Diet and Pre-Intervention Washout Modifies the Effects of Probiotics on Gestational Diabetes Mellitus: A Comprehensive Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Criteria and Selection
2.3. Data Extraction and Management
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Selection and Study Characteristics
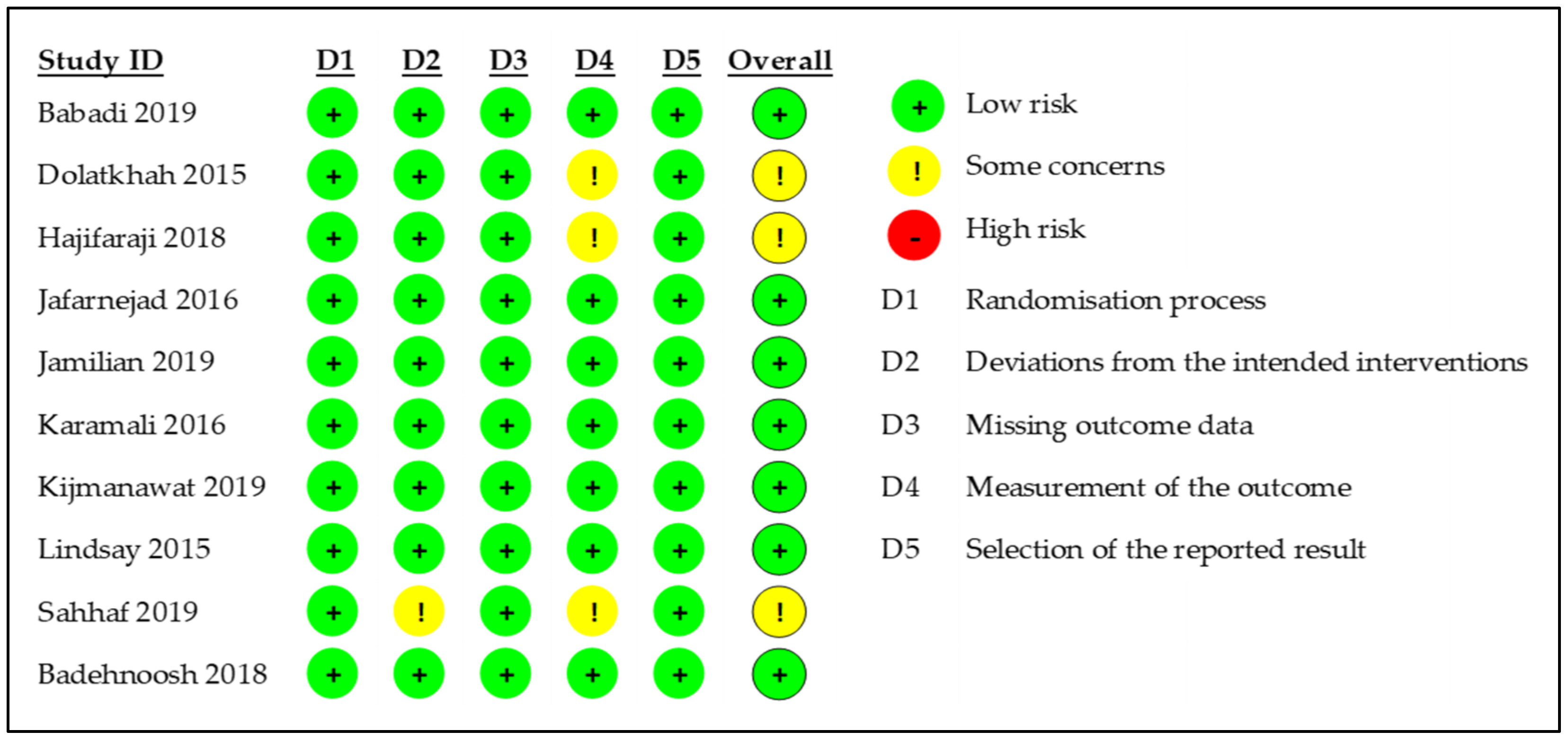
3.2. Quality Assessment
3.3. Effects of Probiotics on Glycemic Control
3.3.1. Sensitivity Analysis
3.3.2. Subgroup Analyses
3.4. Effects of Probiotics on Lipid Parameters
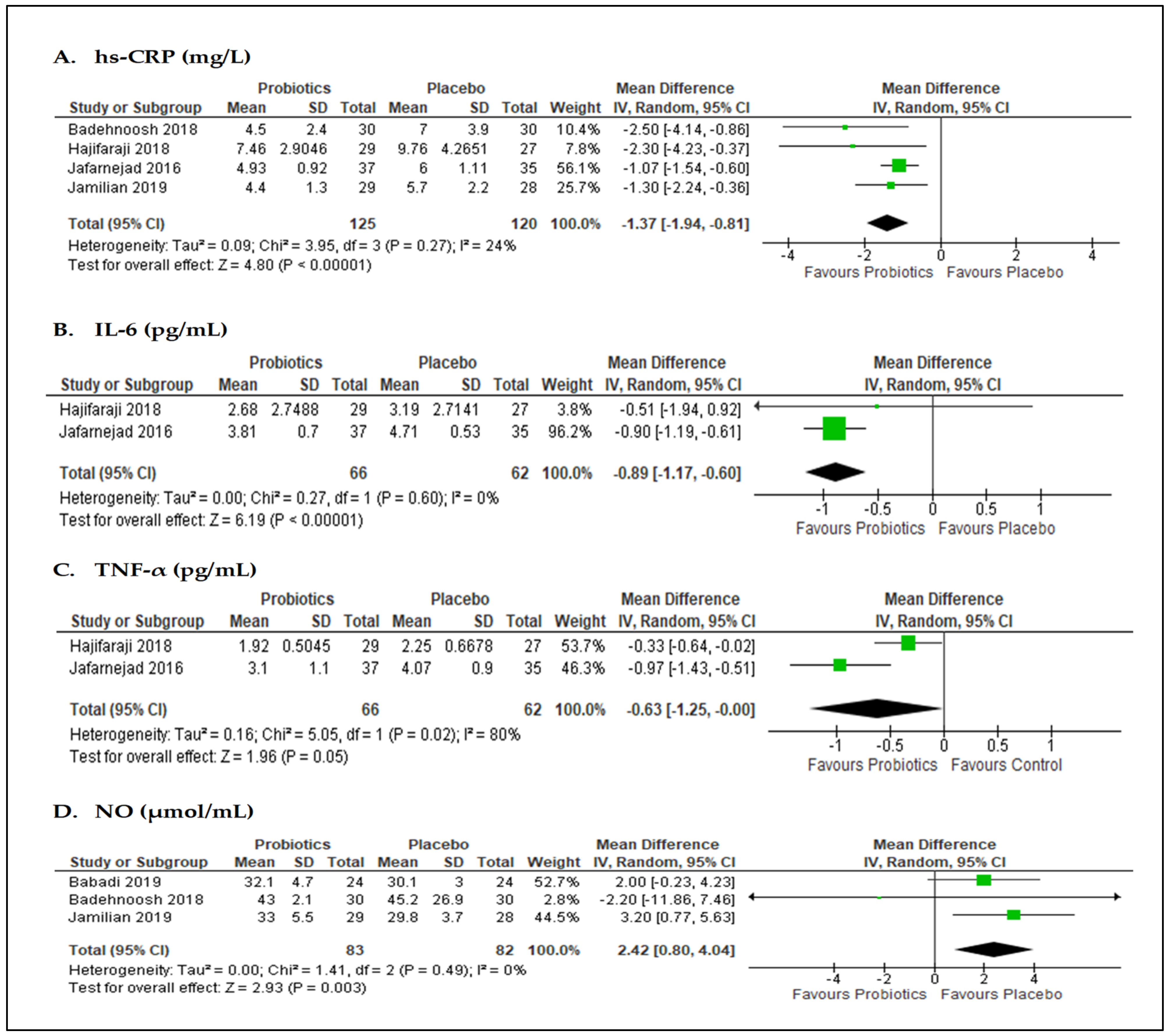
3.5. Effects of Probiotics on Inflammatory Biomarkers
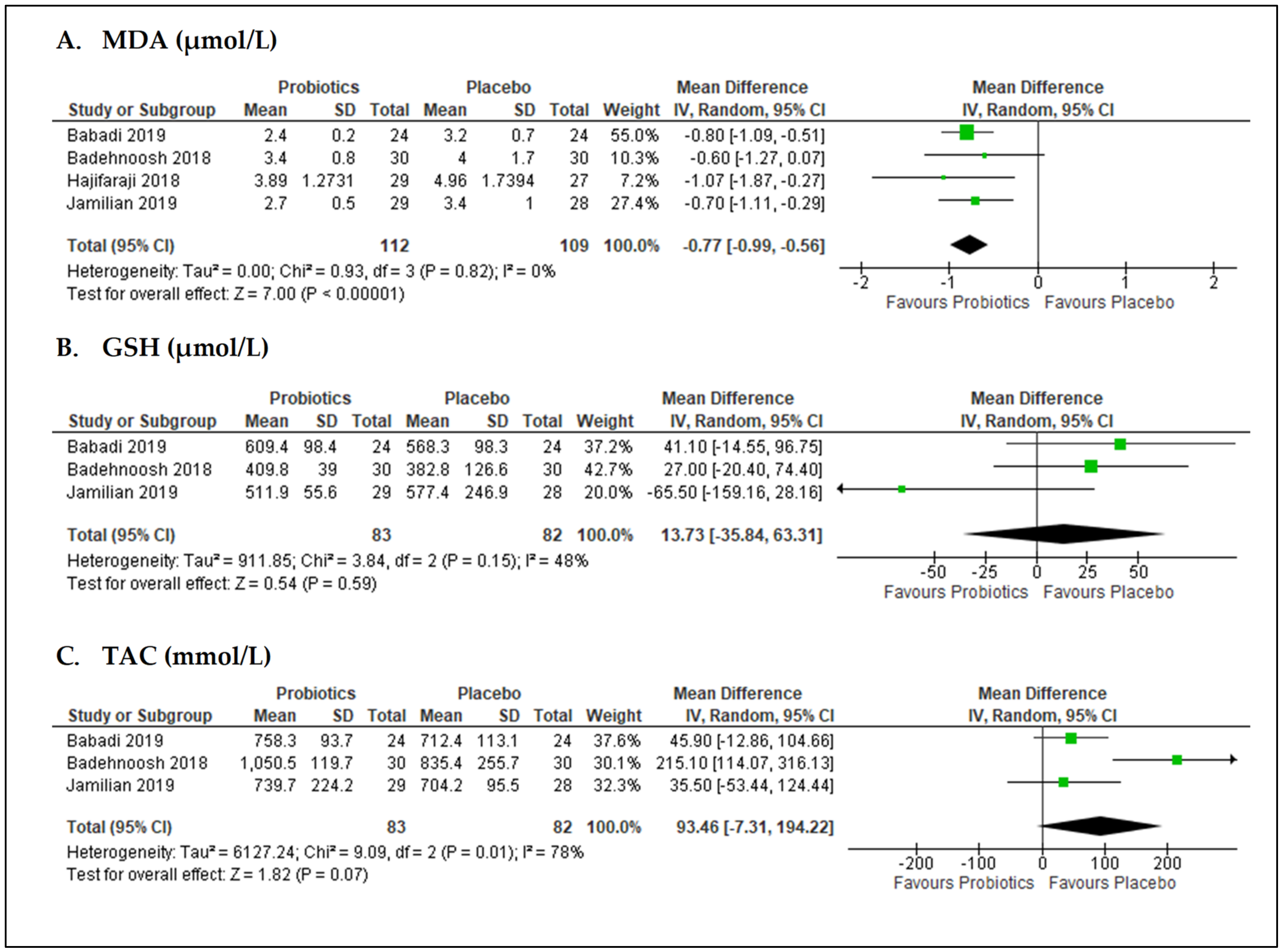
3.6. Effects of Probiotics on Oxidative Stress Biomarkers
3.7. Effects of Probiotics on Maternal Outcomes
3.8. Effects of Probiotics on Neonatal Outcomes
4. Discussion
4.1. Summary of the Findings
4.2. Limitations, Strengths, and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Jiwani, A.; Marseille, E.; Lohse, N.; Damm, P.; Hod, M.; Kahn, J.G. Gestational Diabetes Mellitus: Results from a Survey of Country Prevalence and Practices. J. Matern. Neonatal Med. 2012, 25, 600–610. [Google Scholar] [CrossRef]
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Roura, L.C.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on Gestational Diabetes Mellitus: A Pragmatic Guide for Diagnosis, Management, and Care. Int. J. Gynecol. Obstet. 2015, 131, S173–S211. [Google Scholar] [CrossRef] [Green Version]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 Diabetes Mellitus after Gestational Diabetes: A Systematic Review and Meta-Analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Kim, C. Maternal Outcomes and Follow-up after Gestational Diabetes Mellitus. Diabet. Med. 2014, 31, 292–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S200–S210. [Google Scholar] [CrossRef] [PubMed]
- Dipla, K.; Zafeiridis, A.; Mintziori, G.; Boutou, A.K.; Goulis, D.G.; Hackney, A.C. Exercise as a Therapeutic Intervention in Gestational Diabetes Mellitus. Endocrines 2021, 2, 65–78. [Google Scholar] [CrossRef]
- Ruchat, S.-M.; Mottola, M.F. The Important Role of Physical Activity in the Prevention and Management of Gestational Diabetes Mellitus. Diabetes Metab. Res. Rev. 2013, 29, 334–346. [Google Scholar] [CrossRef]
- Mustafa, S.; Harding, J.; Wall, C.; Crowther, C. Sociodemographic Factors Associated with Adherence to Dietary Guidelines in Women with Gestational Diabetes: A Cohort Study. Nutrients 2021, 13, 1884. [Google Scholar] [CrossRef]
- Balas-Nakash, M.; Rodríguez-Cano, A.; Muñoz-Manrique, C.; Vásquez-Peña, P.; Perichart-Perera, O. Adherence to a Medical Nutrition Therapy Program in Pregnant Women with Diabetes, Measured by Three Methods, and Its Association with Glycemic Control. Rev. Investig. Clin. 2010, 62, 235–243. [Google Scholar]
- Brown, J.; Alwan, N.A.; West, J.; Brown, S.; Mckinlay, C.J.D.; Farrar, D.; Crowther, C.A. Lifestyle Interventions for the Treatment of Women with Gestational Diabetes. Cochrane Database Syst. Rev. 2017, 2017, CD011970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarry-Adkins, J.L.; Ozanne, S.E.; Aiken, C.E. Impact of Metformin Treatment during Pregnancy on Maternal Outcomes: A Systematic Review/Meta-Analysis. Sci. Rep. 2021, 11, 9240. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.M. Intrauterine Factors, Adiposity, and Hyperinsulinaemia. Br. Med. J. 2003, 327, 880–881. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Zhang, L.; Wang, M.; Chen, Y.; Gu, S.; Wang, K.; Leng, J.; Gu, Y.; Xie, X. The Gut Microbiota in Women Suffering from Gestational Diabetes Mellitus with the Failure of Glycemic Control by Lifestyle Modification. J. Diabetes Res. 2019, 2019, 6081248. [Google Scholar] [CrossRef]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational Diabetes Is Associated with Change in the Gut Microbiota Composition in Third Trimester of Pregnancy and Postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef]
- Ferrocino, I.; Ponzo, V.; Gambino, R.; Zarovska, A.; Leone, F.; Monzeglio, C.; Goitre, I.; Rosato, R.; Romano, A.; Grassi, G.; et al. Changes in the Gut Microbiota Composition during Pregnancy in Patients with Gestational Diabetes Mellitus (GDM). Sci. Rep. 2018, 8, 12216. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of Nitric Oxide in Inflammatory Diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Mohamed Ismail, N.A.; Razalli, N.H.; Gnanou, J.V.; Raja Ali, R.A. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Badehnoosh, B.; Karamali, M.; Zarrati, M.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Jafari, P.; Rahmani, E.; Asemi, Z. The Effects of Probiotic Supplementation on Biomarkers of Inflammation, Oxidative Stress and Pregnancy Outcomes in Gestational Diabetes. J. Matern. Neonatal Med. 2018, 31, 1128–1136. [Google Scholar] [CrossRef] [Green Version]
- Mazloom, Z.; Yousefinejad, A.; Dabbaghmanesh, M.H. Effect of Probiotics on Lipid Profile, Glycemic Control, Insulin Action, Oxidative Stress, and Inflammatory Markers in Patients with Type 2 Diabetes: A Clinical Trial. Iran. J. Med. Sci. 2013, 38, 38–43. [Google Scholar]
- Ardeshirlarijani, E.; Tabatabaei-Malazy, O.; Mohseni, S.; Qorbani, M.; Larijani, B.; Baradar Jalili, R. Effect of Probiotics Supplementation on Glucose and Oxidative Stress in Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Trials. DARU J. Pharm. Sci. 2019, 27, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Ziętek, M.; Celewicz, Z.; Szczuko, M. Short-chain Fatty Acids, Maternal Microbiota and Metabolism in Pregnancy. Nutrients 2021, 13, 1244. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaharuddin, L.; Mokhtar, N.M.; Muhammad Nawawi, K.N.; Raja Ali, R.A. A Randomized Double-Blind Placebo-Controlled Trial of Probiotics in Post-Surgical Colorectal Cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Yue, R.; Zhang, B.; Li, Z.; Shui, J.; Huang, X. Effects of Probiotics on Blood Glucose, Biomarkers of Inflammation and Oxidative Stress in Pregnant Women with Gestational Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Med. Clin. 2020, 154, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-Y.; Ahn, Y.-T.; Park, S.-H.; Huh, C.-S.; Yoo, S.-R.; Yu, R.; Sung, M.-K.; McGregor, R.A.; Choi, M.-S. Supplementation of Lactobacillus Curvatus HY7601 and Lactobacillus Plantarum KY1032 in Diet-Induced Obese Mice Is Associated with Gut Microbial Changes and Reduction in Obesity. PLoS ONE 2013, 8, e59470. [Google Scholar] [CrossRef] [Green Version]
- Firouzi, S.; Majid, H.A.; Ismail, A.; Kamaruddin, N.A.; Barakatun-Nisak, M.Y. Effect of Multi-Strain Probiotics (Multi-Strain Microbial Cell Preparation) on Glycemic Control and Other Diabetes-Related Outcomes in People with Type 2 Diabetes: A Randomized Controlled Trial. Eur. J. Nutr. 2017, 56, 1535–1550. [Google Scholar] [CrossRef]
- Ibrahim, A.; Ali, R.A.R.; Manaf, M.R.A.; Ahmad, N.; Tajurruddin, F.W.; Qin, W.Z.; Desa, S.H.M.; Ibrahim, N.M. Multi-Strain Probiotics (Hexbio) Containing MCP BCMC Strains Improved Constipation and Gut Motility in Parkinson’s Disease: A Randomised Controlled Trial. PLoS ONE 2020, 15, e0244680. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Ponzo, V.; Fedele, D.; Goitre, I.; Leone, F.; Lezo, A.; Monzeglio, C.; Finocchiaro, C.; Ghigo, E.; Bo, S. Diet-Gut Microbiota Interactions and Gestational Diabetes Mellitus (GDM). Nutrients 2019, 11, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Probiotics and Prebiotics|World Gastroenterology Organisation. Available online: https://www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics (accessed on 1 July 2021).
- Evans, S.R. Clinical Trial Structures. J. Exp. Stroke Transl. Med. 2010, 3, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.; Sha, L.; Dong, J.; Yi, J.; Liu, Y.; Guo, Z.; Hu, B. Effects of Nutritional Strategies on Glucose Homeostasis in Gestational Diabetes Mellitus: A Systematic Review and Network Meta-Analysis. J. Diabetes Res. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Peng, T.-R.; Wu, T.-W.; Chao, Y.-C. Effect of Probiotics on the Glucose Levels of Pregnant Women: A Meta-Analysis of Randomized Controlled Trials. Medicina 2018, 54, 77. [Google Scholar] [CrossRef] [Green Version]
- Łagowska, K.; Malinowska, A.M.; Zawieja, B.; Zawieja, E. Improvement of Glucose Metabolism in Pregnant Women through Probiotic Supplementation Depends on Gestational Diabetes Status: Meta-Analysis. Sci. Rep. 2020, 10, 17796. [Google Scholar] [CrossRef]
- Ramanathan, K.; Sirala Jagadeesh, N.; Vishwanath, U.; Dayal, C.; Chandrababu, R.; Hayter, M. Efficacy of Supplementation of Probiotics on Maternal Glycaemic Control—A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Epidemiol. Glob. Health 2021, 10, 100674. [Google Scholar] [CrossRef]
- Taylor, B.L.; Woodfall, G.E.; Sheedy, K.E.; O’Riley, M.L.; Rainbow, K.A.; Bramwell, E.L.; Kellow, N.J. Effect of Probiotics on Metabolic Outcomes in Pregnant Women with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 461. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Feng, Q.; Zheng, S.; Xiao, X. The Effects of Probiotics Supplementation on Metabolic Health in Pregnant Women: An Evidence Based Meta-Analysis. PLoS ONE 2018, 13, e0197771. [Google Scholar]
- Masulli, M.; Vitacolonna, E.; Fraticelli, F.; Della Pepa, G.; Mannucci, E.; Monami, M. Effects of Probiotic Supplementation during Pregnancy on Metabolic Outcomes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Res. Clin. Pract. 2020, 162, 108111. [Google Scholar] [CrossRef]
- Pan, J.; Pan, Q.; Chen, Y.; Zhang, H.; Zheng, X. Efficacy of Probiotic Supplement for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Matern. Neonatal Med. 2019, 32, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-M.; Sun, J.-F.; Su, X.-H.; Peng, Y.-F.; Goyal, H.; Wu, C.-H.; Zhu, X.-Y.; Li, L. Probiotics Improve Glucose and Lipid Metabolism in Pregnant Women: A Meta-Analysis. Ann. Transl. Med. 2019, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, S.; Wu, S.; Guo, C.; Long, S.; Tan, H. Effects of Probiotic Supplement in Pregnant Women with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Diabetes Res. 2019, 2019, 5364730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Dolatkhah, N.; Hajifaraji, M.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Mehrabi, Y.; Abbasi, M.M. Is There a Value for Probiotic Supplements in Gestational Diabetes Mellitus? A Randomized Clinical Trial. J. Health Popul. Nutr. 2015, 33, 25. [Google Scholar] [CrossRef] [Green Version]
- Hajifaraji, M.; Jahanjou, F.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Abbasi, M.M.; Dolatkhah, N. Effect of Probiotic Supplements in Women with Gestational Diabetes Mellitus on Inflammation and Oxidative Stress Biomarkers: A Randomized Clinical Trial. Asia Pac. J. Clin. Nutr. 2018, 27, 581–591. [Google Scholar] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, 14898. [Google Scholar] [CrossRef] [Green Version]
- Review Manager (RevMan) [Computer Program]. Version 5.4; The Cochrane Collaboration: London, UK, 2020.
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Babadi, M.; Khorshidi, A.; Aghadavood, E.; Samimi, M.; Kavossian, E.; Bahmani, F.; Mafi, A.; Shafabakhsh, R.; Satari, M.; Asemi, Z. The Effects of Probiotic Supplementation on Genetic and Metabolic Profiles in Patients with Gestational Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1227–1235. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a Multispecies Probiotic Mixture on Glycemic Control and Inflammatory Status in Women with Gestational Diabetes: A Randomized Controlled Clinical Trial. J. Nutr. Metab. 2016, 2016, 5190846. [Google Scholar] [CrossRef] [Green Version]
- Jamilian, M.; Amirani, E.; Asemi, Z. The Effects of Vitamin D and Probiotic Co-Supplementation on Glucose Homeostasis, Inflammation, Oxidative Stress and Pregnancy Outcomes in Gestational Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2019, 38, 2098–2105. [Google Scholar] [CrossRef]
- Karamali, M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Effects of Probiotic Supplementation on Glycaemic Control and Lipid Profiles in Gestational Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Metab. 2016, 42, 234–241. [Google Scholar] [CrossRef]
- Sahhaf Ebrahimi, F.; Homayouni Rad, A.; Mosen, M.; Abbasalizadeh, F.; Tabrizi, A.; Khalili, L. Effect of L. Acidophilus and B. Lactis on Blood Glucose in Women with Gestational Diabetes Mellitus: A Randomized Placebo-Controlled Trial. Diabetol. Metab. Syndr. 2019, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Kijmanawat, A.; Panburana, P.; Reutrakul, S.; Tangshewinsirikul, C. Effects of Probiotic Supplements on Insulin Resistance in Gestational Diabetes Mellitus: A Double-Blind Randomized Controlled Trial. J. Diabetes Investig. 2019, 10, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, O.C.; Smith, T.; Curran, S.; Coffey, M.; Foley, M.E.; Hatunic, M.; Shanahan, F.; et al. Impact of Probiotics in Women with Gestational Diabetes Mellitus on Metabolic Health: A Randomized Controlled Trial. Am. J. Obstet. Gynecol. 2015, 212, e1–e496. [Google Scholar]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mor, G.; Cardenas, I. The Immune System in Pregnancy: A Unique Complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, Y.-S.; Lu, J.-H.; Li, S.-H.; Li, J.-H.; Yuan, M.-Y.; He, J.-R.; Chen, N.-N.; Xiao, W.-Q.; Shen, S.-Y.; Qiu, L.; et al. Connections between the Human Gut Microbiome and Gestational Diabetes Mellitus. Gigascience 2017, 6, gix058. [Google Scholar] [CrossRef]
- Liu, H.; Pan, L.-L.; Lv, S.; Yang, Q.; Zhang, H.; Chen, W.; Lv, Z.; Sun, J. Alterations of Gut Microbiota and Blood Lipidome in Gestational Diabetes Mellitus With Hyperlipidemia. Front. Physiol. 2019, 10, 1015. [Google Scholar] [CrossRef] [Green Version]
- Le Barz, M.; Daniel, N.; Varin, T.V.; Naimi, S.; Demers-Mathieu, V.; Pilon, G.; Audy, J.; Laurin, É.; Roy, D.; Urdaci, M.C.; et al. In Vivo Screening of Multiple Bacterial Strains Identifies Lactobacillus Rhamnosus Lb102 and Bifidobacterium Animalis Ssp. Lactis Bf141 as Probiotics That Improve Metabolic Disorders in a Mouse Model of Obesity. FASEB J. 2019, 33, 4921–4935. [Google Scholar] [CrossRef]
- Davoren, M.J.; Liu, J.; Castellanos, J.; Rodríguez-Malavé, N.I.; Schiestl, R.H. A Novel Probiotic, Lactobacillus Johnsonii 456, Resists Acid and Can Persist in the Human Gut beyond the Initial Ingestion Period. Gut Microbes 2019, 10, 458–480. [Google Scholar] [CrossRef]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Role of Commercial Probiotic Strains against Human Pathogen Adhesion to Intestinal Mucus. Lett. Appl. Microbiol. 2007, 45, 454–460. [Google Scholar] [CrossRef]
- Rodes, L.; Khan, A.; Paul, A.; Coussa-Charley, M.; Marinescu, D.; Tomaro-Duchesneau, C.; Shao, W.; Kahouli, I.; Prakash, S. Effect of Probiotics Lactobacillus and Bifidobacterium on Gut-Derived Lipopolysaccharides and Inflammatory Cytokines: An in Vitro Study Using a Human Colonic Microbiota Model. J. Microbiol. Biotechnol. 2013, 23, 518–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonucci, L.B.; Olbrich dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical Application of Probiotics in Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal Mucosal Adherence and Translocation of Commensal Bacteria at the Early Onset of Type 2 Diabetes: Molecular Mechanisms and Probiotic Treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araújo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. Probiotics Modulate Gut Microbiota and Improve Insulin Sensitivity in DIO Mice. J. Nutr. Biochem. 2017, 50, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Rueangdetnarong, H.; Sekararithi, R.; Jaiwongkam, T.; Kumfu, S.; Chattipakorn, N.; Tongsong, T.; Jatavan, P. Comparisons of the Oxidative Stress Biomarkers Levels in Gestational Diabetes Mellitus (GDM) and Non-GDM among Thai Population: Cohort Study. Endocr. Connect. 2018, 7, 681–687. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, H.; Geng, Q.; Ma, Q.; Long, Y.; Zhou, C.; Chen, M. Association of Oxidative Stress Biomarkers with Gestational Diabetes Mellitus in Pregnant Women: A Case-Control Study. PLoS ONE 2015, 10, e0126490. [Google Scholar]
- Didari, T.; Solki, S.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. A Systematic Review of the Safety of Probiotics. Expert Opin. Drug Saf. 2014, 13, 227–239. [Google Scholar] [CrossRef]
- Hendijani, F.; Akbari, V. Probiotic Supplementation for Management of Cardiovascular Risk Factors in Adults with Type II Diabetes: A Systematic Review and Meta-Analysis. Clin. Nutr. 2018, 37, 532–541. [Google Scholar] [CrossRef]
- Homayoni Rad, A.; Mehrabany, E.V.; Alipoor, B.; Mehrabany, L.V.; Javadi, M. Do Probiotics Act More Efficiently in Foods than in Supplements? Nutrition 2012, 28, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.; Vidanarachchi, J.; Rocha, R.; Cruz, A.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Saxelin, M.; Lassig, A.; Karjalainen, H.; Tynkkynen, S.; Surakka, A.; Vapaatalo, H.; Järvenpää, S.; Korpela, R.; Mutanen, M.; Hatakka, K. Persistence of Probiotic Strains in the Gastrointestinal Tract When Administered as Capsules, Yoghurt, or Cheese. Int. J. Food Microbiol. 2010, 144, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, T.A.; Mainville, I.; Arcand, Y. The Impact of Meals on a Probiotic during Transit through a Model of the Human Upper Gastrointestinal Tract. Benef. Microbes 2011, 2, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Samah, S.; Ramasamy, K.; Lim, S.M.; Neoh, C.F. Probiotics for the Management of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Res. Clin. Pract. 2016, 118, 172–182. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas. 2019. Available online: https://diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf (accessed on 3 July 2021).
- Smith, T.J.; Anderson, D.; Margolis, L.M.; Sikes, A.; Young, A.J. Persistence of Lactobacillus reuteri DSM17938 in the Human Intestinal Tract: Response to Consecutive and Alternate-Day Supplementation. J. Am. Coll. Nutr. 2011, 30, 259–264. [Google Scholar] [CrossRef]
- Duc, L.H.; Hong, H.A.; Barbosa, T.M.; Henriques, A.O.; Cutting, S.M. Characterization of Bacillus Probiotics Available for Human Use. Appl. Environ. Microbiol. 2004, 70, 2161–2171. [Google Scholar] [CrossRef] [Green Version]
- Elvers, K.T.; Wilson, V.J.; Hammond, A.; Duncan, L.; Huntley, A.L.; Hay, A.D.; van der Werf, E.T. Antibiotic-Induced Changes in the Human Gut Microbiota for the Most Commonly Prescribed Antibiotics in Primary Care in the UK: A Systematic Review. BMJ Open 2020, 10, e035677. [Google Scholar] [CrossRef]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of Gut Microbiota of Healthy Adults Following Antibiotic Exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef]
- Nagulesapillai, V.; Belvis, J.; Tompkins, T.; Girard, S.-A. Detection and Quantification of Probiotic Strains in Clinical Fecal Samples of Healthy Adults by Real-Time PCR. FASEB J. 2017, 31, lb207. [Google Scholar]
- Valerio, F.; de Candia, S.; Lonigro, S.L.; Russo, F.; Riezzo, G.; Orlando, A.; De Bellis, P.; Sisto, A.; Lavermicocca, P. Role of the Probiotic Strain Lactobacillus Paracasei LMGP22043 Carried by Artichokes in Influencing Faecal Bacteria and Biochemical Parameters in Human Subjects. J. Appl. Microbiol. 2011, 111, 155–164. [Google Scholar] [CrossRef]
- Meance, S.; Cayuela, C.; Raimondi, A.; Turchet, P.; Lucas, C.; Antoine, J.M. Recent Advances in the Use of Functional Foods: Effects of the Commercial Fermented Milk with Bifidobacterium Animalis Strain DN-173 010 and Yoghurt Strains on Gut Transit Time in the Elderly. Microb. Ecol. Health Dis. 2003, 15, 15–22. [Google Scholar] [CrossRef]
- Divella, R.; De Palma, G.; Tufaro, A.; Pelagio, G.; Gadaleta-Caldarola, G.; Bringiotti, R.; Paradiso, A. Diet, Probiotics and Physical Activity: The Right Allies for a Healthy Microbiota. Anticancer Res. 2021, 41, 2759–2772. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I.; Di Cagno, R.; De Angelis, M.; Turroni, S.; Vannini, L.; Bancalari, E.; Rantsiou, K.; Cardinali, G.; Neviani, E.; Cocolin, L. Fecal Microbiota in Healthy Subjects Following Omnivore, Vegetarian and Vegan Diets: Culturable Populations and RRNA DGGE Profiling. PLoS ONE 2015, 10, e0128669. [Google Scholar]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory Fitness as a Predictor of Intestinal Microbial Diversity and Distinct Metagenomic Functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in Fecal Microbiota Composition by Probiotic Supplementation in Healthy Adults: A Systematic Review of Randomized Controlled Trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Hasain, Z.; Raja Ali, R.A.; Abdul Razak, S.; Azizan, K.A.; El-Omar, E.; Razalli, N.H.; Mokhtar, N.M. Gut Microbiota Signature Among Asian Post-Gestational Diabetes Women Linked to Macronutrient Intakes and Metabolic Phenotypes. Front. Microbiol. 2021, 12, 1559. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Dante, G.; Petrella, E.; Neri, I. Dietary Interventions, Lifestyle Changes, and Dietary Supplements in Preventing Gestational Diabetes Mellitus: A Literature Review. Obstet. Gynecol. Surv. 2014, 69, 669–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef] [Green Version]



| First Author | Study Design | Sample Size | Study Population | Washout Period | Probiotics Intervention | Requirement of Hypoglycemic Agents during Intervention | Compliance (%) Side Effects | Outcomes Measured | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year, Reference | Country Dx of GDM | Total | Gravida Status | Age (Years) | Probiotics Antibiotics | Probiotic Species | Vehicle Dosage (CFU) Frequency Duration | |||||
| I | C | Gestational Age | I | C | ||||||||
| Babadi 2019, [51] | P, RCT, DB Iran 2-h 75 g OGTT | 48 24 24 | Primigravida 24–28 weeks | 28.3 ± 4.3 | 29.0 ± 4.2 | NR NR | B. bifidum L. acidophilus L. casei L. fermentum | Capsule 2 × 109 CFU/g each Once daily 6 weeks | No | >90% No | Genetic Glycemic Lipid Inflammatory Oxidative stress Weight gain | |
| Badehnoosh 2018, [20] | P, RCT, DB Iran 2-h 75 g OGTT | 60 30 30 | Primigravida 24–28 weeks | 28.8 ± 5.4 | 27.8 ± 3.7 | NR NR | B. bifidum L. acidophilus L. casei | Capsule 2 × 109 CFU/g each Once daily 6 weeks | Yes—5 women (3-I and 2-C) | 100% NR | Inflammatory Glycemic Oxidative stress Pregnancy 3 | |
| Dolatkhah 1 2015, [46] Hajifaraji 2 2018, [47] | P, RCT, DB Iran 2-h 75 g OGTT | 56 29 27 | Primigravida 24–28 weeks | 28.1 ± 6.2 | 26.5 ± 5.2 | 2 weeks 4 weeks | B. BB-12 L. acidophilus LA-5 L. delbrueckii Bulgaricus LBY-27 Streptococcus thermophilus STY-31 | Capsule 4 biocap > 4 × 109 CFU Once daily 8 weeks | Yes—4 women (2-I and 2-C) | 100% No | Glycemic Inflammatory Oxidative stress Weight gain | |
| Jafarnejad 2016, [52] | P, RCT, DB Iran 2-h 75 g OGTT | 72 37 35 | Primigravida, Multigravida ~26 weeks | 32.4 ± 3.1 | 31.9 ± 4.0 | 10 days NR | B. breve, B. longum B. infantis, L. acidophilus L. plantarum, L. paracasei L. delbrueckii subsp. Bulgaricus Streptococcus thermophilus | VSL#3 capsule 112.5 × 109 CFU/capsule Twice daily 8 weeks | Yes—7 women (2-I and 5-C) | NR No | Glycemic Inflammatory | |
| Jamilian 2019,[53] | P, RCT, DB Iran 2-h 75 g OGTT | 57 29 28 | Primigravida 24–28 weeks | 31.2 ± 5.9 | 29.9 ± 3.7 | 3 months NR | B. bifidum L. acidophilus L. casei L. fermentum | Capsule 8 × 109 CFU/g Once daily 6 weeks | Yes—3 women (1-I and 2-C) | 100% NR | Glycemic Lipid Inflammatory Oxidative stres Pregnancy 3 | |
| Karamali 2016, [54] | P, RCT, DB Iran 2-h 75 g OGTT | 60 30 30 | Primigravida 24–28 weeks | 31.8 ± 6.0 | 29.7 ± 4.0 | NR NR | B. bifidum L. acidophilus L. casei | Capsule 2 × 109 CFU/g each Once daily 6 weeks | No | >90% No | Glycemic Lipid Weight gain | |
| Kijmanawat 2019, [56] | P, RCT, DB Thailand 2-h 75 g OGTT | 57 28 29 | Primigravida 24–28 weeks | 32.5 ± 5.0 | 30.7 ± 5.1 | 2 weeks 4 weeks | B. bifidum L. acidophilus | Capsule 1 × 109 CFU/each Once daily after meal 4 weeks | No | >90% No | Glycemic Weight gain Neonatal | |
| Lindsay 2015, [57] | P, RCT, DB Ireland 3-h 100 g OGTT | 100 48 52 | Primigravida 18–34 weeks Included both IGT and GDM | 33.5 ± 5.0 | 32.6 ± 4.5 | NR NR | L. salivarius UCC118 | Capsule 100 mg at 109 CFU Once daily after meal 4–6 weeks | Yes—15 women (9-I and 6-C) | NR NR | Glycemic Lipid Pregnancy 3 | |
| Sahhaf Ebrahimi 2019, [55] | P, RCT, DB Iran 2-h 75 g OGTT | 84 42 42 | NR 24–28 weeks | 31.6 ± 6.0 | 31.6 ± 5.5 | 1 week NR | B. lactis L. acidophilus | Yogurt 300 g/day (106 CFU) Once daily 8 weeks | No | NR NR | Glycemic Neonatal | |
| Analysis | No. | References | Random-Effects Model | Fixed-Effect Model | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| MD (95% CI) | p | MD (95% CI) | p | I2 | p | |||
| Subgroup 1: Duration of intervention | 9 | |||||||
| ≤6 weeks | 6 | [20,51,53,54,56,57] | −3.26 [−5.25, −1.27] | 0.001 | −3.10 [−4.72, −1.49] | 0.002 | 32% | 0.2 |
| >6 weeks | 3 | [46,52,55] | −2.73 [−7.06, 1.59] | 0.22 | −2.90 [−4.05, −1.75] | <0.001 | 91% | <0.001 |
| Subgroup 2: Number of species | 9 | |||||||
| ≤3 species | 5 | [20,54,55,56,57] | −2.67 [−4.49, −0.85] | 0.004 | −2.64 [−4.41, −0.86] | 0.004 | 5% | 0.38 |
| >3 species | 4 | [46,51,52,53] | −3.28 [−6.94, 0.37] | 0.08 | −3.10 [−4.20, −1.99] | <0.001 | 88% | <0.001 |
| Subgroup 3: Probiotics washout period | 5 | |||||||
| <2 weeks | 2 | [52,55] | −0.06 [−1.78, 1.65] | 0.94 | −0.06 [−1.78, 1.65] | 0.94 | 53% | 0.14 |
| ≥2 weeks | 3 | [46,53,56] | −5.27 [−6.63, −3.91] | <0.001 | −5.27 [−6.63, −3.91] | <0.001 | 0% | 0.56 |
| Subgroup 4: Dietary intervention | 9 | |||||||
| Received dietary advice | 4 | [46,51,56,57] | −3.16 [−5.90, −0.41] | 0.02 | −4.07 [−5.33, −2.80] | <0.001 | 67% | 0.03 |
| Maintain regular diet | 5 | [20,52,53,54,55] | −3.14 [−6.09, −0.18] | 0.04 | −1.63 [−3.02, −0.23] | 0.02 | 69% | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasain, Z.; Che Roos, N.A.; Rahmat, F.; Mustapa, M.; Raja Ali, R.A.; Mokhtar, N.M. Diet and Pre-Intervention Washout Modifies the Effects of Probiotics on Gestational Diabetes Mellitus: A Comprehensive Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 3045. https://doi.org/10.3390/nu13093045
Hasain Z, Che Roos NA, Rahmat F, Mustapa M, Raja Ali RA, Mokhtar NM. Diet and Pre-Intervention Washout Modifies the Effects of Probiotics on Gestational Diabetes Mellitus: A Comprehensive Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2021; 13(9):3045. https://doi.org/10.3390/nu13093045
Chicago/Turabian StyleHasain, Zubaidah, Nur Aishah Che Roos, Frhana Rahmat, Marami Mustapa, Raja Affendi Raja Ali, and Norfilza Mohd Mokhtar. 2021. "Diet and Pre-Intervention Washout Modifies the Effects of Probiotics on Gestational Diabetes Mellitus: A Comprehensive Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 13, no. 9: 3045. https://doi.org/10.3390/nu13093045
APA StyleHasain, Z., Che Roos, N. A., Rahmat, F., Mustapa, M., Raja Ali, R. A., & Mokhtar, N. M. (2021). Diet and Pre-Intervention Washout Modifies the Effects of Probiotics on Gestational Diabetes Mellitus: A Comprehensive Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 13(9), 3045. https://doi.org/10.3390/nu13093045






