Dietary Bioactive Ingredients Modulating the cAMP Signaling in Diabetes Treatment
Abstract
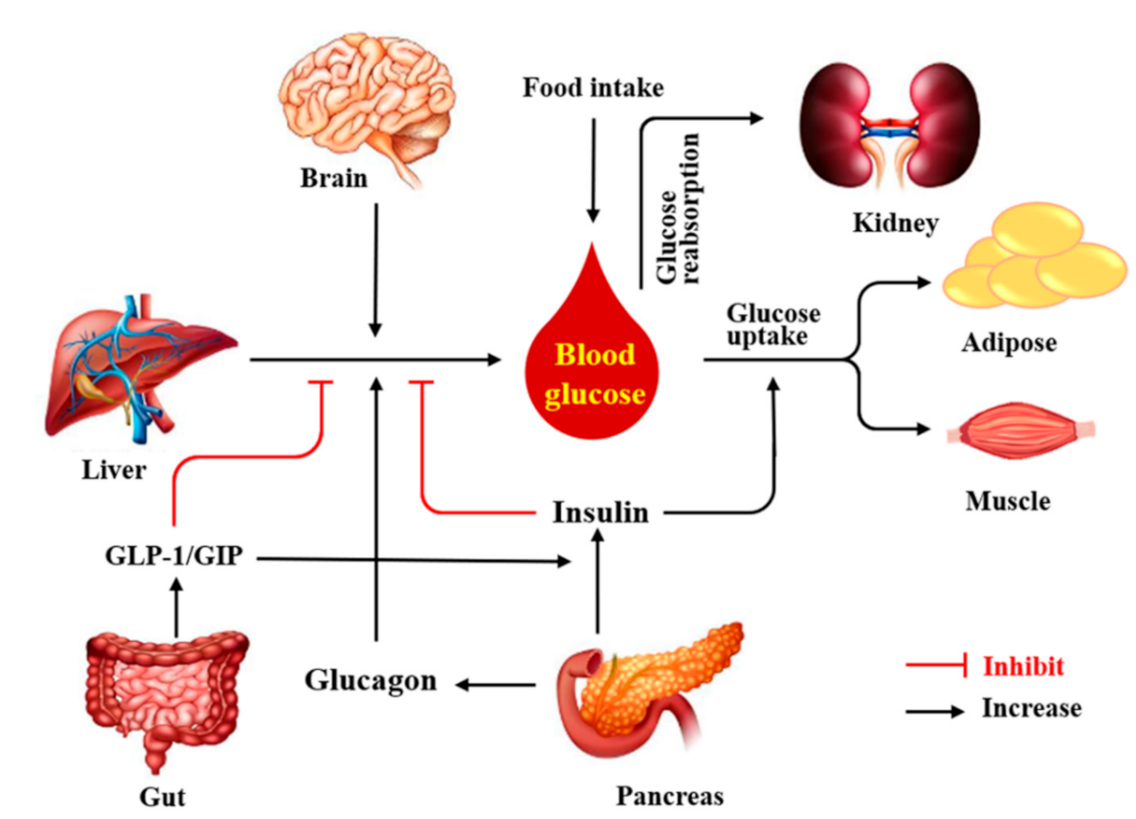
:1. Introduction
2. What Is a cAMP?
3. Pancreas
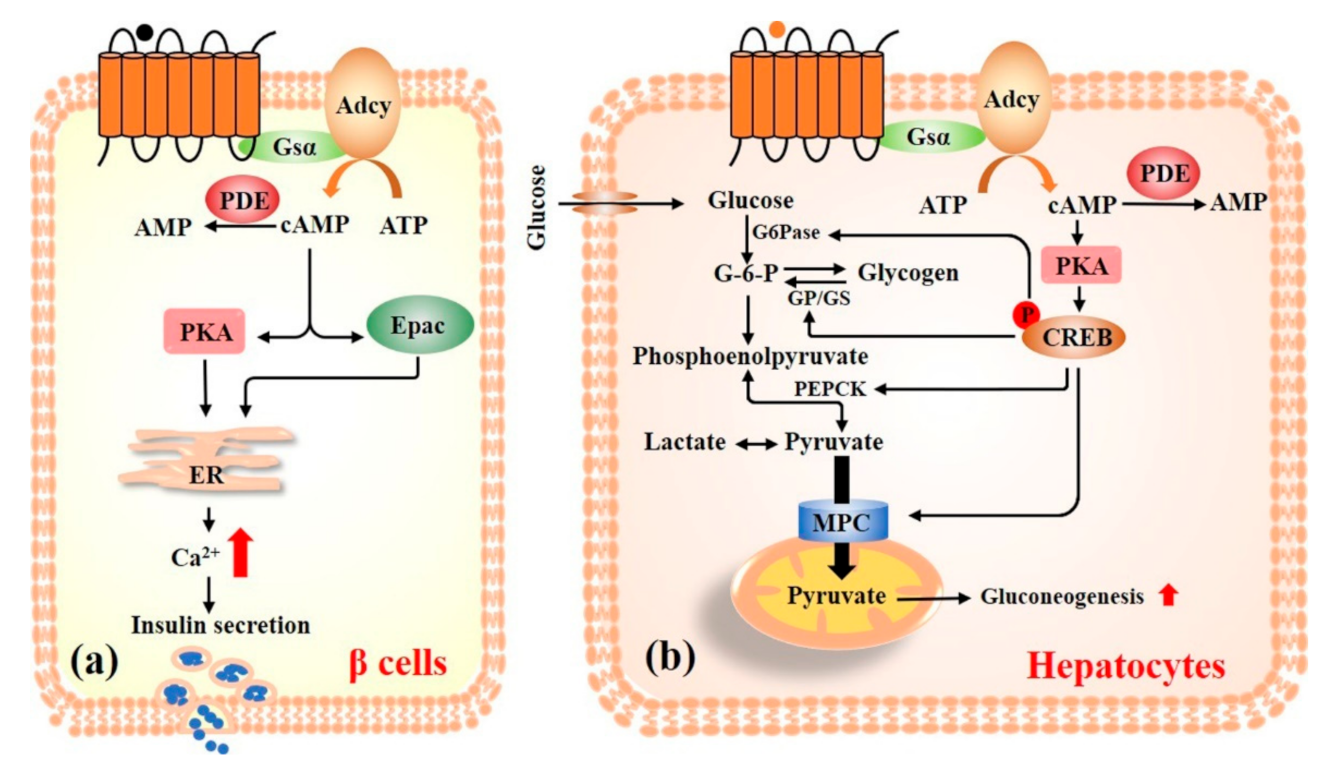
3.1. cAMP Signaling Pathway
3.2. The Dietary Bioactive Ingredients Targeting Pancreas cAMP Signaling for Glucose Homeostasis
4. Liver
4.1. cAMP Signaling Pathway
4.2. The Dietary Bioactive Ingredients Targeting Liver cAMP Signaling for Glucose Homeostasis
5. Gut
5.1. cAMP Signaling Pathway
5.2. The Dietary Bioactive Ingredients Targeting Gut cAMP Signaling for Glucose Homeostasis
6. Skeletal Muscle
6.1. cAMP Signaling Pathway
6.2. The Dietary Bioactive Ingredients Targeting Skeletal Muscle cAMP Signaling for Glucose Homeostasis
7. Adipose Tissue
7.1. cAMP Signaling Pathway
7.2. The Dietary Bioactive Ingredients Targeting Adipose Tissue cAMP Signaling for Glucose Homeostasis
8. Brain
9. Kidney
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Diabetes. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 25 August 2021).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashiesh, H.M.; Meeran, M.F.N.; Sharma, C.; Sadek, B.; Kaabi, J.A.; Ojha, S.K. Therapeutic Potential of β-Caryophyllene: A Dietary Cannabinoid in Diabetes and Associated Complications. Nutrients 2020, 12, 2963. [Google Scholar] [CrossRef]
- Adisakwattana, S. Cinnamic Acid and Its Derivatives: Mechanisms for Prevention and Management of Diabetes and Its Complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Kang, J.; Liu, Q.; Tong, T.; Quan, H. Fighting diabetes mellitus: Pharmacological and non-pharmacological approaches. Curr. Pharm. Des. 2020, 26, 4992–5001. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.; Fang, J.; Geng, R.; Li, M.; Zhao, Y.; Kang, S.G.; Huang, K.; Tong, T. Oleuropein ameliorates advanced stage of type 2 diabetes in db/db mice by regulating gut microbiota. Nutrients 2021, 13, 2131. [Google Scholar] [CrossRef]
- Hughes, J.W.; Ustione, A.; Lavagnino, Z.; Piston, D.W. Regulation of islet glucagon secretion: Beyond calcium. Diabetes Obes. Metab. 2018, 20 (Suppl. S2), 127–136. [Google Scholar] [CrossRef] [Green Version]
- Lei, H.; Preitner, F.; Labouèbe, G.; Thorens, B. Glucose transporter 2 mediates the hypoglycemia-induced increase in cerebral blood flow. J. Cereb. Blood Flow Metab. 2019, 39, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Volta, F.; Scerbo, M.J.; Seelig, A.; Wagner, R.; O’Brien, N.; Gerst, F.; Fritsche, A.; Häring, H.U.; Zeigerer, A.; Ullrich, S.; et al. Glucose homeostasis is regulated by pancreatic β-cell cilia via endosomal EphA-processing. Nat. Commun. 2019, 10, 5686. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.X.; Gong, J.; Chen, Z.; Sun, J.; Xiao, Y.; Wang, L.; Li, Y.; Liu, J.; Xu, X.Z.S.; Lin, J.D. Glucose sensing by skeletal myocytes couples nutrient signaling to systemic homeostasis. Mol. Cell 2017, 66, 332–344.e334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierre, S.; Eschenhagen, T.; Geisslinger, G.; Scholich, K. Capturing adenylyl cyclases as potential drug targets. Nat. Rev. Drug Discov. 2009, 8, 321–335. [Google Scholar] [CrossRef]
- Tong, T.; Ryu, S.E.; Min, Y.; de March, C.A.; Bushdid, C.; Golebiowski, J.; Moon, C.; Park, T. Olfactory receptor 10J5 responding to α-cedrene regulates hepatic steatosis via the cAMP-PKA pathway. Sci. Rep. 2017, 7, 9471. [Google Scholar] [CrossRef] [Green Version]
- Tong, T.; Park, J.; Moon, C.; Park, T. Regulation of adipogenesis and thermogenesis through mouse olfactory receptor 23 stimulated by alpha-cedrene in 3T3-L1 cells. Nutrients 2018, 10, 1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, T.; Shen, Y.; Lee, H.W.; Yu, R.; Park, T. Adenylyl cyclase 3 haploinsufficiency confers susceptibility to diet-induced obesity and insulin resistance in mice. Sci. Rep. 2016, 6, 34179. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Huang, S.; Wu, X.; Feng, Y.; Shen, Y.; Zhao, Q.S.; Leng, Y. Activation of SIK1 by phanginin A inhibits hepatic gluconeogenesis by increasing PDE4 activity and suppressing the cAMP signaling pathway. Mol. Metab. 2020, 41, 101045. [Google Scholar] [CrossRef]
- Vollert, S.; Kaessner, N.; Heuser, A.; Hanauer, G.; Dieckmann, A.; Knaack, D.; Kley, H.P.; Beume, R.; Weiss-Haljiti, C. The glucose-lowering effects of the PDE4 inhibitors roflumilast and roflumilast-N-oxide in db/db mice. Diabetologia 2012, 55, 2779–2788. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Chu, Q.; Xie, J.; Foretz, M.; Viollet, B.; Birnbaum, M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013, 494, 256–260. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhen, W.; Yang, Z.; Carter, J.D.; Si, H.; Reynolds, K.A. Genistein acutely stimulates insulin secretion in pancreatic beta-cells through a cAMP-dependent protein kinase pathway. Diabetes 2006, 55, 1043–1050. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, D.; She, L.; Zhang, Y.; Wei, Q.; Aa, J.; Wang, G.; Liu, B.; Xie, Y. Curcumin restrains hepatic glucose production by blocking cAMP/PKA signaling and reducing acetyl CoA accumulation in high-fat diet (HFD)-fed mice. Mol. Cell. Endocrinol. 2018, 474, 127–136. [Google Scholar] [CrossRef]
- Berthet, J.; Rall, T.W.; Sutherland, E.W. The relationship of epinephrine and glucagon to liver phosphorylase. IV. Effect of epinephrine and glucagon on the reactivation of phosphorylase in liver homogenates. J. Biol. Chem. 1957, 224, 463–475. [Google Scholar]
- Pons, J.; Kitlinska, J.; Jacques, D.; Perreault, C.; Nader, M.; Everhart, L.; Zhang, Y.; Zukowska, Z. Interactions of multiple signaling pathways in neuropeptide Y-mediated bimodal vascular smooth muscle cell growth. Can. J. Physiol. Pharmacol. 2008, 86, 438–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wei, Y.; Zheng, F.; Guan, Y.; Zhang, X. Prostaglandin E2 in the regulation of water transport in renal collecting ducts. Int. J. Mol. Sci. 2017, 18, 2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Ye, Y.; Feng, Y.; Xu, T.; Huang, S.; Shen, J.; Leng, Y. Linderane suppresses hepatic gluconeogenesis by inhibiting the cAMP/PKA/CREB pathway through indirect activation of PDE 3 via ERK/STAT3. Front. Pharmacol. 2018, 9, 476. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, H.; Springett, G.M.; Mochizuki, N.; Toki, S.; Nakaya, M.; Matsuda, M.; Housman, D.E.; Graybiel, A.M. A family of cAMP-binding proteins that directly activate Rap1. Science 1998, 282, 2275–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.J.; Kim, D.H.; Lee, J.Y.; Lee, B.C.; Kang, I.; Kook, M.G.; Kong, D.; Choi, S.W.; Woo, H.M.; Kim, D.I.; et al. cAMP/EPAC signaling enables ETV2 to induce endothelial cells with high angiogenesis potential. Mol. Ther. 2020, 28, 466–478. [Google Scholar] [CrossRef]
- Jeevaratnam, K.; Salvage, S.C.; Li, M.; Huang, C.L. Regulatory actions of 3’,5’-cyclic adenosine monophosphate on osteoclast function: Possible roles of Epac-mediated signaling. Ann. N. Y. Acad. Sci. 2018, 1433, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokman, G.; Qin, Y.; Booij, T.H.; Ramaiahgari, S.; Lacombe, M.; Dolman, M.E.; van Dorenmalen, K.M.; Teske, G.J.; Florquin, S.; Schwede, F.; et al. Epac-Rap signaling reduces oxidative stress in the tubular epithelium. J. Am. Soc. Nephrol. 2014, 25, 1474–1485. [Google Scholar] [CrossRef]
- Hagren, O.I.; Tengholm, A. Glucose and insulin synergistically activate phosphatidylinositol 3-kinase to trigger oscillations of phosphatidylinositol 3,4,5-trisphosphate in beta-cells. J. Biol. Chem. 2006, 281, 39121–39127. [Google Scholar] [CrossRef] [Green Version]
- Grill, V.; Cerasi, E. Activation by glucose of adenyl cyclase in pancreatic islets of the rat. FEBS Lett. 1973, 33, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Hellman, B.; Idahl, L.A.; Lernmark, A.; Täljedal, I.B. The pancreatic beta-cell recognition of insulin secretagogues: Does cyclic AMP mediate the effect of glucose? Proc. Natl. Acad. Sci. USA 1974, 71, 3405–3409. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Nagasawa, M.; Medina, J.; Kojima, I. Glucose evokes rapid Ca2+ and cyclic AMP signals by activating the cell-surface glucose-sensing receptor in pancreatic β-cells. PLoS ONE 2015, 10, e0144053. [Google Scholar]
- Tian, G.; Sol, E.R.; Xu, Y.; Shuai, H.; Tengholm, A. Impaired cAMP generation contributes to defective glucose-stimulated insulin secretion after long-term exposure to palmitate. Diabetes 2015, 64, 904–915. [Google Scholar] [CrossRef] [Green Version]
- Dyachok, O.; Idevall-Hagren, O.; Sågetorp, J.; Tian, G.; Wuttke, A.; Arrieumerlou, C.; Akusjärvi, G.; Gylfe, E.; Tengholm, A. Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metab. 2008, 8, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lavoie, G.; Méant, A.; Aubert, L.; Cargnello, M.; Haman, A.; Hoang, T.; Roux, P.P. Extracellular signal-regulated kinases 1 and 2 phosphorylate Gab2 to promote a negative-feedback loop that attenuates phosphoinositide 3-kinase/Akt signaling. Mol. Cell. Biol. 2017, 37, e00357-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Z.; Zhang, W.; Zhen, W.; Lum, H.; Nadler, J.; Bassaganya-Riera, J.; Jia, Z.; Wang, Y.; Misra, H.; Liu, D. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology 2010, 151, 3026–3037. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Shuai, H.; Ahooghalandari, P.; Gylfe, E.; Tengholm, A. Glucose controls glucagon secretion by directly modulating cAMP in alpha cells. Diabetologia 2019, 62, 1212–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, H.; Gylfe, E.; Hellman, B. Cyclic AMP raises cytoplasmic calcium in pancreatic alpha 2-cells by mobilizing calcium incorporated in response to glucose. Cell Calcium 1989, 10, 205–211. [Google Scholar] [CrossRef]
- Johansson, H.; Gylfe, E.; Hellman, B. The actions of arginine and glucose on glucagon secretion are mediated by opposite effects on cytoplasmic Ca2+. Biochem. Biophys. Res. Commun. 1987, 147, 309–314. [Google Scholar] [CrossRef]
- Gromada, J.; Bokvist, K.; Ding, W.G.; Barg, S.; Buschard, K.; Renström, E.; Rorsman, P. Adrenaline stimulates glucagon secretion in pancreatic A-cells by increasing the Ca2+ current and the number of granules close to the L-type Ca2+ channels. J. Gen. Physiol. 1997, 110, 217–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.J.; Vieira, E.; Gylfe, E. A store-operated mechanism determines the activity of the electrically excitable glucagon-secreting pancreatic alpha-cell. Cell Calcium 2004, 35, 357–365. [Google Scholar] [CrossRef]
- De Marinis, Y.Z.; Salehi, A.; Ward, C.E.; Zhang, Q.; Abdulkader, F.; Bengtsson, M.; Braha, O.; Braun, M.; Ramracheya, R.; Amisten, S.; et al. GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis. Cell Metab. 2010, 11, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Rouse, M.; Younès, A.; Egan, J.M. Resveratrol and curcumin enhance pancreatic β-cell function by inhibiting phosphodiesterase activity. J. Endocrinol. 2014, 223, 107–117. [Google Scholar] [CrossRef]
- Brouwer, S.; Hoffmeister, T.; Gresch, A.; Schönhoff, L.; Düfer, M. Resveratrol influences pancreatic islets by opposing effects on electrical activity and insulin release. Mol. Nutr. Food. Res. 2018, 62, 1700902. [Google Scholar] [CrossRef]
- Samad, M.B.; Mohsin, M.; Razu, B.A.; Hossain, M.T.; Mahzabeen, S.; Unnoor, N.; Muna, I.A.; Akhter, F.; Kabir, A.U.; Hannan, J.M.A. [6]-Gingerol, from Zingiber officinale, potentiates GLP-1 mediated glucose-stimulated insulin secretion pathway in pancreatic β-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Lepr(db/db) type 2 diabetic mice. BMC Complement. Altern. Med. 2017, 17, 395. [Google Scholar]
- Ohno, T.; Kato, N.; Ishii, C.; Shimizu, M.; Ito, Y.; Tomono, S.; Kawazu, S. Genistein augments cyclic adenosine 3’5’-monophosphate(cAMP) accumulation and insulin release in MIN6 cells. Endocr. Res. 1993, 19, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, S.; Bao, B.; Li, X.; Wang, S. Structure characterization of an arabinogalactan from green tea and its anti-diabetic effect. Carbohydr. Polym. 2015, 124, 98–108. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, J.; Ma, Z.; Zhang, H.; Xie, F. Effects of fucoidan on insulin stimulation and pancreatic protection via the cAMP signaling pathway in vivo and in vitro. Mol. Med. Rep. 2015, 12, 4501–4507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.P.; Rajagopal, S.; Mahavadi, S.; Mirshahi, F.; Grider, J.R.; Murthy, K.S.; Sanyal, A.J. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells. Biochem. Biophys. Res. Commun. 2012, 427, 600–605. [Google Scholar] [CrossRef] [Green Version]
- Hameed, A.; Hafizur, R.M.; Khan, M.I.; Jawed, A.; Wang, H.; Zhao, M.; Matsunaga, K.; Izumi, T.; Siddiqui, S.; Khan, F.; et al. Coixol amplifies glucose-stimulated insulin secretion via cAMP mediated signaling pathway. Eur. J. Pharmacol. 2019, 858, 172514. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, S.; Lin, Y.; Xu, W.; Ye, D.; Xiong, Y.; Zhao, S.; Guan, K.L. Acetylation negatively regulates glycogen phosphorylase by recruiting protein phosphatase 1. Cell Metab. 2012, 15, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Quinn, P.G. Inhibition by insulin of protein kinase A-induced transcription of the phosphoenolpyruvate carboxykinase gene. Mediation by the activation domain of cAMP response element-binding protein (CREB) and factors bound to the TATA box. J. Biol. Chem. 1994, 269, 14375–14378. [Google Scholar] [CrossRef]
- Leahy, P.; Crawford, D.R.; Grossman, G.; Gronostajski, R.M.; Hanson, R.W. CREB binding protein coordinates the function of multiple transcription factors including nuclear factor I to regulate phosphoenolpyruvate carboxykinase (GTP) gene transcription. J. Biol. Chem. 1999, 274, 8813–8822. [Google Scholar] [CrossRef] [Green Version]
- Streeper, R.S.; Hornbuckle, L.A.; Svitek, C.A.; Goldman, J.K.; Oeser, J.K.; O’Brien, R.M. Protein kinase A phosphorylates hepatocyte nuclear factor-6 and stimulates glucose-6-phosphatase catalytic subunit gene transcription. J. Biol. Chem. 2001, 276, 19111–19118. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–890S. [Google Scholar] [CrossRef] [Green Version]
- Johanns, M.; Lai, Y.C.; Hsu, M.F.; Jacobs, R.; Vertommen, D.; Van Sande, J.; Dumont, J.E.; Woods, A.; Carling, D.; Hue, L.; et al. AMPK antagonizes hepatic glucagon-stimulated cyclic AMP signalling via phosphorylation-induced activation of cyclic nucleotide phosphodiesterase 4B. Nat. Commun. 2016, 7, 10856. [Google Scholar] [CrossRef]
- He, L.; Sabet, A.; Djedjos, S.; Miller, R.; Sun, X.; Hussain, M.A.; Radovick, S.; Wondisford, F.E. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 2009, 137, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Zhao, A.Z.; Shinohara, M.M.; Huang, D.; Shimizu, M.; Eldar-Finkelman, H.; Krebs, E.G.; Beavo, J.A.; Bornfeldt, K.E. Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J. Biol. Chem. 2000, 275, 11348–11354. [Google Scholar] [CrossRef] [Green Version]
- Xiao, N.; Lou, M.D.; Lu, Y.T.; Yang, L.L.; Liu, Q.; Liu, B.; Qi, L.W.; Li, P. Ginsenoside Rg5 attenuates hepatic glucagon response via suppression of succinate-associated HIF-1α induction in HFD-fed mice. Diabetologia 2017, 60, 1084–1093. [Google Scholar] [CrossRef] [Green Version]
- Du, Q.; Zhang, S.; Li, A.; Mohammad, I.S.; Liu, B.; Li, Y. Astragaloside IV inhibits adipose lipolysis and reduces hepatic glucose production via Akt dependent PDE3B expression in HFD-fed mice. Front. Physiol. 2018, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, Y.; Xu, L.; Tang, D.; Dorfman, R.G.; Zhou, Q.; Yin, Y.; Li, Y.; Zhou, L.; Zhao, S.; et al. Berberine promotes glucose uptake and inhibits gluconeogenesis by inhibiting deacetylase SIRT3. Endocrine 2018, 62, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Lou, M.D.; Li, J.; Cheng, Y.; Xiao, N.; Ma, G.; Li, P.; Liu, B.; Liu, Q.; Qi, L.W. Glucagon up-regulates hepatic mitochondrial pyruvate carrier 1 through cAMP-responsive element-binding protein; inhibition of hepatic gluconeogenesis by ginsenoside Rb1. Br. J. Pharmacol. 2019, 176, 2962–2976. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Zhang, Y.; Liu, X.; Wu, Z.; Gilbert, R.G.; Deng, B.; Wang, K. Dendrobium officinale polysaccharide ameliorates diabetic hepatic glucose metabolism via glucagon-mediated signaling pathways and modifying liver-glycogen structure. J. Ethnopharmacol. 2020, 248, 112308. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Shen, J.Z.; Pan, Q.; Yang, W.; Yan, H.; Liu, H.; Ai, W.; Liao, W.; Guo, S. Epigallocatechin gallate inhibits hepatic glucose production in primary hepatocytes via downregulating PKA signaling pathways and transcriptional factor FoxO1. J. Agric. Food Chem. 2019, 67, 3651–3661. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.; Tao, S.; Wei, G.; Huang, Y.; Chen, D.; Wu, C. Purification, characterization and biological activity of polysaccharides from Dendrobium officinale. Molecules 2016, 21, 701. [Google Scholar] [CrossRef]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef] [Green Version]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Fujii, Y.; Osaki, N.; Hase, T.; Shimotoyodome, A. Ingestion of coffee polyphenols increases postprandial release of the active glucagon-like peptide-1 (GLP-1(7-36)) amide in C57BL/6J mice. J. Nutr. Sci. 2015, 4, e9. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S.; Lee, I.S.; Kim, K.H.; Park, J.; Kim, Y.; Choi, J.H.; Choi, J.S.; Jang, H.J. Activation of intestinal olfactory receptor stimulates glucagon-like peptide-1 secretion in enteroendocrine cells and attenuates hyperglycemia in type 2 diabetic mice. Sci. Rep. 2017, 7, 13978. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, J.; Teuber, S.; Morera, F.J.; Ojeda, C.; Flores, C.A.; Hidalgo, M.A.; Núñez, L.; Villalobos, C.; Burgos, R.A. Delphinidin reduces glucose uptake in mice jejunal tissue andhHuman intestinal cells lines through FFA1/GPR40. Int. J. Mol. Sci. 2017, 18, 750. [Google Scholar] [CrossRef] [Green Version]
- Klinger, S.; Breves, G. Resveratrol inhibits porcine intestinal glucose and alanine transport: Potential roles of Na⁺/K⁺-ATPase activity, protein kinase A, AMP-activated protein kinase and the association of selected nutrient transport proteins with detergent resistant membranes. Nutrients 2018, 10, 302. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.Z.; Yang, G.; Jiang, X.M.; Qin, D.Y.; Li, Q.M.; Zha, X.Q.; Pan, L.H.; Jin, C.S.; Luo, J.P. Polygonatum cyrtonema Hua polysaccharide promotes GLP-1 secretion from enteroendocrine L-cells through sweet taste receptor-mediated cAMP signaling. J. Agric. Food. Chem. 2020, 68, 6864–6872. [Google Scholar] [CrossRef]
- Cui, H.X.; Zhang, L.S.; Luo, Y.; Yuan, K.; Huang, Z.Y.; Guo, Y. A purified anthraquinone-glycoside preparation from rhubarb ameliorates type 2 diabetes mellitus by modulating the gut microbiota and reducing inflammation. Front. Microbiol. 2019, 10, 1423. [Google Scholar] [CrossRef]
- Bala, V.; Rajagopal, S.; Kumar, D.P.; Nalli, A.D.; Mahavadi, S.; Sanyal, A.J.; Grider, J.R.; Murthy, K.S. Release of GLP-1 and PYY in response to the activation of G protein-coupled bile acid receptor TGR5 is mediated by Epac/Epac/PLC-ε pathway and modulated by endogenous H2S. Front. Physiol. 2014, 5, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.F.; Wang, J.T.; Zhang, L.X.; Xing, S.F.; Wang, Y.X.; Wang, K.; Deng, S.L.; Zhang, J.Q.; Tang, L.; Wu, H.S. Oleanolic acid derivative DKS26 exerts antidiabetic and hepatoprotective effects in diabetic mice and promotes glucagon-like peptide-1 secretion and expression in intestinal cells. Br. J. Pharmacol. 2017, 174, 2912–2928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.E.; Kang, C.W.; Oh, J.H.; Park, S.H.; Ku, C.R.; Cho, Y.H.; Lee, M.K.; Lee, E.J. Olfactory receptor OR51E1 mediates GLP-1 secretion in human and rodent enteroendocrine L cells. J. Endocr. Soc. 2018, 2, 1251–1258. [Google Scholar] [CrossRef]
- Mączka, W.; Wińska, K.; Grabarczyk, M. One hundred faces of geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Ren, J.M.; Marshall, B.A.; Gulve, E.A.; Gao, J.; Johnson, D.W.; Holloszy, J.O.; Mueckler, M. Evidence from transgenic mice that glucose transport is rate-limiting for glycogen deposition and glycolysis in skeletal muscle. J. Biol. Chem. 1993, 268, 16113–16115. [Google Scholar] [CrossRef]
- Molina, S.A.; Moriarty, H.K.; Infield, D.T.; Imhoff, B.R.; Vance, R.J.; Kim, A.H.; Hansen, J.M.; Hunt, W.R.; Koval, M.; McCarty, N.A. Insulin signaling via the PI3-kinase/Akt pathway regulates airway glucose uptake and barrier function in a CFTR-dependent manner. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L688–L702. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Dehvari, N.; Oberg, A.I.; Dallner, O.S.; Sandström, A.L.; Olsen, J.M.; Csikasz, R.I.; Summers, R.J.; Hutchinson, D.S.; Bengtsson, T. Improving type 2 diabetes through a distinct adrenergic signaling pathway involving mTORC2 that mediates glucose uptake in skeletal muscle. Diabetes 2014, 63, 4115–4129. [Google Scholar] [CrossRef] [Green Version]
- Mukaida, S.; Sato, M.; Öberg, A.I.; Dehvari, N.; Olsen, J.M.; Kocan, M.; Halls, M.L.; Merlin, J.; Sandström, A.L.; Csikasz, R.I.; et al. BRL37344 stimulates GLUT4 translocation and glucose uptake in skeletal muscle via β2-adrenoceptors without causing classical receptor desensitization. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R666–R677. [Google Scholar] [CrossRef]
- Jensen, J.; Rustad, P.I.; Kolnes, A.J.; Lai, Y.C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front. Physiol. 2011, 2, 112. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Yang, L. Targeting cAMP/PKA pathway for glycemic control and type 2 diabetes therapy. J. Mol. Endocrinol. 2016, 57, R93–R108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldfarb, A.H.; Bruno, J.F.; Buckenmeyer, P.J. Intensity and duration of exercise effects on skeletal muscle cAMP, phosphorylase, and glycogen. J. Appl. Physiol. 1989, 66, 190–194. [Google Scholar] [CrossRef]
- Cheong, S.H.; Furuhashi, K.; Ito, K.; Nagaoka, M.; Yonezawa, T.; Miura, Y.; Yagasaki, K. Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and improves glucose homeostasis in Type 2 diabetic model mice. J. Nutr. Biochem. 2014, 25, 136–143. [Google Scholar] [CrossRef]
- Matsukawa, T.; Motojima, H.; Sato, Y.; Takahashi, S.; Villareal, M.O.; Isoda, H. Upregulation of skeletal muscle PGC-1α through the elevation of cyclic AMP levels by Cyanidin-3-glucoside enhances exercise performance. Sci. Rep. 2017, 7, 44799. [Google Scholar] [CrossRef] [Green Version]
- Kang, W.; Zhang, K.; Tong, T.; Park, T. Improved glucose intolerance through a distinct mouse olfactory receptor 23-induced signaling pathway mediating glucose uptake in myotubes and adipocytes. Mol. Nutr. Food Res. 2020, 11, e1901329. [Google Scholar] [CrossRef]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Kim, C.H.; Hoang, D.M.; Kim, B.Y.; Sohn, C.B.; Kim, M.R.; Ahn, J.S. Genistein-derivatives from Tetracera scandens stimulate glucose-uptake in L6 myotubes. Biol. Pharm. Bull. 2009, 32, 504–508. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.H.; Gerhart-Hines, Z.; Dominy, J.E.; Lee, Y.; Kim, S.; Tabata, M.; Xiang, Y.K.; Puigserver, P. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1α complex. J. Biol. Chem. 2013, 288, 7117–7126. [Google Scholar] [CrossRef] [Green Version]
- Tardif, N.; Salles, J.; Landrier, J.F.; Mothe-Satney, I.; Guillet, C.; Boue-Vaysse, C.; Combaret, L.; Giraudet, C.; Patrac, V.; Bertrand-Michel, J.; et al. Oleate-enriched diet improves insulin sensitivity and restores muscle protein synthesis in old rats. Clin. Nutr. 2011, 30, 799–806. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health benefits and molecular mechanisms of resveratrol: A narrative review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, J.M.; Sato, M.; Dallner, O.S.; Sandström, A.L.; Pisani, D.F.; Chambard, J.C.; Amri, E.Z.; Hutchinson, D.S.; Bengtsson, T. Glucose uptake in brown fat cells is dependent on mTOR complex 2-promoted GLUT1 translocation. J. Cell. Biol. 2014, 207, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Pagnon, J.; Matzaris, M.; Stark, R.; Meex, R.C.; Macaulay, S.L.; Brown, W.; O’Brien, P.E.; Tiganis, T.; Watt, M.J. Identification and functional characterization of protein kinase A phosphorylation sites in the major lipolytic protein, adipose triglyceride lipase. Endocrinology 2012, 153, 4278–4289. [Google Scholar] [CrossRef]
- Krintel, C.; Osmark, P.; Larsen, M.R.; Resjö, S.; Logan, D.T.; Holm, C. Ser649 and Ser650 are the major determinants of protein kinase A-mediated activation of human hormone-sensitive lipase against lipid substrates. PLoS ONE 2008, 3, e3756. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, H.; Perfield, J.W., 2nd; Souza, S.C.; Shen, W.J.; Zhang, H.H.; Stancheva, Z.S.; Kraemer, F.B.; Obin, M.S.; Greenberg, A.S. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J. Biol. Chem. 2007, 282, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; He, J.; Xu, C.; Zu, L.; Jiang, H.; Pu, S.; Guo, X.; Xu, G. Mechanisms of metformin inhibiting lipolytic response to isoproterenol in primary rat adipocytes. J. Mol. Endocrinol. 2009, 42, 57–66. [Google Scholar] [CrossRef]
- Zhao, W.; Li, A.; Feng, X.; Hou, T.; Liu, K.; Liu, B.; Zhang, N. Metformin and resveratrol ameliorate muscle insulin resistance through preventing lipolysis and inflammation in hypoxic adipose tissue. Cell. Signal. 2016, 28, 1401–1411. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chang, W.L.; Huang, S.F.; Lin, C.Y.; Lin, H.C.; Chang, T.C. Pachymic acid stimulates glucose uptake through enhanced GLUT4 expression and translocation. Eur. J. Pharmacol. 2010, 648, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, B.; Huang, F.; Liu, B.; Xie, Y. Curcumin inhibits lipolysis via suppression of ER stress in adipose tissue and prevents hepatic insulin resistance. J. Lipid Res. 2016, 57, 1243–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, N.; Yang, L.L.; Yang, Y.L.; Liu, L.W.; Li, J.; Liu, B.; Liu, K.; Qi, L.W.; Li, P. Ginsenoside Rg5 inhibits succinate-associated lipolysis in adipose tissue and prevents muscle insulin resistance. Front. Pharmacol. 2017, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.; Narayan, V.P.; Sung, M.K.; Park, T. Piperonal attenuates visceral adiposity in mice fed a high-fat diet: Potential involvement of the adenylate cyclase-protein kinase A dependent pathway. Mol. Nutr. Food. Res. 2017, 61, 1601124. [Google Scholar] [CrossRef]
- Meriga, B.; Parim, B.; Chunduri, V.R.; Naik, R.R.; Nemani, H.; Suresh, P.; Ganapathy, S.; Sathibabu Uddandrao, V.V. Antiobesity potential of Piperonal: Promising modulation of body composition, lipid profiles and obesogenic marker expression in HFD-induced obese rats. Nutr. Metab. 2017, 14, 72. [Google Scholar] [CrossRef]
- Moon, Y.; Tong, T.; Kang, W.; Park, T. Filbertone ameliorates adiposity in mice fed a high-fat diet via activation of cAMP signaling. Nutrients 2019, 11, 1749. [Google Scholar] [CrossRef] [Green Version]
- Locher, K.P. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat. Struct. Mol. Biol. 2016, 23, 487–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, J.M.; Schwartz, M.W. Control of hepatic glucose metabolism by islet and brain. Diabetes Obes. Metab. 2014, 16 (Suppl. S1), 33–40. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.W.; Seeley, R.J.; Tschöp, M.H.; Woods, S.C.; Morton, G.J.; Myers, M.G.; D’Alessio, D. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 2013, 503, 59–66. [Google Scholar] [CrossRef]
- Grayson, B.E.; Seeley, R.J.; Sandoval, D.A. Wired on sugar: The role of the CNS in the regulation of glucose homeostasis. Nat. Rev. Neurosci. 2013, 14, 24–37. [Google Scholar] [CrossRef]
- Yang, L. Neuronal cAMP/PKA Signaling and Energy Homeostasis. Adv. Exp. Med. Biol. 2018, 1090, 31–48. [Google Scholar]
- Morton, G.J.; Schwartz, M.W. Leptin and the central nervous system control of glucose metabolism. Physiol. Rev. 2011, 91, 389–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mighiu, P.I.; Yue, J.T.; Filippi, B.M.; Abraham, M.A.; Chari, M.; Lam, C.K.; Yang, C.S.; Christian, N.R.; Charron, M.J.; Lam, T.K. Hypothalamic glucagon signaling inhibits hepatic glucose production. Nat. Med. 2013, 19, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Wu, P.F.; Long, L.H.; Chen, Y.; Hu, Z.L.; Ni, L.; Wang, F.; Chen, J.G. Resveratrol promotes cellular glucose utilization in primary cultured cortical neurons via calcium-dependent signaling pathway. J. Nutr. Biochem. 2013, 24, 629–637. [Google Scholar] [CrossRef]
- Wilding, J.P. The role of the kidneys in glucose homeostasis in type 2 diabetes: Clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metabolism 2014, 63, 1228–1237. [Google Scholar] [CrossRef]
- Gerich, J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: Therapeutic implications. Diabet. Med. 2010, 27, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landau, B.R.; Wahren, J.; Chandramouli, V.; Schumann, W.C.; Ekberg, K.; Kalhan, S.C. Contributions of gluconeogenesis to glucose production in the fasted state. J. Clin. Investig. 1996, 98, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Sunilkumar, S.; Ford, S.M. Elevated glucose concentration in culture media decreases membrane trafficking of SGLT2 in LLC-PK(1) cells via a cAMP/PKA-dependent pathway. Am. J. Physiol. Cell Physiol. 2019, 316, C913–C924. [Google Scholar] [CrossRef]
- Aschenbach, J.R.; Borau, T.; Gäbel, G. Glucose uptake via SGLT-1 is stimulated by beta(2)-adrenoceptors in the ruminal epithelium of sheep. J. Nutr. 2002, 132, 1254–1257. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, Y.; Eguchi, T.; Ishida, H. Mechanism of beta-adrenergic agonist-induced transmural transport of glucose in rat small intestine. Regulation of phosphorylation of SGLT1 controls the function. Biochim. Biophys. Acta 1997, 1357, 306–318. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Takeo, J.; Aoyama, C.; Kawahara, H. Na+-glucose cotransporter (SGLT) inhibitory flavonoids from the roots of Sophora flavescens. Bioorg. Med. Chem. 2007, 15, 3445–3449. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J.; Hao, J.; Lv, Y.; Chen, L.; Lin, Q.; Yuan, J.; Yang, X. A novel flavonoid kushenol Z from sophora flavescens mediates mTOR pathway by Inhibiting phosphodiesterase and Akt activity to induce apoptosis in non-small-cell lung cancer cells. Molecules 2019, 24, 4425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, R.; Xiao, Z.T.; Zheng, Y.J.; Zhang, Y.L.; Xu, J.W.; Huang, J.H.; Zhou, W.L.; Li, P.B.; Su, W.W. Naringenin regulates CFTR activation and expression in airway epithelial cells. Cell. Physiol. Biochem. 2017, 44, 1146–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhang, Y.; Sun, Y.; Zhang, G.; Bai, J.; Guo, J.; Su, X.; Du, H.; Cao, X.; Yang, J.; et al. Naringenin improves insulin sensitivity in gestational diabetes mellitus mice through AMPK. Nutr. Diabetes 2019, 9, 28. [Google Scholar]
- Blaschek, W. Natural products as lead compounds for sodium glucose cotransporter (SGLT) inhibitors. Planta Med. 2017, 83, 985–993. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Main Source of Food | Concentration of cAMP | Main Effect | Mechanism of Action | Model | Refs. |
|---|---|---|---|---|---|---|
| Resveratrol | Peanut; grape (red wine); mulberry | • ↑ cAMP | • ↓ Blood glucose • ↑ Insulin sensitivity | • Inhibition of PDE activity | • MIN6 cells • HP62 cells | [43] |
| • ↑ cAMP | • ↑ Insulin secretion | • Activation of cAMP/Epac1signaling pathway | • C57BL/6N mice | [44] | ||
| [6]-Gingerol | Ginger | • ↑ cAMP | • ↑ Insulin secretion | • Activation of cAMP/PKA signaling pathway | • db/db mice | [45] |
| Curcumin | Ginger | • ↑ cAMP | • ↑ Insulin sensitivity | • Inhibition of PDE activity | • MIN6 cells • HP62 cells | [43] |
| Genistein | Soy; locust horn | • ↑ cAMP | • ↑ Pancreatic β-cell proliferation | • Activation of cAMP/PKA MEK/ERK signaling • Phosphorylation of ERK1/2 | • INS-1 cells • PANC1s cells | [36] |
| • ↑ cAMP | • ↑ Insulin secretion | • Activation of the Adcy/cAMP/PKA signaling pathway | • INS-1 cells • MIN6 cells | [18] | ||
| • ↑ cAMP | • ↑ Insulin secretion | • Accumulation of cAMP and Ca2+ | • MIN6 cells | [46] | ||
| • ↑ cAMP | • ↓ Hyperglycemia • ↑ Glucose tolerance | • Improvement of islet β-cell proliferation, survival, and mass | • C57BL/6J mice | [36] | ||
| 7WA | Green tea | • ↑ cAMP | • ↑ Insulin secretion | • Activation of cAMP/PKA signaling pathway | • RIN-5F cells | [47] |
| Fucoidan | Seaweed Fucus vesiculosus | • ↑ cAMP | • ↑ Insulin secretion • ↓ Hyperglycemia • ↑ Pancreatic β-cell proliferation | • Activation of cAMP/PKA and PI3K/Akt signaling pathway | • INS-1E cells • Human islets | [48] |
| Oleanolic acid | Jujube; papaya | • ↑ cAMP | • ↑ Insulin secretion | • Activation Gs/cAMP/Ca2+ signaling pathway | • MIN6 cells | [49] |
| Coixol | Coix chinensis | • ↑ cAMP | • ↑ Insulin secretion | • Activation of cAMP/PKA signaling pathway | • MIN6 cells | [50] |
| Compounds | Main Source of Food | Concentration of cAMP | Main Effect | Mechanism of Action | Model | Refs. |
|---|---|---|---|---|---|---|
| Curcumin | Ginger | • ↓ cAMP | • ↓ Hepatic glucose production | • Preservation of PDE4B activity • Inhibition of acetyl CoA production | • ICR mice • Primary hepatocytes • HepG2 cells | [19] |
| Ginsenoside Rg5 | Ginseng | • ↓ cAMP | • ↓ Hepatic gluconeogenesis | • Preservation of PDE4B activity | • C57BL/6J mice • Primary hepatocytes | [59] |
| Phanginin A | Caesalpinia sappan | • ↓ cAMP | • ↓ Hepatic gluconeogenesis | • Activation of PDE4 activity • Inhibition of cAMP/PKA/CREB signaling pathway | • C57BL/6J mice • Primary hepatocytes | [15] |
| Astragaloside IV | Astragalus membranaceus | • ↓ cAMP | • ↓ Hepatic lipid deposition • ↓ Hepatic glucose production | • Preservation of PDE3B activity | • ICR mice | [60] |
| Berberine | Cortex phellodendri; coptis chinensis | • ↓ cAMP | • ↓ Hepatic gluconeogenesis | • Activation of PDE activity | • ob/ob mice | [61] |
| • ↓ cAMP | • ↓ Hepatic gluconeogenesis | • Inhibition of glucagon signaling | • Primary hepatocytes | |||
| Ginsenoside Rb1 | Ginseng | • ↓ cAMP | • ↓ Hepatic gluconeogenesis | • Inhibition of Adcy activity • Inactivation of CREB | • C57BL/6J mice • Primary hepatocytes | [62] |
| Dendrobium officinale polysaccharide | Dendrobium officinale | • ↓ cAMP | • ↑ Hepatic glycogen synthesis • ↓ Hepatic glycogen degradation • ↓ Hepatic gluconeogenesis | • Inactivation of Adcy activity • Reduction the expression of PKA | • C57BL/6J mice | [63] |
| Epigallocatechin gallate | Green tea | ND | • ↓ Hepatic glucose production | • Inhibition of cAMP/PKA/CREB signaling pathway | • Primary hepatocytes | [64] |
| Compounds | Main Source of Food | Concentration of cAMP | Main Effect | Mechanism of Action | Model | Refs. |
|---|---|---|---|---|---|---|
| Coffee polyphenols | Coffee, goji berries | • ↑ cAMP | • ↑ GLP-1 secretion | • Activation of cAMP-dependent pathway | • NCI-H716 cells | [69] |
| Geraniol | Geranium; lemon | • ↑ cAMP | • ↑ GLP-1 secretion | • Activation of OR1G1 /cAMP/PKA signaling pathway | • NCI-H716 cells | [70] |
| Citronellal | Kaffir lime leaves | • ↑ cAMP | • ↑ GLP-1 secretion | • Activation of OR1G1/cAMP/PKA signaling pathway | • NCI-H716 cells | [70] |
| Delphinidin | Bilberry; Maqui berry | • ↑ cAMP | • ↓ Glucose uptake | • Activation of FFA1/GPR40/cAMP signaling pathway | • RF/J mice • HT-29 cells • Caco-2 cells • NCM460 cells | [71] |
| Resveratrol | Peanut; grape (red wine); mulberry | • ↑ cAMP | • ↓ Glucose and alanine transport | • Inhibition of PDE activity | • Porcine jejunum and ileum | [72] |
| Polygonatum cyrtonema polysaccharide | Polygonatum cyrtonema | • ↑ cAMP | • ↑ GLP-1 secretion | • Activation of the T1R2/T1R3/cAMP signaling pathway | • NCI-H716 cells | [73] |
| Anthraquinone-glycoside | Rhubarb | • ↑ cAMP | • ↑ GLP-1 secretion | • Activation of cAMP signaling pathway | • SD rats | [74] |
| Oleanolic acid | Jujube; Papaya | • ↑ cAMP | • ↑ GLP-1 and PYY secretion | • Activation of TGR5/cAMP/Epac/Ca2+ signaling pathway | • STC-1 cells | [75] |
| Oleanolic acid derivative DKS26 | Hawthorn | • ↑ cAMP | • ↑ GLP-1 secretion | • Activation of the cAMP/PKA signaling pathway | • db/db mice • NCI-H716 cells | [76] |
| Nonanoic Acid | Royal jelly | • ↑ cAMP | • ↑ GLP-1 and PYY secretion | • Activation of OR51E1/cAMP and p-ERK signaling pathway | • SD rats • NCI-H716 cells | [77] |
| Butyrate | Cheese | • ↑ cAMP | • ↑ Insulin sensitivity | • Activation of cAMP dependent pathway | • SD rats • Caco-2 cells | [68] |
| Compounds | Main Source of Food | Concentration of cAMP | Main Effect | Mechanism | Model | Refs. |
|---|---|---|---|---|---|---|
| Daidzein | Soy; celery | • ↑ cAMP | • ↑ Glucose uptake | • Inhibition of PDE4 activity • Improved the AMPK phosphorylation | • L6 cells • db/db mice | [87] |
| Cyanidin-3-O-β-glucoside | Black soybean; blueberry | • ↑ cAMP | • ↑ Exercise performance | • Inhibition of PDE activity | • ICR mice • C2C12 cells | [88] |
| α-Cedrene | Cedar wood oil | • ↑ cAMP | • ↑ Glucose uptake | • Activation of OR23/cAMP/PKA signaling pathway | • C2C12 cells | [89] |
| [6]-Gingerol | Ginger | • ↑ cAMP | • ↑ Glycogen deposition | • Activation of cAMP/PKA/CREB signaling pathway | • db/db mice | [45] |
| Resveratrol | Grape; mulberry | • ↑ cAMP | • ↑ Glucose uptake | • Inhibition of PDE4 activity | • C2C12 cells | [19,90] |
| Genistein | Soy | • ↑ cAMP | • ↑ Glucose uptake | • Inhibition of PDE activity | • L6 cells | [91] |
| Oleic acid | Walnuts; nuts; almonds | • ↑ cAMP | • ↑ Oxidation of fatty acids | • Activation of Sirtin1-PGC1α complex | • C57BL/6 mice | [64,92] |
| • ↑ cAMP | • ↑ Insulin sensitivity | / | • Wistar rats | [93] |
| Compounds | Main Source of Food | Concentration of cAMP | Main Effect | Mechanism of Action | Model | Refs. |
|---|---|---|---|---|---|---|
| α-Cedrene | Cedar wood oil | • ↑ cAMP | • ↑ Glucose uptake | • Stimulation of mOR23/cAMP/PKA signaling pathway | • 3T3-L1 adipocytes | [89] |
| Pachymic acid | Poria cocos | • ↓ cAMP | • ↑ Glucose uptake • ↓ Lipolysis | • Stimulation of GLUT4 expression and redistribution | • 3T3-L1 adipocytes | [102] |
| Curcumin | Ginger | • ↓ cAMP | • ↓ Lipolysis | • Reduction of ER stress | • C57BL/6 mice • 3T3-L1 adipocytes | [103] |
| Ginsenoside Rg5 | Ginseng | • ↓ cAMP | • ↓ Lipolysis • ↑ Insulin resistance | • Preservation of PDE3B activity | • 3T3-L1 adipocytes | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Q.; Kang, S.-G.; Huang, K.; Tong, T. Dietary Bioactive Ingredients Modulating the cAMP Signaling in Diabetes Treatment. Nutrients 2021, 13, 3038. https://doi.org/10.3390/nu13093038
Wang Y, Liu Q, Kang S-G, Huang K, Tong T. Dietary Bioactive Ingredients Modulating the cAMP Signaling in Diabetes Treatment. Nutrients. 2021; 13(9):3038. https://doi.org/10.3390/nu13093038
Chicago/Turabian StyleWang, Yanan, Qing Liu, Seong-Gook Kang, Kunlun Huang, and Tao Tong. 2021. "Dietary Bioactive Ingredients Modulating the cAMP Signaling in Diabetes Treatment" Nutrients 13, no. 9: 3038. https://doi.org/10.3390/nu13093038







