development of hypertension in adolescents with pre-hypertension. J. Pediatr. 2012, 160, 98–103. [Google Scholar] [CrossRef]
- Litwin, M.; Niemirska, A.; Śladowska, J.; Antoniewicz, J.; Daszkowska, J.; Wierzbicka-Rucinska, A.; Wawer, Z.; Grenda, R. Left ventricular hypertrophy and arterial wall thickening in children with essential hypertension. Pediatr. Nephrol. 2006, 21, 811–819. [Google Scholar] [CrossRef]
- Schuler, G.; Adams, V.; Goto, Y. Role of exercise in the prevention of cardiovascular disease: Results, mechanisms, and new perspectives. Eur. Heart J. 2013, 34, 1790–1799. [Google Scholar] [CrossRef]
- García-Hermoso, A.; González-Ruíz, K.; Triana-Reina, H.R.; Olloquequi, J.; Ramírez-Vélez, R. Effects of Exercise on Carotid Arterial Wall Thickness in Obese Pediatric Populations: A meta-analysis of randomized controlled trials. Child. Obes. 2017, 13, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Escalante, Y.; Saavedra, J.M.; García-Hermoso, A.; Domínguez, A.M. Improvement of the lipid profile with exercise in obese children: A systematic review. Prev. Med. 2012, 54, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Murray, T.D.; Eldridge, J.; Squires, J.W.G.; Silvius, P.; Silvius, E.; Squires, W.G. The association between waist circumference and FITNESSGRAM® aerobic capacity classification in sixth-grade children. Pediatr. Exerc. Sci. 2015, 27, 488–493. [Google Scholar] [CrossRef]
- Coutinho, T.; Borlaug, B.A.; Pellikka, P.A.; Turner, S.T.; Kullo, I.J. Sex differences in arterial stiffness and ventricular-arterial interactions. J. Am. Coll. Cardiol. 2015, 61, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Carvalho, L.P.; Marcangeli, V.; Dulac, M.; Hajj Boutros, G.; Gouspillou, G.; Gaudreau, P.; Noirez, P.; Aubertin-Leheudre, M. High intensity interval training combined with L-Citrulline supplementation: Effects on physical performance in healthy older adults. Exp. Gerontol. 2020, 140, 111036. [Google Scholar] [CrossRef]
- Lucotti, P.; Setola, E.; Monti, L.D.; Galluccio, E.; Costa, S.; Sandoli, E.P.; Fermo, I.; Rabaiotti, G.; Gatti, R.; Piatti, P. Beneficial effects of a long-term oral L-Arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E906–E912. [Google Scholar] [CrossRef]
- Buckinx, F.; Gouspillou, G.; Carvalho, L.P.; Marcangeli, V.; Boutros, G.E.H.; Dulac, M.; Noirez, P.; Morais, J.A.; Gaudreau, P.; Aubertin-Leheudre, M. Effect of high-intensity interval training combined with L-Citrulline supplementation on functional capacities and muscle function in Dynapenic-obese older adults. J. Clin. Med. 2018, 7, 561. [Google Scholar] [CrossRef] [PubMed]


| Article | Total Sample | Group | Intervention Group | Exercise Training Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Female | Male | Age (Years) | Body Mass Index (kg/m2) | Training | Intensity | Session (Minutes) | Frequency (Days/Week) | Duration (Weeks) | |||
| [35] | 175 | Exercise | 90 | 55 | 35 | 9.7 (9.5, 9.8) | 25.9 (25.0, 26.9) | Jump rope | HR >140 bpm | 40 | 5 | 32 |
| Control | 85 | 47 | 38 | 9.7 (9.5, 9.9) | 25 (24.4, 26.8) | N/A | N/A | N/A | N/A | N/A | ||
| [32] | 48 | HIIT | 11 | 0 | 11 | 11± 0.3 | 24.2 ± 1.0 | HIIT: 8 × 2 min intervals | 90% PPO | 24 | 3 | 12 |
| Supra-HIIT | 15 | 0 | 15 | 11± 0.2 | 26.5 ± 0.9 | Supra HIIT: 8 × 20 s intervals | 170% PPO | 4 | 3 | 12 | ||
| Control | 11 | 0 | 11 | 10.6 ± 0.3 | 53.6 ± 4.0 | N/A | N/A | N/A | N/A | N/A | ||
| [34] | 118 | Aerobic exercise | 38 | 13 | 25 | 14.4 ± 1.6 | >85th percentile | Walking/jogging | 60–65% VO2 | 40–60 | 3 | 24 |
| [31] | 40 | Exercise | 20 | 20 | 0 | 15 ± 1 | 26 ± 3 | Jump rope | 40–70% HRR | 50 | 5 | 12 |
| Control | 20 | 20 | 0 | 15 ± 1 | 25 ± 2 | N/A | N/A | N/A | N/A | N/A | ||
| [33] | 38 | Exercise | 25 | 13 | 12 | 15.1 ± 1 | 28 ± 3 | HIIT: 2–6 (100 m running sprints) | NP | 10–22 | 3 | 12 |
| Articles | Group | SBP (mmHg) | FMD (%) | cIMT (mm) | PWV (m/s) | SBP (mmHg) | LDL-C (mg/dL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post/ Mean Changes | Baseline | Post/ Mean Changes | Baseline | Post/ Mean Changes | Baseline | Post/ Mean Changes | Baseline | Post/ Mean Changes | Baseline | Post/ Mean Changes | ||
| [35] | Exercise | 105 (103, 107) | 0.1 (−1.5, 1.7) | NM | 105 (103, 107) | 0.1 (−1.5, 1.7) | 5.1 (4.9, 5.3) b | −0.02 (−0.23, 0.2) | 105 (103,107) | 0.1 (−1.5, 1.7) | 102 (95,110) | −6 (−10, −2) | |
| Control | 102 (100, 104) | 0.3 (−1.3, 2) | NM | 102 (100, 104) | 0.3 (−1.3, 2) | 5.1 (4.9, 5.2) b | −0.04 (−0.18, 0.26) | 102 (100,104) | 0.3 (−1.3, 2) | 103 (96,110) | −5 (−9, −1) | ||
| [32] | HIIT | 128 ± 4 | 125 ± 4 | 8.9 | 11.1 *# | 128 ± 4 | 125 ± 4 | 9.97 a | 9.3 *# | 128 ± 4 | 125 ± 4 | 112 ± 6 | 88 ± 6 * |
| Supra HIIT | 127 ± 4 | 116 ± 3 * | 7.9 | 10.1 * | 127 ± 4 | 116 ± 3 * | 9.86 a | 9.06 *# | 127 ± 4 | 116 ± 3 * | 105 ± 6 | 81 ± 5 *# | |
| Control | 121 ± 4 | 121 ± 4 | 8.3 | 7.4 | 121 ± 4 | 121 ± 4 | 10.04 a | 10.2 | 121 ± 4 | 121 ± 4 | 111 ± 7 | 104 ± 6 | |
| [34] | Aerobic exercise | 112 ± 8 | 0.9 ± 1.4 | NM | 112 ± 8 | 0.9 ± 1.4 | 5.97 ± 0.75 b | −0.19 ± 0.14 | 112 ± 8 | 0.9 ± 1.4 | 87 ± 24 | −4 ± 2 | |
| [31] | Exercise | 126 ± 3 | 120 ± 2 *# | NM | 126 ± 3 | 120 ± 2 *# | 8.2 ± 1 a | 7.4 ± 0.2 *,# | 126 ± 3 | 120 ± 2 *# | NM | ||
| Control | 126 ± 4 | 127 ± 5.3 | NM | 126 ± 4 | 127 ± 5.3 | 8.2 ± 0.5 a | 8.1 ± 0.2 | 126 ± 4 | 127 ± 5.3 | NM | |||
| [33] | Exercise | NM | 7.9 | 12.2 * | NM | NM | NM | 90 ± 23 | 99 ± 25 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Ramírez, A.G.; Tovar-Villegas, V.I.; Maharaj, A.; Garay-Sevilla, M.E.; Figueroa, A. Effects of L-Citrulline Supplementation and Aerobic Training on Vascular Function in Individuals with Obesity across the Lifespan. Nutrients 2021, 13, 2991. https://doi.org/10.3390/nu13092991
Flores-Ramírez AG, Tovar-Villegas VI, Maharaj A, Garay-Sevilla ME, Figueroa A. Effects of L-Citrulline Supplementation and Aerobic Training on Vascular Function in Individuals with Obesity across the Lifespan. Nutrients. 2021; 13(9):2991. https://doi.org/10.3390/nu13092991
Chicago/Turabian StyleFlores-Ramírez, Anaisa Genoveva, Verónica Ivette Tovar-Villegas, Arun Maharaj, Ma Eugenia Garay-Sevilla, and Arturo Figueroa. 2021. "Effects of L-Citrulline Supplementation and Aerobic Training on Vascular Function in Individuals with Obesity across the Lifespan" Nutrients 13, no. 9: 2991. https://doi.org/10.3390/nu13092991
APA StyleFlores-Ramírez, A. G., Tovar-Villegas, V. I., Maharaj, A., Garay-Sevilla, M. E., & Figueroa, A. (2021). Effects of L-Citrulline Supplementation and Aerobic Training on Vascular Function in Individuals with Obesity across the Lifespan. Nutrients, 13(9), 2991. https://doi.org/10.3390/nu13092991







